Impaired Expression of Humanin during Adrenocortical Carcinoma
Abstract
1. Introduction
2. Results
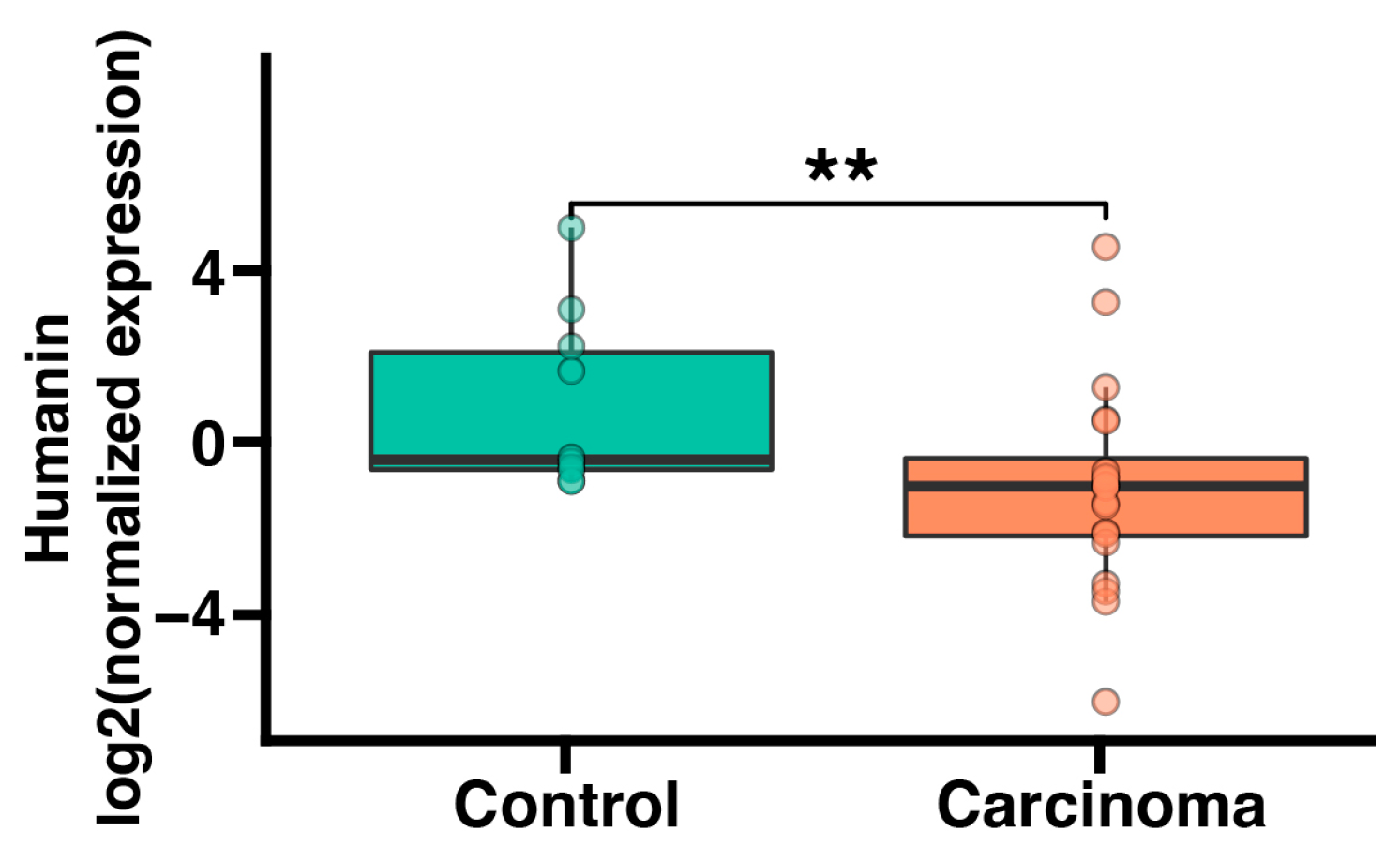
2.1. Humanin mRNA Expression Is Significantly Downregulated in Adrenocortical Carcinoma
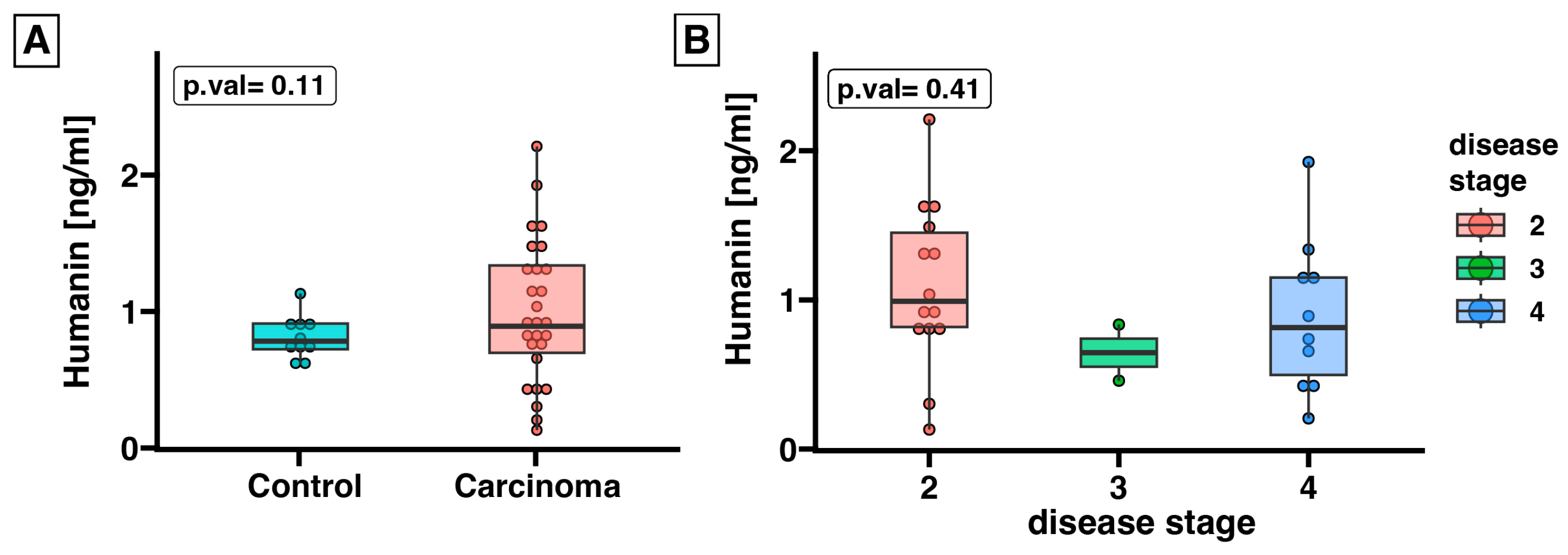
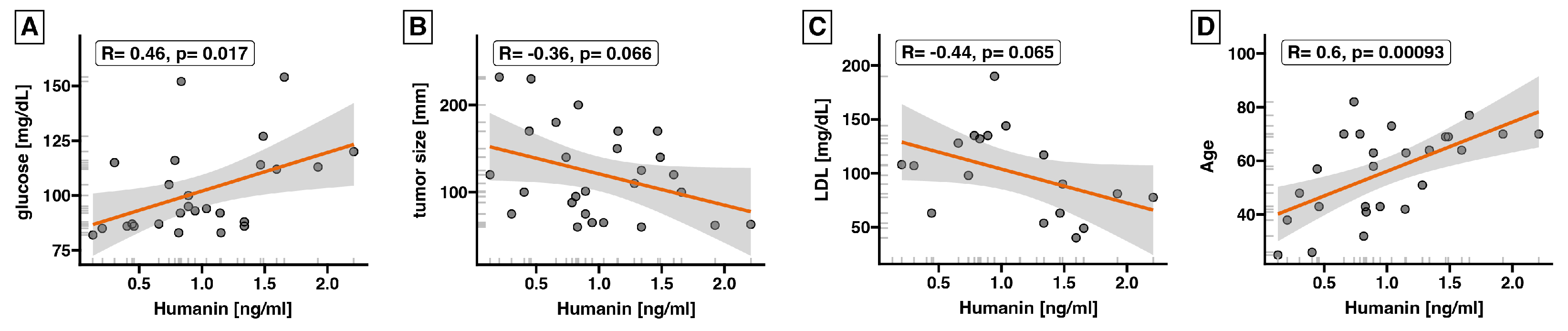
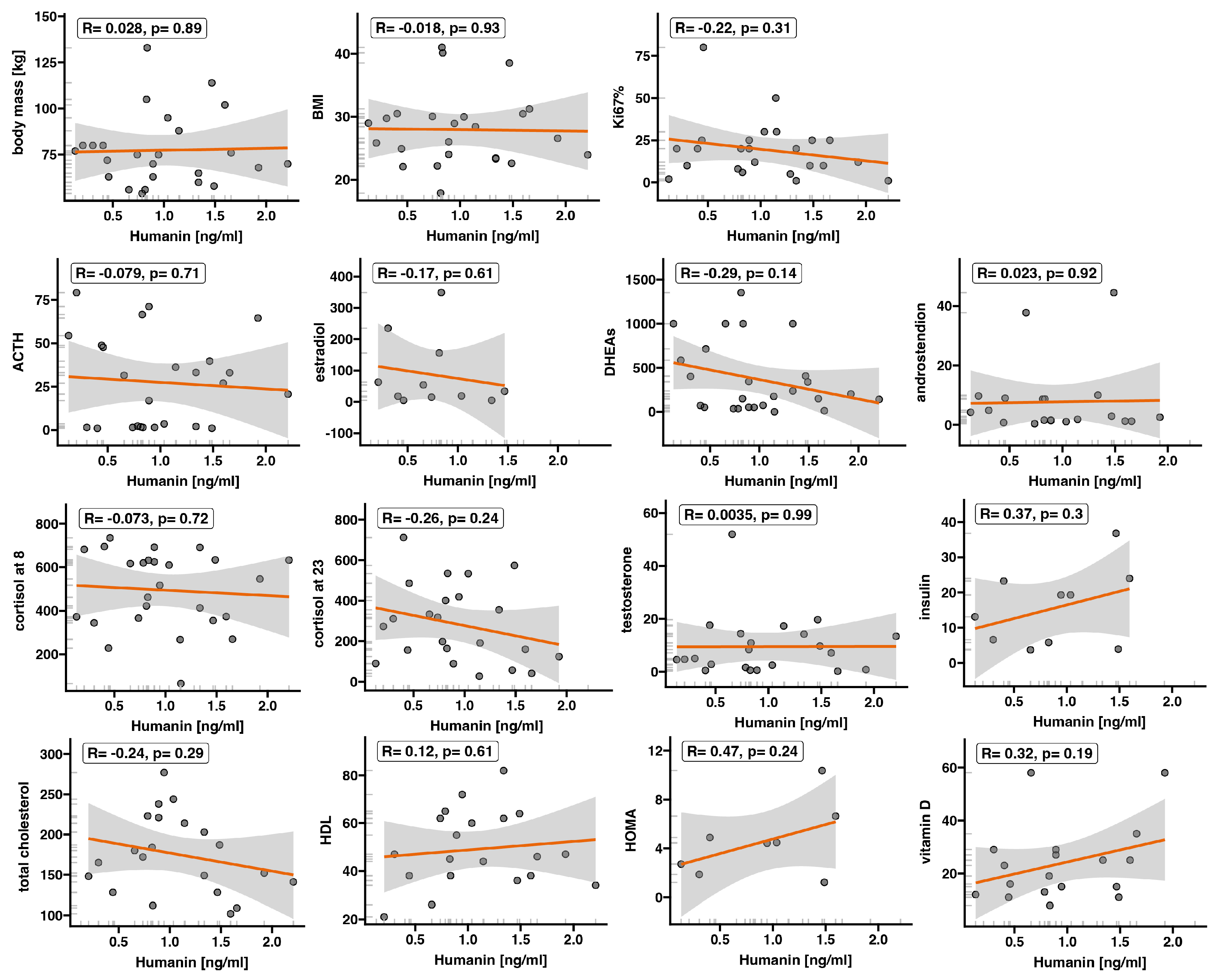
2.2. Expression of Humanin at the Protein Level, Determined in Blood Serum, Does Not Differ between the Control Group and ACC
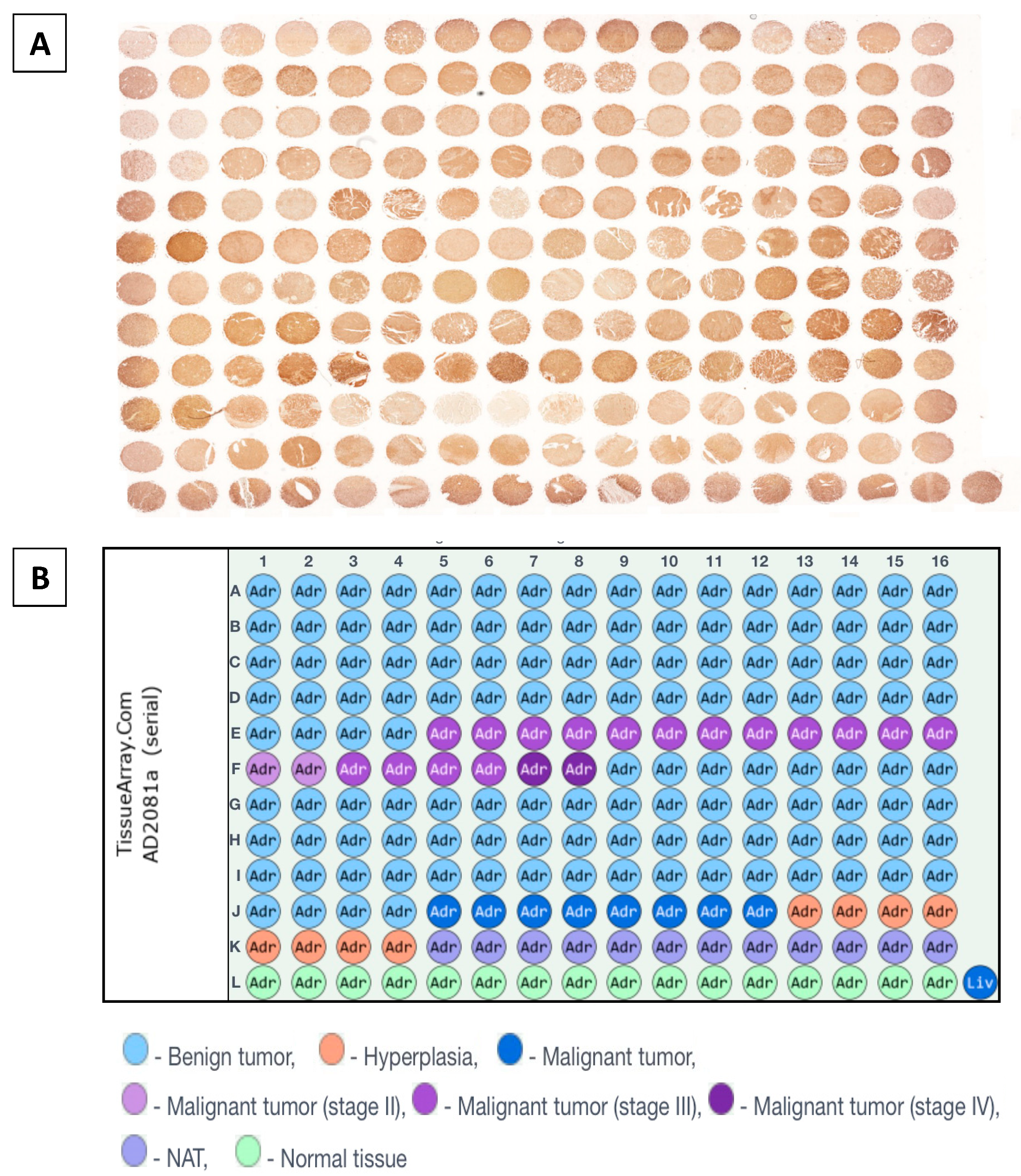
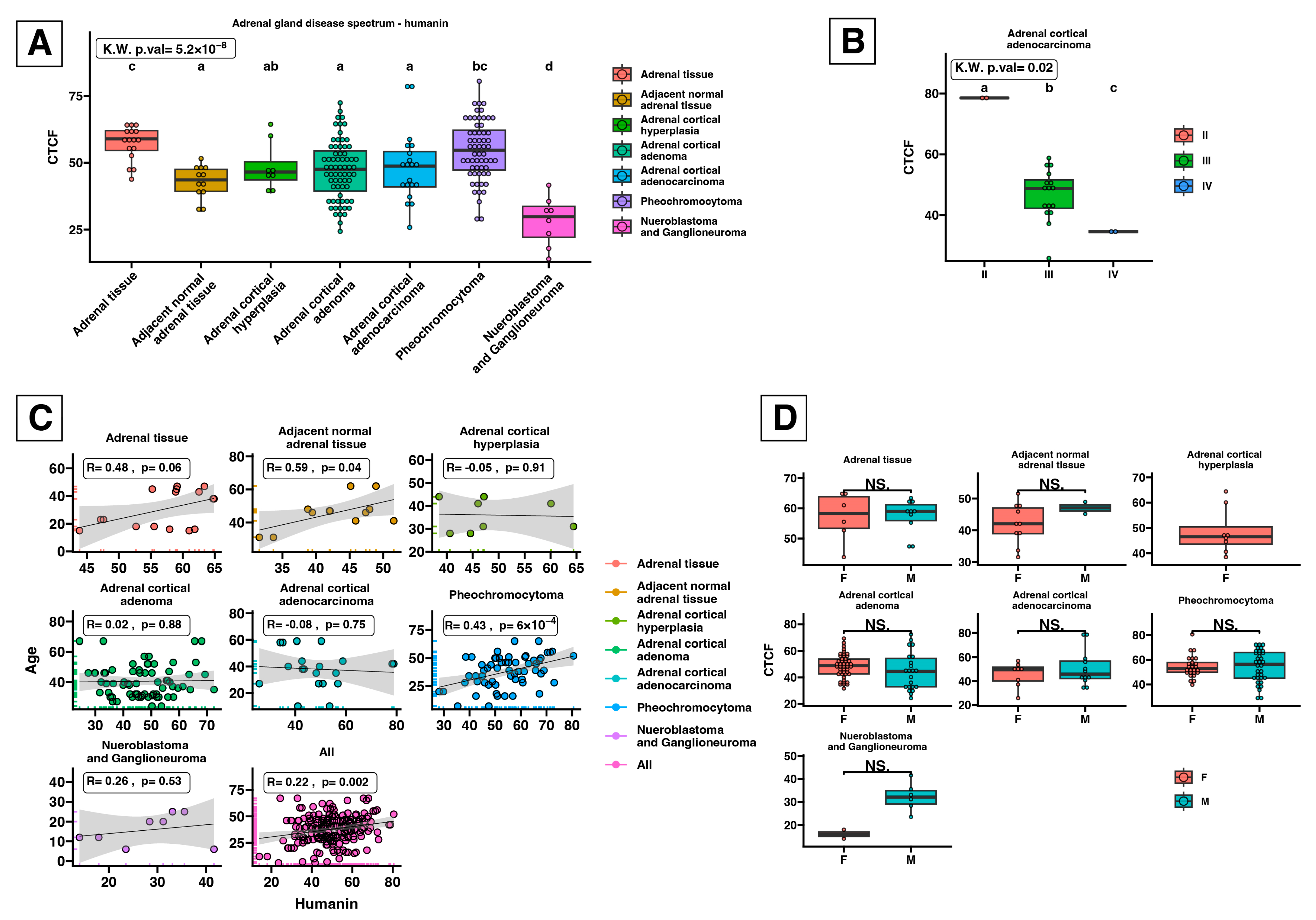
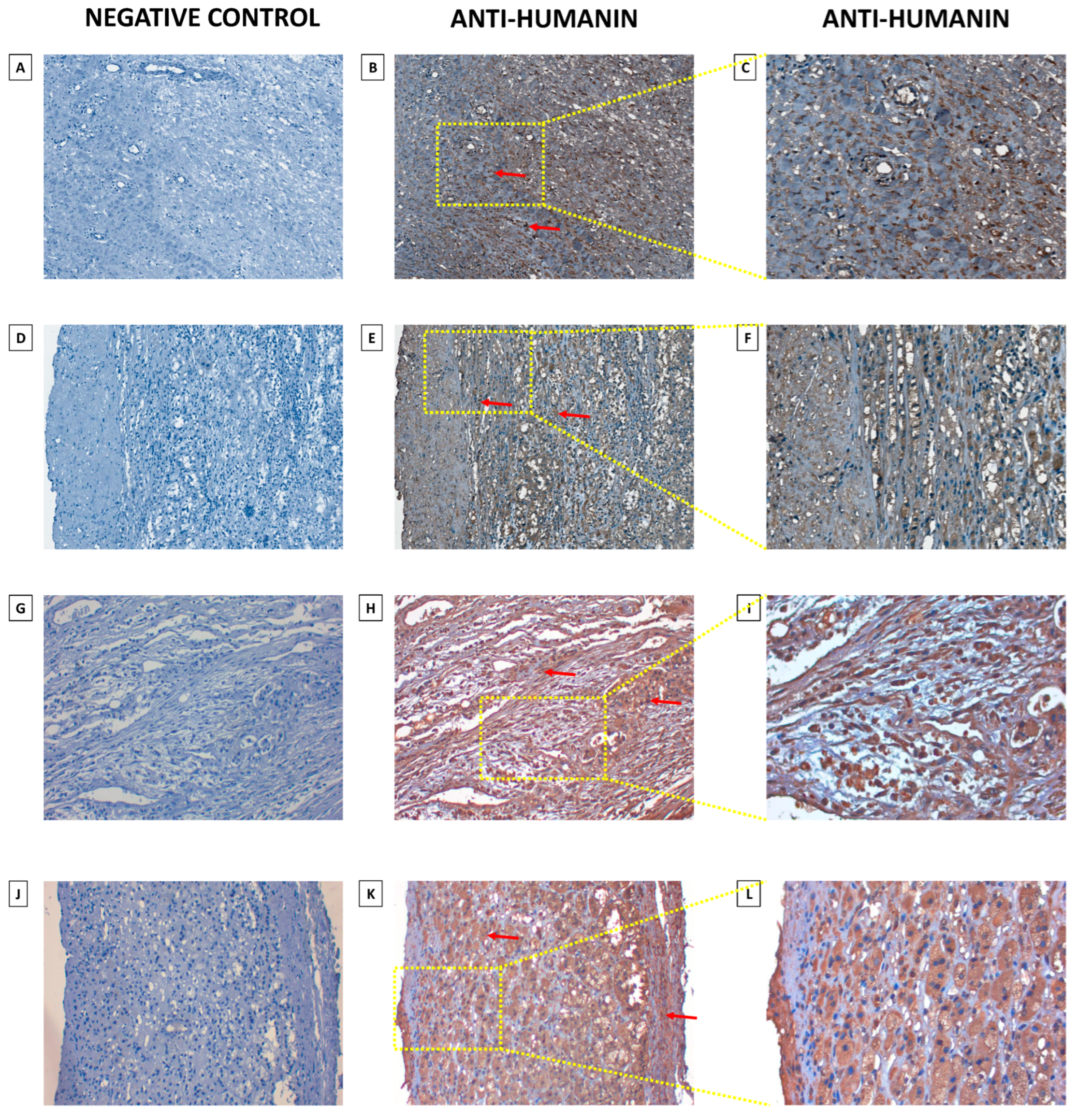
2.3. Humanin Expression at the Protein Level in the Adrenal Glands Is Downregulated in the following Groups: Adjacent Normal Adrenal Tissue, Adrenal Cortical Hyperplasia, Adrenal Cortical Adenoma, Adrenal Cortical Adenocarcinoma, and Neuroblastoma and Ganglioneuroma
3. Discussion
4. Materials and Methods
4.1. Patients’ Characteristics
4.2. RNA Isolation and qPCR Performing
4.3. ELISA
4.4. Immunohistochemical Analysis
4.5. Densitometric Analysis of Tissue Microarray Slide and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cobb, L.J.; Lee, C.; Xiao, J.; Yen, K.; Wong, R.G.; Nakamura, H.K.; Mehta, H.H.; Gao, Q.; Ashur, C.; Huffman, D.M.; et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging 2016, 8, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Niikura, T.; Tajima, H.; Yasukawa, T.; Sudo, H.; Ito, Y.; Kita, Y.; Kawasumi, M.; Kouyama, K.; Doyu, M.; et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc. Natl. Acad. Sci. USA 2001, 98, 6336–6341. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Kim, S.J.; Mehta, H.H.; Cao, K.; Kumagai, H.; Thumaty, N.; Leelaprachakul, N.; Braniff, R.G.; Jiao, H.; Vaughan, J.; et al. Mitochondrial DNA variation in Alzheimer’s disease reveals a unique microprotein called SHMOOSE. Mol. Psychiatry 2023, 28, 1813–1826. [Google Scholar] [CrossRef]
