Betulonic Acid Inhibits Type-2 Porcine Reproductive and Respiratory Syndrome Virus Replication by Downregulating Cellular ATP Production
Abstract
1. Introduction
2. Results
2.1. BA Inhibits PRRSV Replication in Marc-145 Cells
2.2. BA Inhibits PRRSV Replication in PAMs
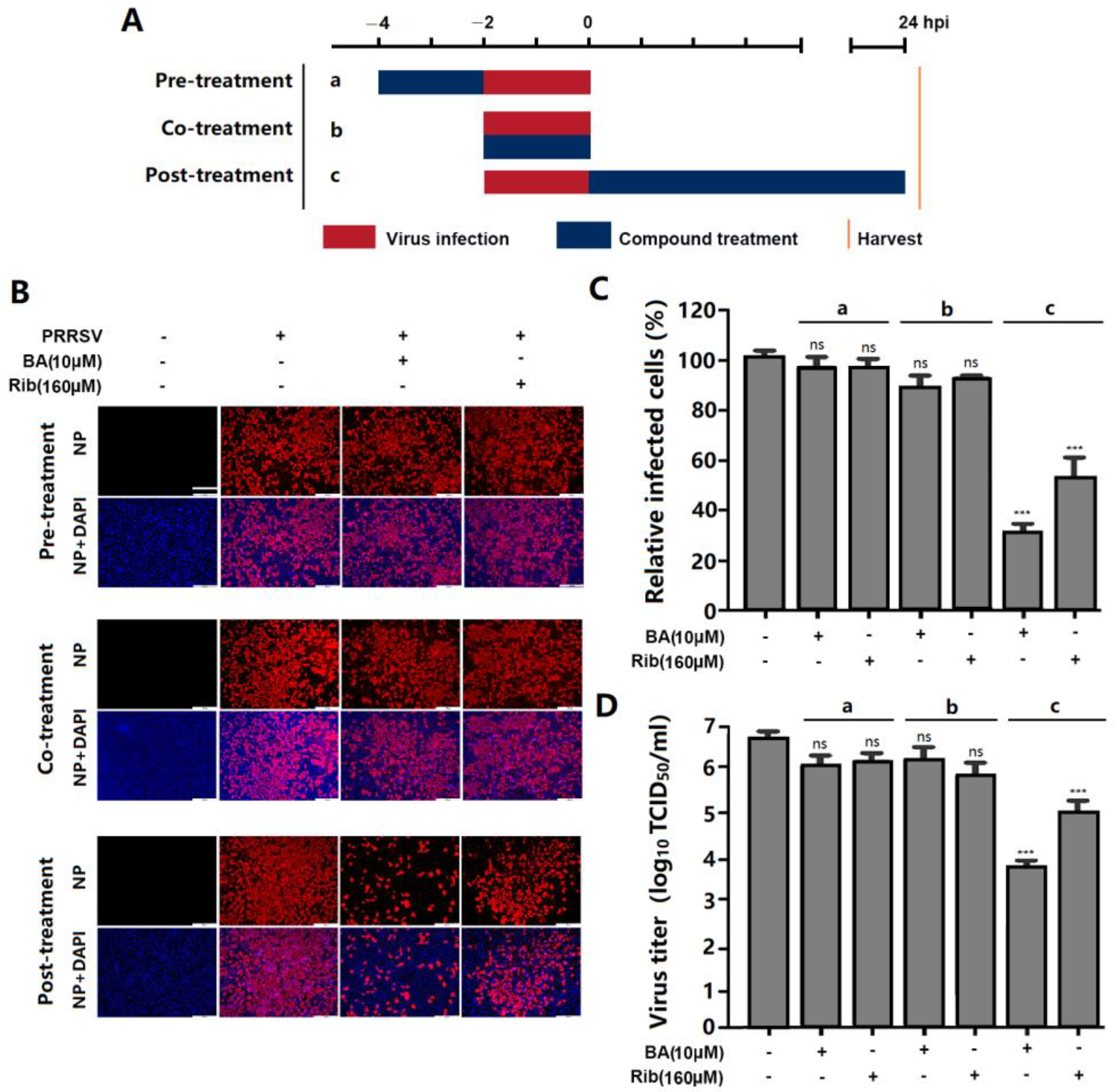
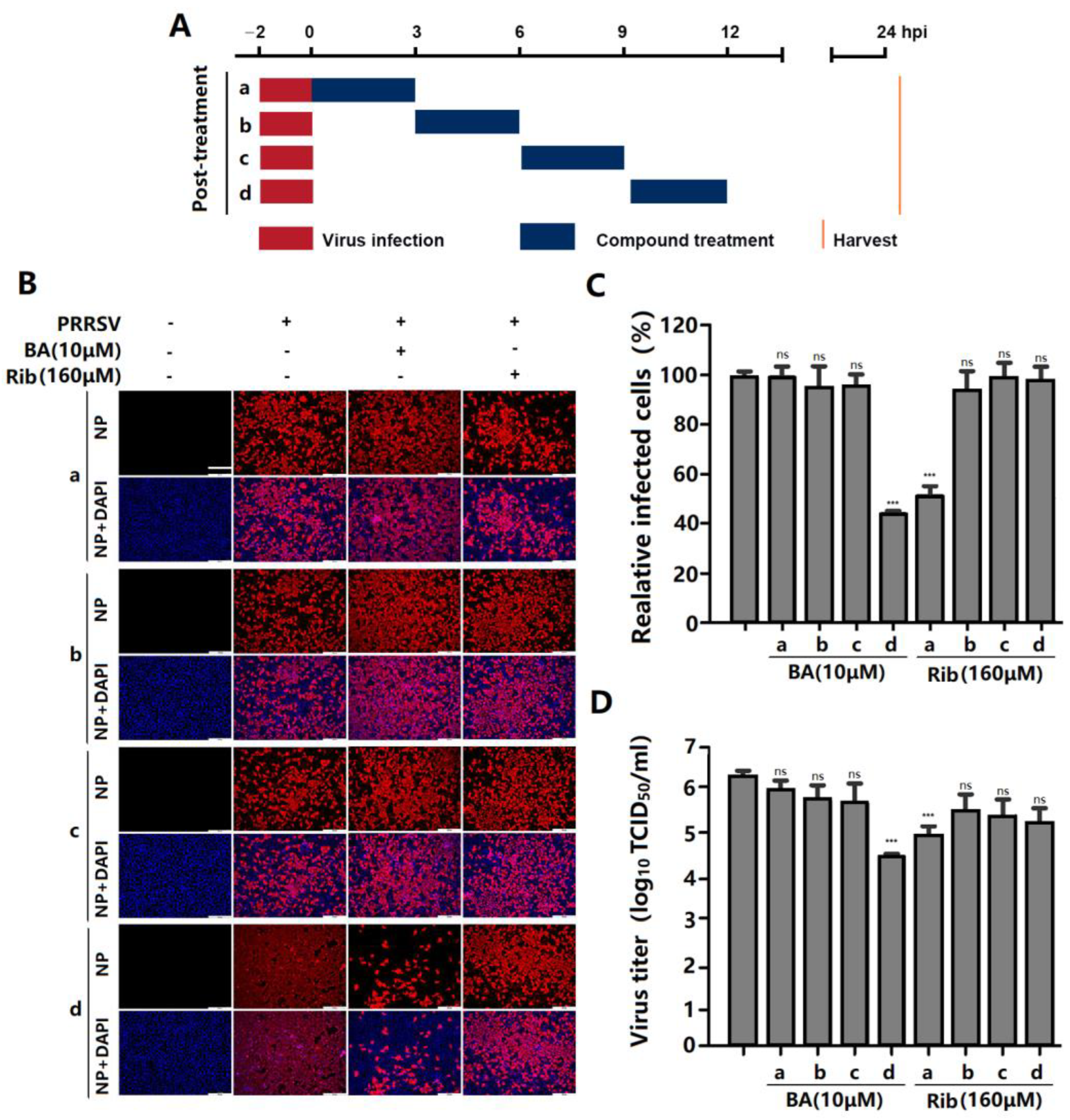
2.3. BA Inhibits PRRSV Replication by Interfering with the Late Stage of the Virus Replication Cycle Rather than Its Entry
2.4. BA Inhibits PRRSV Replication by Reducing ATP Production
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Virus Strains
4.2. Antibodies, Chemicals, and Reagents
4.3. Cytotoxicity Assay
4.4. Quantitative Real-Time PCR (qPCR)
4.5. Indirect Immunofluorescence Assay (IFA)
4.6. Western Blot Analysis
4.7. Time-of-Addition Assay
4.8. Determination of ATP Content
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Shen, D.; Cui, J.; Zhao, B. Accelerated evolution of PRRSV during recent outbreaks in China. Virus Genes 2010, 41, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Feng, N.; Li, Z.; Wang, P.; Qi, Z.; Liang, W.; Zhou, X.; Xu, X.; Liu, B. 2′,5′-Oligoadenylate synthetase 1(OAS1) inhibits PRRSV replication in Marc-145 cells. Antivir. Res. 2016, 132, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Khatun, A.; Shabir, N.; Yoon, K.J.; Kim, W.I. Effects of ribavirin on the replication and genetic stability of porcine reproductive and respiratory syndrome virus. BMC Vet. Res. 2015, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Gao, Y.; Zhang, M.; Liu, Z.; Lin, Q.; Gong, L.; Guo, J.; Chen, L.; An, T.; Chen, J. Andrographolide and Its Derivative Potassium Dehydrographolide Succinate Suppress PRRSV Replication in Primary and Established Cells via Differential Mechanisms of Action. Virol. Sin. 2021, 36, 1626–1643. [Google Scholar] [CrossRef]
