Nutrient Combinations Sensed by L-Cell Receptors Potentiate GLP-1 Secretion
Abstract
1. Introduction
2. Results
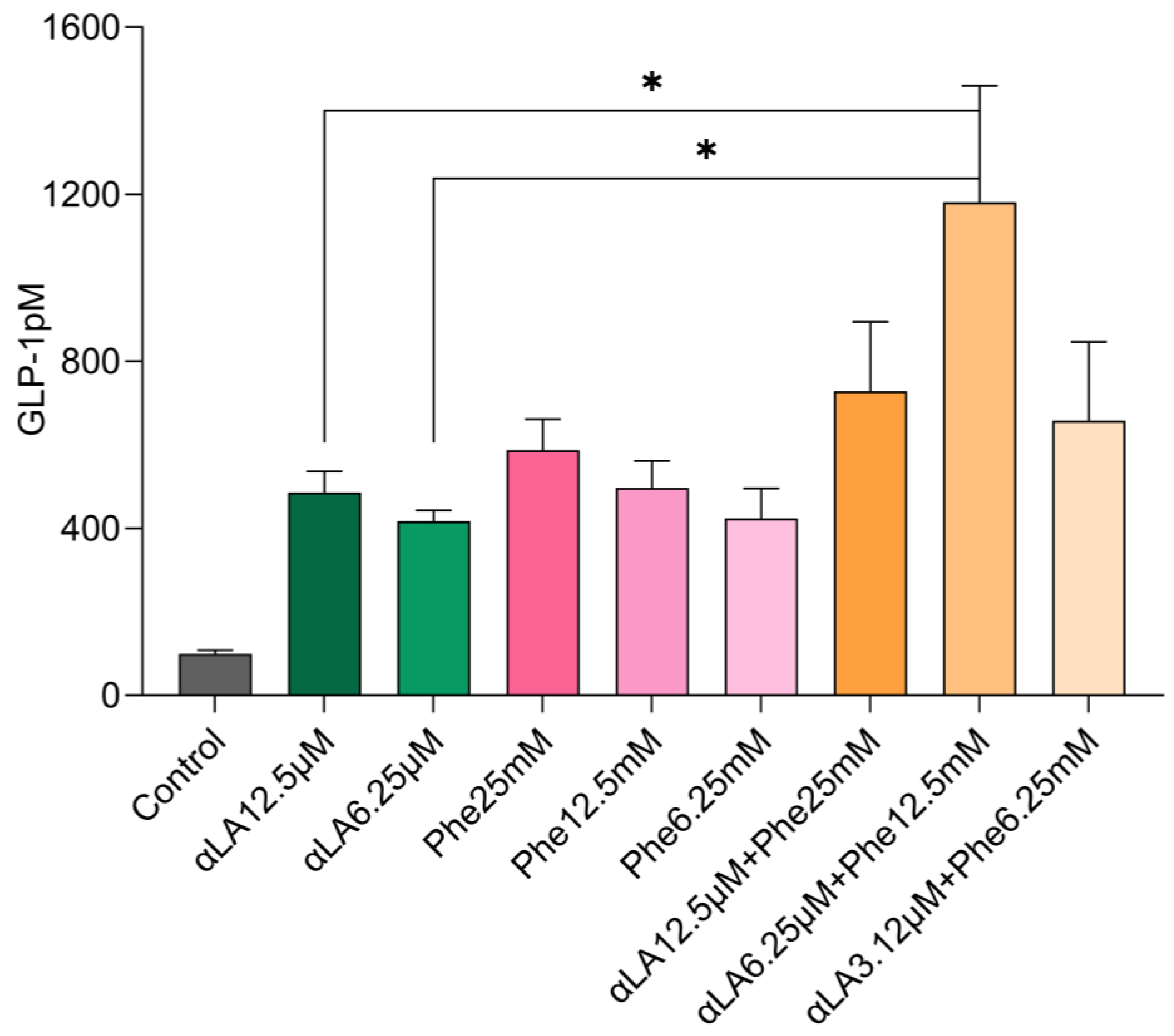
2.1. In Vitro GLP-1 Secretions in STC-1 Cells
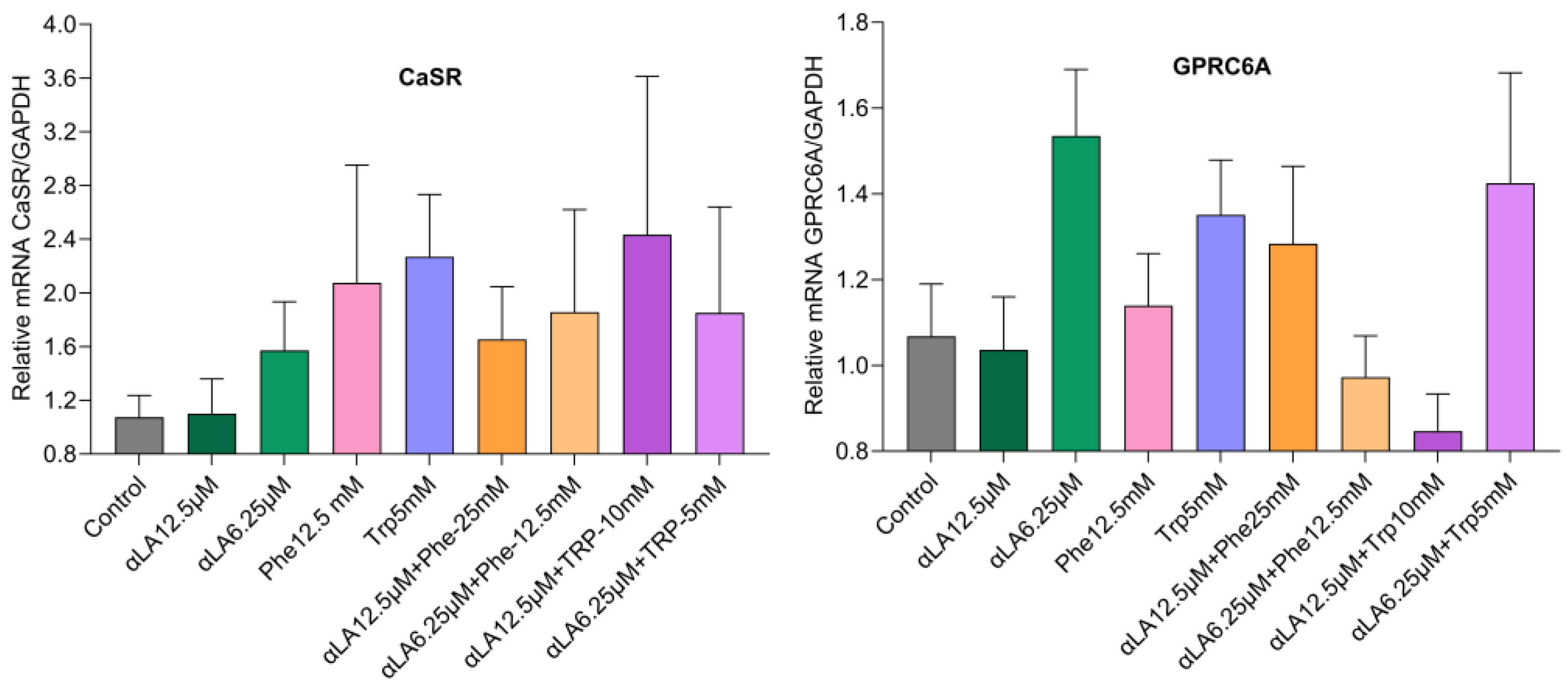
2.2. Gene Expression
mRNA Expression Levels of Fatty Acid and Amino Acid Receptors
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. STC-1 Cell Culture
4.3. In Vitro GLP-1 Secretions from STC-1 Cells
4.4. Gene Expression (RT-PCR)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why Does Obesity Cause Diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like Peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Karhunen, L.J.; Juvonen, K.R.; Huotari, A.; Purhonen, A.K.; Herzig, K.H. Effect of Protein, Fat, Carbohydrate and Fibre on Gastrointestinal Peptide Release in Humans. Regul. Pept. 2008, 149, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kovalainen, M.; Mönkäre, J.; Riikonen, J.; Pesonen, U.; Vlasova, M.; Salonen, J.; Lehto, V.P.; Järvinen, K.; Herzig, K.H. Novel Delivery Systems for Improving the Clinical Use of Peptides. Pharmacol. Rev. 2015, 67, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Huotari, A.; Xu, W.; Mönkäre, J.; Kovalainen, M.; Herzig, K.H.; Lehto, V.P.; Järvinen, K. Effect of Surface Chemistry of Porous Silicon Microparticles on Glucagon-like Peptide-1 (GLP-1) Loading, Release and Biological Activity. Int. J. Pharm. 2013, 454, 67–73. [Google Scholar] [CrossRef]
- Harden, C.J.; Perez-Carrion, K.; Babakordi, Z.; Plummer, S.F.; Hepburn, N.; Barker, M.E.; Wright, P.C.; Evans, C.A.; Corfe, B.M. Evaluation of the Salivary Proteome as a Surrogate Tissue for Systems Biology Approaches to Understanding Appetite. J. Proteom. 2012, 75, 2916–2923. [Google Scholar] [CrossRef]
- Thomsen, C.; Storm, H.; Holst, J.J.; Hermansen, K. Differential Effects of Saturated and Monounsaturated Fats on Postprandial Lipemia and Glucagon-like Peptide 1 Responses in Patients with Type 2 Diabetes. Am. J. Clin. Nutr. 2003, 77, 605–611. [Google Scholar] [CrossRef]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Smout, A.J.P.M.; Wishart, J.; Pilichiewicz, A.N.; Rades, T.; Chapman, I.M.; Feinle-Bisset, C. Effects of Intraduodenal Fatty Acids on Appetite, Antropyloroduodenal Motility, and Plasma CCK and GLP-1 in Humans Vary with Their Chain Length. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R524–R533. [Google Scholar] [CrossRef]