- Kienzle, L.; Bettinazzi, S.; Choquette, T.; Brunet, M.; Khorami, H.H.; Jacques, J.F.; Moreau, M.; Roucou, X.; Landry, C.R.; Angers, A.; et al. A small protein coded within the mitochondrial canonical gene nd4 regulates mitochondrial bioenergetics. BMC Biol. 2023, 21, 111. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Zeller, M.; Cottin, Y.; Vergely, C. Role of humanin, a mitochondrial-derived peptide, in cardiovascular disorders. Arch. Cardiovasc. Dis. 2020, 113, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Xiao, J.; Wan, J.; Cohen, P.; Yen, K. Mitochondrially derived peptides as novel regulators of metabolism. J. Physiol. 2017, 595, 6613–6621. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ito, Y.; Niikura, T.; Shao, Z.; Hata, M.; Oyama, F.; Nishimoto, I. Mechanisms of neuroprotection by a novel rescue factor humanin from Swedish mutant amyloid precursor protein. Biochem. Biophys. Res. Commun. 2001, 283, 460–468. [Google Scholar] [CrossRef]
- Benaki, D.; Zikos, C.; Evangelou, A.; Livaniou, E.; Vlassi, M.; Mikros, E.; Pelecanou, M. Solution structure of humanin, a peptide against Alzheimer’s disease-related neurotoxicity. Biochem. Biophys. Res. Commun. 2005, 329, 152–160. [Google Scholar] [CrossRef]
- Guo, B.; Zhai, D.; Cabezas, E.; Welsh, K.; Nouraini, S.; Satterthwait, A.C.; Reed, J.C. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 2003, 423, 456–461. [Google Scholar] [CrossRef]
- Miller, B.; Kim, S.J.; Kumagai, H.; Yen, K.; Cohen, P. Mitochondria-derived peptides in aging and healthspan. J. Clin. Investig. 2022, 132, e158449. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Son, J.M.; Benayoun, B.A.; Lee, C. The Mitochondrial-Encoded Peptide MOTS-c Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress. Cell Metab. 2018, 28, 516–524.e7. [Google Scholar] [CrossRef] [PubMed]
- Zarate, S.C.; Traetta, M.E.; Codagnone, M.G.; Seilicovich, A.; Reines, A.G. Humanin, a Mitochondrial-Derived Peptide Released by Astrocytes, Prevents Synapse Loss in Hippocampal Neurons. Front. Aging Neurosci. 2019, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Yen, K.; Mehta, H.H.; Kim, S.J.; Lue, Y.; Hoang, J.; Guerrero, N.; Port, J.; Bi, Q.; Navarrete, G.; Brandhorst, S.; et al. The mitochondrial derived peptide humanin is a regulator of lifespan and healthspan. Aging 2020, 12, 11185–11199. [Google Scholar] [CrossRef] [PubMed]