- Long, F.; Zhang, M.; Yang, X.; Liang, X.; Su, L.; An, T.; Zhang, G.; Zeng, Z.; Liu, Y.; Chen, W.; et al. The Antimalaria Drug Ar-tesunate Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication by Activating AMPK and Nrf2/HO-1 Signaling Pathways. J. Virol. 2022, 96, e01487-21. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, C.; Su, L.; Long, F.; Yang, X.; Guo, X.; Song, G.; An, T.; Chen, W.; Chen, J. Toosendanin activates caspase-1 and in-duces maturation of IL-1β to inhibit type 2 porcine reproductive and respiratory syndrome virus replication via an IFI16-dependent pathway. Vet. Res. 2022, 53, 61. [Google Scholar] [CrossRef]
- Liu, X.; Song, Z.; Bai, J.; Nauwynck, H.; Zhao, Y.; Jiang, P. Xanthohumol inhibits PRRSV proliferation and alleviates oxidative stress induced by PRRSV via the Nrf2-HMOX1 axis. Vet. Res. 2019, 50, 61. [Google Scholar] [CrossRef]
- Oliveira-Costa, J.F.; Meira, C.S.; Neves, M.V.G.D.; Dos Reis, B.P.Z.C.; Soares, M.B.P. Anti-Inflammatory Activities of Betulinic Acid: A Review. Front. Pharmacol. 2022, 13, 883857. [Google Scholar] [CrossRef]
- Macasoi, I.; Mioc, A.; Mioc, M.; Racoviceanu, R.; Soica, I.; Cheveresan, A.; Dehelean, C.; Dumitrascu, V. Targeting Mitochondria through the Use of Mitocans as Emerging Anticancer Agents. Curr. Med. Chem. 2020, 27, 5730–5757. [Google Scholar] [CrossRef]
- Huang, Q.-X.; Chen, H.-F.; Luo, X.-R.; Zhang, Y.-X.; Yao, X.; Zheng, X. Structure and Anti-HIV Activity of Betulinic Acid Analogues. Curr. Med. Sci. 2018, 38, 387–397. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Boreko, E.I.; Nigmatullina, L.R.; Pavlova, N.I.; Nikolaeva, S.N.; Savinova, O.V.; Eremin, V.F.; Baltina, L.A.; Galin, F.Z.; Tolstikov, G.A. Synthesis and antiviral activity of hydrazides and substituted benzalhydrazides of betulinic acid and its derivatives. Bioorg. Khim. 2003, 29, 296–301. [Google Scholar] [CrossRef]