- Rocca, A.S.; Brubaker, P.L. Stereospecific Effects of Fatty Acids on Proglucagon-Derived Peptide Secretion in Fetal Rat Intestinal Cultures. Endocrinology 1995, 136, 5593–5599. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, R.; Raza, G.S.; Sodum, N.; Lehto, V.-P.; Kovalainen, M.; Herzig, K.-H. Colonic Delivery of Nutrients for Sustained and Prolonged Release of Gut Peptides: A Novel Strategy for Appetite Management. Mol. Nutr. Food Res. 2022, 66, e2200192. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, E.; Watterson, K.R.; Stocker, C.J.; Sokol, E.; Jenkins, L.; Simon, K.; Grundmann, M.; Petersen, R.K.; Wargent, E.T.; Hudson, B.D.; et al. Activity of Dietary Fatty Acids on FFA1 and FFA4 and Characterisation of Pinolenic Acid as a Dual FFA1/FFA4 Agonist with Potential Effect against Metabolic Diseases. Br. J. Nutr. 2015, 113, 1677–1688. [Google Scholar] [CrossRef]
- Diakogiannaki, E.; Pais, R.; Tolhurst, G.; Parker, H.E.; Horscroft, J.; Rauscher, B.; Zietek, T.; Daniel, H.; Gribble, F.M.; Reimann, F. Oligopeptides Stimulate Glucagon-like Peptide-1 Secretion in Mice through Proton-Coupled Uptake and the Calcium-Sensing Receptor. Diabetologia 2013, 56, 2688–2696. [Google Scholar] [CrossRef] [PubMed]
- Oya, M.; Kitaguchi, T.; Pais, R.; Reimann, F.; Gribble, F.; Tsuboi, T. The G Protein-Coupled Receptor Family C Group 6 Subtype A (GPRC6A) Receptor Is Involved in Amino Acid-Induced Glucagon-like Peptide-1 Secretion from GLUTag Cells. J. Biol. Chem. 2013, 288, 4513. [Google Scholar] [CrossRef] [PubMed]
- Santos-Hernández, M.; Vivanco-Maroto, S.M.; Miralles, B.; Recio, I. Food Peptides as Inducers of CCK and GLP-1 Secretion and GPCRs Involved in Enteroendocrine Cell Signalling. Food Chem. 2023, 402, 134225. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A Gut-Brain Neural Circuit for Nutrient Sensory Transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The Arcuate Nucleus Mediates GLP-1 Receptor Agonist Liraglutide-Dependent Weight Loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like Peptide 1 in Health and Disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, X.; Cao, H.; Qingguo, L.; Li, N.; Liu, Y.; Zhang, X.; Zhang, Y.; Cao, M.; Wan, J.; et al. Impaired Secretion of Total Glucagon-like Peptide-1 in People with Impaired Fasting Glucose Combined Impaired Glucose Tolerance. Int. J. Med. Sci. 2012, 9, 574–581. [Google Scholar] [CrossRef]