- Yen, K.; Wan, J.; Mehta, H.H.; Miller, B.; Christensen, A.; Levine, M.E.; Salomon, M.P.; Brandhorst, S.; Xiao, J.; Kim, S.J.; et al. Humanin Prevents Age-Related Cognitive Decline in Mice and is Associated with Improved Cognitive Age in Humans. Sci. Rep. 2018, 8, 14212. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wan, J.; Miyazaki, B.; Fang, Y.; Guevara-Aguirre, J.; Yen, K.; Longo, V.; Bartke, A.; Cohen, P. IGF-I regulates the age-dependent signaling peptide humanin. Aging Cell 2014, 13, 958–961. [Google Scholar] [CrossRef]
- Kim, S.J.; Miller, B.; Kumagai, H.; Silverstein, A.R.; Flores, M.; Yen, K. Mitochondrial-derived peptides in aging and age-related diseases. Geroscience 2021, 43, 1113–1121. [Google Scholar] [CrossRef]
- Midzak, A.; Papadopoulos, V. Adrenal Mitochondria and Steroidogenesis: From Individual Proteins to Functional Protein Assemblies. Front. Endocrinol. 2016, 7, 106. [Google Scholar] [CrossRef]
- Vietor, C.L.; Creemers, S.G.; van Kemenade, F.J.; van Ginhoven, T.M.; Hofland, L.J.; Feelders, R.A. How to Differentiate Benign from Malignant Adrenocortical Tumors? Cancers 2021, 13, 4383. [Google Scholar] [CrossRef]
- Ayala-Ramirez, M.; Jasim, S.; Feng, L.; Ejaz, S.; Deniz, F.; Busaidy, N.; Waguespack, S.G.; Naing, A.; Sircar, K.; Wood, C.G.; et al. Adrenocortical carcinoma: Clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur. J. Endocrinol. 2013, 169, 891–899. [Google Scholar] [CrossRef]
- Minner, S.; Schreiner, J.; Saeger, W. Adrenal cancer: Relevance of different grading systems and subtypes. Clin. Transl. Oncol. 2021, 23, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Bronswijk, M.J.H.; Laenen, A.; Bechter, O.E. Clinical presentation, treatment modalities and outcome in patients with adrenocortical carcinoma: A single center experience. Neoplasma 2020, 67, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gupta, D.; Albert, S.G. Pheochromocytoma as a reversible cause of cardiomyopathy: Analysis and review of the literature. Int. J. Cardiol. 