- Holzer, A.M.; Granstein, R.D. Role of extracellular adenosine triphosphate in human skin. J. Cutan. Med. Surg. 2004, 8, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, M.; Simmen, T. (Eds.) Hepatitis C Virus Replication. In Organelle Contact Sites: From Molecular Mechanism to Disease; Springer: Singapore, 2017; pp. 199–209. [Google Scholar]
- Stoeck, I.K.; Lee, J.Y.; Tabata, K.; Romero-Brey, I.; Paul, D.; Schult, P.; Lohmann, V.; Kaderali, L.; Bartenschlager, R. Hepatitis C Virus Replication Depends on Endosomal Cholesterol Homeostasis. J. Virol. 2017, 92, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Séror, C.; Melki, M.T.; Subra, F.; Raza, S.Q.; Bras, M.; Saïdi, H.; Nardacci, R.; Voisin, L.; Paoletti, A.; Law, F.; et al. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J. Exp. Med. 2011, 208, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Song, Y.J.; Wang, H.; Zhao, B.R.; Wang, X.W. Mindin Activates Autophagy for Lipid Utilization and Facilitates White Spot Syndrome Virus Infection in Shrimp. mBio 2023, 14, 16. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Semenova, A.A.; Ilzorkina, A.I.; Mikheeva, I.B.; Yashin, V.A.; Penkov, N.V.; Vydrina, V.A.; Ishmuratov, G.Y.; Sharapov, V.A.; Khoroshavina, E.I.; et al. Effect of betulin and betulonic acid on isolated rat liver mitochondria and liposomes. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183383. [Google Scholar] [CrossRef]
- Takahashi, H.; Sawa, H.; Hasegawa, H.; Shoya, Y.; Sata, T.; Hall, W.W.; Nagashima, K.; Kurata, T. Topoisomerase I and ATP activate cDNA synthesis of human immunodeficiency virus type 1. Biochem. Biophys. Res. Commun. 2002, 294, 509–517. [Google Scholar] [CrossRef]
- Nagy, P.D.; Lin, W.W. Taking over Cellular Energy-Metabolism for TBSV Replication: The High ATP Requirement of an RNA Virus within the Viral Replication Organelle. Viruses 2020, 12, 56. [Google Scholar] [CrossRef]