- Larsen, M.P.; Torekov, S.S. Glucagon-Like Peptide 1: A Predictor of Type 2 Diabetes? J. Diabetes Res. 2017, 2017, 7583506. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Vardarli, I.; Deacon, C.F.; Holst, J.J.; Meier, J.J. Secretion of Glucagon-like Peptide-1 (GLP-1) in Type 2 Diabetes: What Is up, What Is Down? Diabetologia 2011, 54, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Faerch, K.; Torekov, S.S.; Vistisen, D.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; Sandbaek, A.; et al. GLP-1 Response to Oral Glucose Is Reduced in Prediabetes, Screen-Detected Type 2 Diabetes, and Obesity and Influenced by Sex: The ADDITION-PRO Study. Diabetes 2015, 64, 2513–2525. [Google Scholar] [CrossRef] [PubMed]
- Muscelli, E.; Mari, A.; Casolaro, A.; Camastra, S.; Seghieri, G.; Gastaldelli, A.; Holst, J.J.; Ferrannini, E. Separate Impact of Obesity and Glucose Tolerance on the Incretin Effect in Normal Subjects and Type 2 Diabetic Patients. Diabetes 2008, 57, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Cajacob, L.; Keller, N.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Peterli, R.; Beglinger, C.; Meyer-Gerspach, A.C. Gut Hormone Secretion, Gastric Emptying, and Glycemic Responses to Erythritol and Xylitol in Lean and Obese Subjects. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1053–E1061. [Google Scholar] [CrossRef] [PubMed]
- Knop, F.K.; Aaboe, K.; Vilsbøll, T.; Vølund, A.; Holst, J.J.; Krarup, T.; Madsbad, S. Impaired Incretin Effect and Fasting Hyperglucagonaemia Characterizing Type 2 Diabetic Subjects Are Early Signs of Dysmetabolism in Obesity. Diabetes. Obes. Metab. 2012, 14, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Calanna, S.; Christensen, M.; Holst, J.J.; Laferrère, B.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Secretion of Glucagon-like Peptide-1 in Patients with Type 2 Diabetes Mellitus: Systematic Review and Meta-Analyses of Clinical Studies. Diabetologia 2013, 56, 965–972. [Google Scholar] [CrossRef]
- Chong, S.C.; Sukor, N.; Robert, S.A.; Ng, K.F.; Kamaruddin, N.A. Fasting and Stimulated Glucagon-like Peptide-1 Exhibit a Compensatory Adaptive Response in Diabetes and Pre-Diabetes States: A Multi-Ethnic Comparative Study. Front. Endocrinol. 2022, 13, 961432. [Google Scholar] [CrossRef]
- Larraufie, P.; Roberts, G.P.; McGavigan, A.K.; Kay, R.G.; Li, J.; Leiter, A.; Melvin, A.; Biggs, E.K.; Ravn, P.; Davy, K.; et al. Important Role of the GLP-1 Axis for Glucose Homeostasis after Bariatric Surgery. Cell Rep. 2019, 26, 1399. [Google Scholar] [CrossRef]