2017, 249, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Plouin, P.F.; Chatellier, G.; Fofol, I.; Corvol, P. Tumor recurrence and hypertension persistence after successful pheochromocytoma operation. Hypertension 1997, 29, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Colvin, A.; Saltzman, A.F.; Walker, J.; Bruny, J.; Cost, N.G. Metastatic Pheochromocytoma in an Asymptomatic 12-Year-Old with von Hippel-Lindau Disease. Urology 2018, 119, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Asa, S.L. Aldosterone-producing adrenal cortical adenoma with oncocytic change and cytoplasmic eosinophilic globular inclusions. Endocr. Pathol. 2009, 20, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Helmberg, A.; Ausserer, B.; Kofler, R. Frame shift by insertion of 2 basepairs in codon 394 of CYP11B1 causes congenital adrenal hyperplasia due to steroid 11 beta-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 1992, 75, 1278–1281. [Google Scholar]
- Hekim, M.G.; Ozcan, S.; Yur, M.; Yildirim, N.; Ozcan, M. Exploring the potential of humanin as a biomarker for early breast cancer detection: A study of serum levels and diagnostic performance. Biomarkers 2023, 28, 555–561. [Google Scholar] [CrossRef]
- Pena Agudelo, J.A.; Pidre, M.L.; Garcia Fallit, M.; Perez Kuper, M.; Zuccato, C.; Nicola Candia, A.J.; Marchesini, A.; Vera, M.B.; De Simone, E.; Giampaoli, C.; et al. Mitochondrial Peptide Humanin Facilitates Chemoresistance in Glioblastoma Cells. Cancers 2023, 15, 4061. [Google Scholar] [CrossRef]
- Mottaghi-Dastjerdi, N.; Soltany-Rezaee-Rad, M.; Sepehrizadeh, Z.; Roshandel, G.; Ebrahimifard, F.; Setayesh, N. Genome expression analysis by suppression subtractive hybridization identified overexpression of Humanin, a target gene in gastric cancer chemoresistance. Daru 2014, 22, 14. [Google Scholar] [CrossRef]
- Omar, N.N.; Tash, R.F.; Shoukry, Y.; ElSaeed, K.O. Breaking the ritual metabolic cycle in order to save acetyl CoA: A potential role for mitochondrial humanin in T2 bladder cancer aggressiveness. J. Egypt. Natl. Canc Inst. 2017, 29, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondria and cancer: Warburg addressed. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype-concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Park, D.H.; Chae, Y.C. Role of Mitochondrial Stress Response in Cancer Progression. Cells 2022, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.; Santoro, A.; Salvioli, S. Mitochondria and mitochondria-induced signalling molecules as longevity determinants. Mech. Ageing Dev. 2017, 165 Pt B, 115–128. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Cuzzocrea, S.; Iavicoli, I.; Rizzarelli, E.; Calabrese, E.J. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol. Asp. Med. 2011, 32, 279–304. [Google Scholar] [CrossRef]
- Conte, M.; Martucci, M.; Chiariello, A.; Franceschi, C.; Salvioli, S. Mitochondria, immunosenescence and inflammaging: A role for mitokines? Semin. Immunopathol. 2020, 42, 607–617. [Google Scholar] [CrossRef]
- Bachar, A.R.; Scheffer, L.; Schroeder, A.S.; Nakamura, H.K.; Cobb, L.J.; Oh, Y.K.; Lerman, L.O.; Pagano, R.E.; Cohen, P.; Lerman, A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc. Res. 2010, 88, 360–366. [Google Scholar] [CrossRef]
- Muzumdar, R.H.; Huffman, D.M.; Atzmon, G.; Buettner, C.; Cobb, L.J.; Fishman, S.; Budagov, T.; Cui, L.; Einstein, F.H.; Poduval, A.; et al. Humanin: A novel central regulator of peripheral insulin action. PLoS ONE 2009, 4, e6334. [Google Scholar] [CrossRef]
- Conte, M.; Ostan, R.; Fabbri, C.; Santoro, A.; Guidarelli, G.; Vitale, G.; Mari, D.; Sevini, F.; Capri, M.; Sandri, M.; et al. Human Aging and Longevity Are Characterized by High Levels of Mitokines. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Fuku, N.; Pareja-Galeano, H.; Zempo, H.; Alis, R.; Arai, Y.; Lucia, A.; Hirose, N. The mitochondrial-derived peptide MOTS-c: A player in exceptional longevity? Aging Cell 2015, 14, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Wan, J.; Lai, V.; Cohen, P.; Cherney, D.Z. The effect of sex on humanin levels in healthy adults and patients with uncomplicated type 1 diabetes mellitus. Can. J. Physiol. Pharmacol. 2015, 93, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Peuchant, E.; Brun, J.L.; Rigalleau, V.; Dubourg, L.; Thomas, M.J.; Daniel, J.Y.; Leng, J.J.; Gin, H. Oxidative and antioxidative status in pregnant women with either gestational or type 1 diabetes. Clin. Biochem. 2004, 37, 293–298. [Google Scholar] [CrossRef]