- Roberts, G.P.; Kay, R.G.; Howard, J.; Hardwick, R.H.; Reimann, F.; Gribble, F.M. Gastrectomy with Roux-En-Y Reconstruction as a Lean Model of Bariatric Surgery. Surg. Obes. Relat. Dis. 2018, 14, 562–568. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Aylwin, S.J.B.; Batterham, R.L.; Borg, C.M.; Coyle, F.; Prasad, V.; Shurey, S.; Ghatei, M.A.; Patel, A.G.; Bloom, S.R. Gut Hormone Profiles Following Bariatric Surgery Favor an Anorectic State, Facilitate Weight Loss, and Improve Metabolic Parameters. Ann. Surg. 2006, 243, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Fourmy, D. Hybrid Peptides in the Landscape of Drug Discovery. Peptides 2017, 90, A1–A2. [Google Scholar] [CrossRef]
- Pan, C.Q.; Buxton, J.M.; Yung, S.L.; Tom, I.; Yang, L.; Chen, H.; MacDougall, M.; Bell, A.; Claus, T.H.; Clairmont, K.B.; et al. Design of a Long Acting Peptide Functioning as Both a Glucagon-like Peptide-1 Receptor Agonist and a Glucagon Receptor Antagonist. J. Biol. Chem. 2006, 281, 12506–12515. [Google Scholar] [CrossRef] [PubMed]
- Pocai, A.; Carrington, P.E.; Adams, J.R.; Wright, M.; Eiermann, G.; Zhu, L.; Du, X.; Petrov, A.; Lassman, M.E.; Jiang, G.; et al. Glucagon-Like Peptide 1/Glucagon Receptor Dual Agonism Reverses Obesity in Mice. Diabetes 2009, 58, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W.; Ottaway, N.; Patterson, J.T.; Gelfanov, V.; Smiley, D.; Gidda, J.; Findeisen, H.; Bruemmer, D.; Drucker, D.J.; Chaudhary, N.; et al. A New Glucagon and GLP-1 Co-Agonist Eliminates Obesity in Rodents. Nat. Chem. Biol. 2009, 5, 749–757. [Google Scholar] [CrossRef]
- Bossart, M.; Wagner, M.; Elvert, R.; Evers, A.; Hübschle, T.; Kloeckener, T.; Lorenz, K.; Moessinger, C.; Eriksson, O.; Velikyan, I.; et al. Effects on Weight Loss and Glycemic Control with SAR441255, a Potent Unimolecular Peptide GLP-1/GIP/GCG Receptor Triagonist. Cell Metab. 2022, 34, 59–74.e10. [Google Scholar] [CrossRef]
- Bailey, C.J. Glucose-Lowering Therapies in Type 2 Diabetes: Opportunities and Challenges for Peptides. Peptides 2018, 100, 9–17. [Google Scholar] [CrossRef]
- Herzig, K.H. Peptides Combined—Physiology Revisited! Peptides 2018, 110, A1–A2. [Google Scholar] [CrossRef]
- Kamakura, R.; Raza, G.S.; Prasannan, A.; Walkowiak, J.; Herzig, K.H. Dipeptidyl Peptidase-4 and GLP-1 Interplay in STC-1 and GLUTag Cell Lines. Peptides 2020, 134, 170419. [Google Scholar] [CrossRef]
- Acar, I.; Cetinkaya, A.; Lay, I.; Ileri-Gurel, E. The Role of Calcium Sensing Receptors in GLP-1 and PYY Secretion after Acute Intraduodenal Administration of L-Tryptophan in Rats. Nutr. Neurosci. 2020, 23, 481–489. [Google Scholar] [CrossRef]