- Lopez-Tinoco, C.; Roca, M.; Garcia-Valero, A.; Murri, M.; Tinahones, F.J.; Segundo, C.; Bartha, J.L.; Aguilar-Diosdado, M. Oxidative stress and antioxidant status in patients with late-onset gestational diabetes mellitus. Acta Diabetol. 2013, 50, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Tseng, L.M.; Lee, H.C. Role of mitochondrial dysfunction in cancer progression. Exp. Biol. Med. 2016, 241, 1281–1295. [Google Scholar] [CrossRef]
- Boland, M.L.; Chourasia, A.H.; Macleod, K.F. Mitochondrial dysfunction in cancer. Front. Oncol. 2013, 3, 292. [Google Scholar] [CrossRef]
- Lee, H.C.; Yin, P.H.; Lin, J.C.; Wu, C.C.; Chen, C.Y.; Wu, C.W.; Chi, C.W.; Tam, T.N.; Wei, Y.H. Mitochondrial genome instability and mtDNA depletion in human cancers. Ann. N. Y. Acad. Sci. 2005, 1042, 109–122. [Google Scholar] [CrossRef]
- Lee, H.C.; Wei, Y.H. Mitochondrial DNA instability and metabolic shift in human cancers. Int. J. Mol. Sci. 2009, 10, 674–701. [Google Scholar] [CrossRef]
- Feng, S.; Xiong, L.; Ji, Z.; Cheng, W.; Yang, H. Correlation between increased copy number of mitochondrial DNA and clinicopathological stage in colorectal cancer. Oncol. Lett. 2011, 2, 899–903. [Google Scholar] [PubMed]
- Wang, Y.; Liu, V.W.; Xue, W.C.; Tsang, P.C.; Cheung, A.N.; Ngan, H.Y. The increase of mitochondrial DNA content in endometrial adenocarcinoma cells: A quantitative study using laser-captured microdissected tissues. Gynecol. Oncol. 2005, 98, 104–110. [Google Scholar] [CrossRef]
- Liang, B.C.; Hays, L. Mitochondrial DNA copy number changes in human gliomas. Cancer Lett. 1996, 105, 167–173. [Google Scholar] [CrossRef]
- Lee, H.C.; Li, S.H.; Lin, J.C.; Wu, C.C.; Yeh, D.C.; Wei, Y.H. Somatic mutations in the D-loop and decrease in the copy number of mitochondrial DNA in human hepatocellular carcinoma. Mutat. Res. 2004, 547, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.M.; Yin, P.H.; Chi, C.W.; Hsu, C.Y.; Wu, C.W.; Lee, L.M.; Wei, Y.H.; Lee, H.C. Mitochondrial DNA mutations and mitochondrial DNA depletion in breast cancer. Genes. Chromosomes Cancer 2006, 45, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, M.F.; Jaita, G.; Magri, M.L.; Zarate, S.; Moreno Ayala, M.; Ferraris, J.; Eijo, G.; Pisera, D.; Candolfi, M.; Seilicovich, A. Antiapoptotic factor humanin is expressed in normal and tumoral pituitary cells and protects them from TNF-alpha-induced apoptosis. PLoS ONE 2014, 9, e111548. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, L.; Cui, Y.; Wu, B.; Liao, Z. Potent humanin analogue (HNG) protects human sperm from freeze-thaw-induced damage. Cryobiology 2019, 88, 47–53. [Google Scholar] [CrossRef]
- Colon, E.; Strand, M.L.; Carlsson-Skwirut, C.; Wahlgren, A.; Svechnikov, K.V.; Cohen, P.; Soder, O. Anti-apoptotic factor humanin is expressed in the testis and prevents cell-death in leydig cells during the first wave of spermatogenesis. J. Cell Physiol. 2006, 208, 373–385. [Google Scholar] [CrossRef]
- Han, K.; Jia, N.; Zhong, Y.; Shang, X. S14G-humanin alleviates insulin resistance and increases autophagy in neurons of APP/PS1 transgenic mouse. J. Cell Biochem. 2018, 119, 3111–3117. [Google Scholar] [CrossRef]
- Kuliawat, R.; Klein, L.; Gong, Z.; Nicoletta-Gentile, M.; Nemkal, A.; Cui, L.; Bastie, C.; Su, K.; Huffman, D.; Surana, M.; et al. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the beta cell. FASEB J. 2013, 27, 4890–4898. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Mandrup-Poulsen, T. A choice of death--the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001, 44, 2115–2133. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Pappas, P.D.; Theodoridis, T.D.; Vavilis, D. Humanin and diabetes mellitus: A review of in vitro and in vivo studies. World J. Diabetes 2022, 13, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Jin, J.; He, F.; Zheng, Y.; Li, T.; Zhang, Y.; He, J. Humanin promotes mitochondrial biogenesis in pancreatic MIN6 beta-cells. Biochem. Biophys. Res. Commun. 2018, 497, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Paharkova, V.; Alvarez, G.; Nakamura, H.; Cohen, P.; Lee, K.W. Rat Humanin is encoded and translated in mitochondria and is localized to the mitochondrial compartment where it regulates ROS production. Mol. Cell Endocrinol. 2015, 413, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.T.; Park, P.; Cobb, L.J.; Paharkova-Vatchkova, V.; Hakimi, M.; Cohen, P.; Lee, K.W. The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism 2010, 59, 343–349. [Google Scholar] [CrossRef]