- Alamshah, A.; Spreckley, E.; Norton, M.; Kinsey-Jones, J.S.; Amin, A.; Ramgulam, A.; Cao, Y.; Johnson, R.; Saleh, K.; Akalestou, E.; et al. L-Phenylalanine Modulates Gut Hormone Release and Glucose Tolerance, and Suppresses Food Intake through the Calcium-Sensing Receptor in Rodents. Int. J. Obes. 2017, 41, 1693–1701. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Narumi, K.; Yamagishi, N.; Nishi, T.; Ito, T.; Iseki, K.; Kobayashi, M.; Kanai, Y. Oral Administration of Linoleic Acid Immediately before Glucose Load Ameliorates Postprandial Hyperglycemia. Front. Pharmacol. 2023, 14, 1197743. [Google Scholar] [CrossRef]
- Adachi, T.; Tanaka, T.; Takemoto, K.; Koshimizu, T.A.; Hirasawa, A.; Tsujimoto, G. Free Fatty Acids Administered into the Colon Promote the Secretion of Glucagon-like Peptide-1 and Insulin. Biochem. Biophys. Res. Commun. 2006, 340, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Swaminath, G.; Cao, Q.; Yang, L.; Guo, Q.; Salomonis, H.; Lu, J.; Houze, J.B.; Dransfield, P.J.; Wang, Y.; et al. Activation of FFA1 Mediates GLP-1 Secretion in Mice. Evidence for Allosterism at FFA1. Mol. Cell. Endocrinol. 2013, 369, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Murthy, K.S.; Grider, J.R. Expression Patterns of L-Amino Acid Receptors in the Murine STC-1 Enteroendocrine Cell Line. Cell Tissue Res. 2019, 378, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.C.E.; Manoliu, B.; Herbillon, B.; Steinert, R.E.; Horowitz, M.; Feinle-Bisset, C. Effects of L-Phenylalanine on Energy Intake and Glycaemia-Impacts on Appetite Perceptions, Gastrointestinal Hormones and Gastric Emptying in Healthy Males. Nutrients 2020, 12, 1788. [Google Scholar] [CrossRef]
- Amin, A.; Frampton, J.; Liu, Z.; Franco-Becker, G.; Norton, M.; Alaa, A.; Li, J.V.; Murphy, K.G. Differential effects of L- and D-phenylalanine on pancreatic and gastrointestinal hormone release in humans: A randomized crossover study. Diabetes Obes. Metab. 2021, 23, 147–157. [Google Scholar] [CrossRef]
- Tocmo, R.; Liang, D.; Lin, Y.; Huang, D. Chemical and Biochemical Mechanisms Underlying the Cardioprotective Roles of Dietary Organopolysulfides. Front. Nutr. 2015, 2, 1. [Google Scholar] [CrossRef]
- Osuga, Y.; Harada, K.; Tsuboi, T. Identification of a Regulatory Pathway of L-Phenylalanine-Induced GLP-1 Secretion in the Enteroendocrine L Cells. Biochem. Biophys. Res. Commun. 2022, 588, 118–124. [Google Scholar] [CrossRef]
- Modvig, I.M.; Kuhre, R.E.; Jepsen, S.L.; Xu, S.F.S.; Engelstoft, M.S.; Egerod, K.L.; Schwartz, T.W.; Ørskov, C.; Rosenkilde, M.M.; Holst, J.J. Amino Acids Differ in Their Capacity to Stimulate GLP-1 Release from the Perfused Rat Small Intestine and Stimulate Secretion by Different Sensing Mechanisms. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E874–E885. [Google Scholar] [CrossRef]
- Steinert, R.E.; Ullrich, S.S.; Geary, N.; Asarian, L.; Bueter, M.; Horowitz, M.; Feinle-Bisset, C. Comparative Effects of Intraduodenal Amino Acid Infusions on Food Intake and Gut Hormone Release in Healthy Males. Physiol. Rep. 2017, 5, e13492. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Gerspach, A.C.; Häfliger, S.; Meili, J.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Beglinger, C.; Wölnerhanssen, B. Effect of L-Tryptophan and L-Leucine on Gut Hormone Secretion, Appetite Feelings and Gastric Emptying Rates in Lean and Non-Diabetic Obese Participants: A Randomized, Double-Blind, Parallel-Group Trial. PLoS ONE 2016, 11, e0166758. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Tamini, S.; Cicolini, S.; De Col, A.; Caroli, D.; Mai, S.; Rondinelli, E.; Saezza, A.; Cella, S.G.; Sartorio, A. Evaluation of an Amino Acid Mix on the Secretion of Gastrointestinal Peptides, Glucometabolic Homeostasis, and Appetite in Obese Adolescents Administered with a Fixed-Dose or Ad Libitum Meal. J. Clin. Med. 2020, 9, 3054. [Google Scholar] [CrossRef] [PubMed]
- Elovaris, R.A.; Hutchison, A.T.; Lange, K.; Horowitz, M.; Feinle-Bisset, C.; Luscombe-Marsh, N.D. Plasma Free Amino Acid Responses to Whey Protein and Their Relationships with Gastric Emptying, Blood Glucose- and Appetite-Regulatory Hormones and Energy Intake in Lean Healthy Men. Nutrients 2019, 11, 2465. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Hartmann, B.; Deacon, C.F.; Holst, J.J. Measurement of the Incretin Hormones: Glucagon-like Peptide-1 and Glucose-Dependent Insulinotropic Peptide. J. Diabetes Complicat. 2015, 29, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Deacon, C.F. Glucagon-like Peptide-1 Mediates the Therapeutic Actions of DPP-IV Inhibitors. Diabetologia 2005, 48, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Hjørne, A.P.; Modvig, I.M.; Holst, J.J. The Sensory Mechanisms of Nutrient-Induced GLP-1 Secretion. Metabolites 2022, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- McVeay, C.; Fitzgerald, P.C.E.; Ullrich, S.S.; Steinert, R.E.; Horowitz, M.; Feinle-Bisset, C. Effects of Intraduodenal Administration of Lauric Acid and L-Tryptophan, Alone and Combined, on Gut Hormones, Pyloric Pressures, and Energy Intake in Healthy Men. Am. J. Clin. Nutr. 2019, 109, 1335–1343. [Google Scholar] [CrossRef]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The Orphan G Protein-Coupled Receptor GPR40 Is Activated by Medium and Long Chain Fatty Acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef]