- Gidlund, E.K.; von Walden, F.; Venojarvi, M.; Riserus, U.; Heinonen, O.J.; Norrbom, J.; Sundberg, C.J. Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol. Rep. 2016, 4, e13063. [Google Scholar] [CrossRef]
- Blatkiewicz, M.; Kaminski, K.; Szyszka, M.; Al-Shakarchi, Z.; Olechnowicz, A.; Stelcer, E.; Komarowska, H.; Tyczewska, M.; Klimont, A.; Karczewski, M.; et al. The Enhanced Expression of ZWILCH Predicts Poor Survival of Adrenocortical Carcinoma Patients. Biomedicines 2023, 11, 1233. [Google Scholar] [CrossRef]
- The Open Lab Book. Available online: https://tholb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html (accessed on 16 November 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blatkiewicz, M.; Szyszka, M.; Olechnowicz, A.; Kamiński, K.; Jopek, K.; Komarowska, H.; Tyczewska, M.; Klimont, A.; Wierzbicki, T.; Karczewski, M.; et al. Impaired Expression of Humanin during Adrenocortical Carcinoma. Int. J. Mol. Sci. 2024, 25, 1038. https://doi.org/10.3390/ijms25021038
Blatkiewicz M, Szyszka M, Olechnowicz A, Kamiński K, Jopek K, Komarowska H, Tyczewska M, Klimont A, Wierzbicki T, Karczewski M, et al. Impaired Expression of Humanin during Adrenocortical Carcinoma. International Journal of Molecular Sciences. 2024; 25(2):1038. https://doi.org/10.3390/ijms25021038
Chicago/Turabian StyleBlatkiewicz, Małgorzata, Marta Szyszka, Anna Olechnowicz, Kacper Kamiński, Karol Jopek, Hanna Komarowska, Marianna Tyczewska, Anna Klimont, Tomasz Wierzbicki, Marek Karczewski, and et al. 2024. "Impaired Expression of Humanin during Adrenocortical Carcinoma" International Journal of Molecular Sciences 25, no. 2: 1038. https://doi.org/10.3390/ijms25021038
APA StyleBlatkiewicz, M., Szyszka, M., Olechnowicz, A., Kamiński, K., Jopek, K., Komarowska, H., Tyczewska, M., Klimont, A., Wierzbicki, T., Karczewski, M., Ruchała, M., & Rucinski, M. (2024). Impaired Expression of Humanin during Adrenocortical Carcinoma. International Journal of Molecular Sciences, 25(2), 1038. https://doi.org/10.3390/ijms25021038