- Watson, S.J.; Brown, A.J.H.; Holliday, N.D. Differential Signaling by Splice Variants of the Human Free Fatty Acid Receptor GPR120. Mol. Pharmacol. 2012, 81, 631–642. [Google Scholar] [CrossRef]
- Lizarzaburu, M.; Turcotte, S.; Du, X.; Duquette, J.; Fu, A.; Houze, J.; Li, L.; Liu, J.; Murakoshi, M.; Oda, K.; et al. Discovery and Optimization of a Novel Series of GPR142 Agonists for the Treatment of Type 2 Diabetes Mellitus. Bioorg. Med. Chem. Lett. 2012, 22, 5942–5947. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Efanov, A.M.; Fang, X.; Beavers, L.S.; Wang, X.; Wang, J.; Gonzalez Valcarcel, I.C.; Ma, T. GPR142 Controls Tryptophan-Induced Insulin and Incretin Hormone Secretion to Improve Glucose Metabolism. PLoS ONE 2016, 11, e0157298. [Google Scholar] [CrossRef] [PubMed]
- Glassmeier, G.; Herzig, K.H.; Höpfner, M.; Lemmer, K.; Jansen, A.; Scherübl, H. Expression of Functional GABAA Receptors in Cholecystokinin-Secreting Gut Neuroendocrine Murine STC-1 Cells. J. Physiol. 1998, 510 Pt 3, 805–814. [Google Scholar] [CrossRef]
- Purhonen, A.K.; Louhivuori, L.M.; Kiehne, K.; Kerman, K.E.; Herzig, K.H. TRPA1 Channel Activation Induces Cholecystokinin Release via Extracellular Calcium. FEBS Lett. 2008, 582, 229–232. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodum, N.; Mattila, O.; Sharma, R.; Kamakura, R.; Lehto, V.-P.; Walkowiak, J.; Herzig, K.-H.; Raza, G.S. Nutrient Combinations Sensed by L-Cell Receptors Potentiate GLP-1 Secretion. Int. J. Mol. Sci. 2024, 25, 1087. https://doi.org/10.3390/ijms25021087
Sodum N, Mattila O, Sharma R, Kamakura R, Lehto V-P, Walkowiak J, Herzig K-H, Raza GS. Nutrient Combinations Sensed by L-Cell Receptors Potentiate GLP-1 Secretion. International Journal of Molecular Sciences. 2024; 25(2):1087. https://doi.org/10.3390/ijms25021087
Chicago/Turabian StyleSodum, Nalini, Orvokki Mattila, Ravikant Sharma, Remi Kamakura, Vesa-Pekka Lehto, Jaroslaw Walkowiak, Karl-Heinz Herzig, and Ghulam Shere Raza. 2024. "Nutrient Combinations Sensed by L-Cell Receptors Potentiate GLP-1 Secretion" International Journal of Molecular Sciences 25, no. 2: 1087. https://doi.org/10.3390/ijms25021087
APA StyleSodum, N., Mattila, O., Sharma, R., Kamakura, R., Lehto, V.-P., Walkowiak, J., Herzig, K.-H., & Raza, G. S. (2024). Nutrient Combinations Sensed by L-Cell Receptors Potentiate GLP-1 Secretion. International Journal of Molecular Sciences, 25(2), 1087. https://doi.org/10.3390/ijms25021087