- Appleby, T.C.; Anderson, R.; Fedorova, O.; Pyle, A.M.; Wang, R.; Liu, X.H.; Brendza, K.M.; Somoza, J.R. Visualizing ATP-Dependent RNA Translocation by the NS3 Helicase from HCV. J. Mol. Biol. 2011, 405, 1139–1153. [Google Scholar] [CrossRef]
- Paslaru, L.; Bindea, G.; Nastase, A.; Sorop, A.; Zimbru, C.; Herlea, V.; Hrehoret, D.; Brasoveanu, V.; Zamfir, R.; Dima, S.; et al. Comparative RNA-Sequencing Analysis Reveals High Complexity and Heterogeneity of Transcriptomic and Immune Profiles in Hepatocellular Carcinoma Tumors of Viral (HBV, HCV) and Non-Viral Etiology. Med. Lith. 2022, 58, 39. [Google Scholar] [CrossRef]
- Niu, B.; Ma, L.J.; Yao, L.X.; Zhang, Y.T.; Su, H. HCV affects KATP channels through GnT-IVa-mediated N-glycosylation of GLUT2 on the surface of pancreatic β-cells leading to impaired insulin secretion. Endocrine 2023, 14, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Horváth, G.; Molnár, E.; Szabó, Z.; Kecskeméti, G.; Juhász, L.; Tallósy, S.P.; Nyári, J.; Bogdanov, A.; Somogyvári, F.; Endrész, V.; et al. Carnosic Acid Inhibits Herpes Simplex Virus Replication by Suppressing Cellular ATP Synthesis. Int. J. Mol. Sci. 2024, 25, 4983. [Google Scholar] [CrossRef] [PubMed]
- Li Puma, D.D.; Marcocci, M.E.; Lazzarino, G.; De Chiara, G.; Tavazzi, B.; Palamara, A.T.; Piacentini, R.; Grassi, C. Ca2+-dependent release of ATP from astrocytes affects herpes simplex virus Type 1 infection of neurons. Glia 2021, 69, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Pujhari, S.; Zakhartchouk, A.N. Porcine reproductive and respiratory syndrome virus envelope (E) protein interacts with mitochondrial proteins and induces apoptosis. Arch. Virol. 2016, 161, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudabadi, G.; Milo, R.; Phillips, R. Energetic cost of building a virus. Proc. Natl. Acad. Sci. USA 2017, 114, E4324–E4333. [Google Scholar] [CrossRef]
- Kuzmiak-Glancy, S.; Glancy, B.; Kay, M.W. Ischemic damage to every segment of the oxidative phosphorylation cascade elevates ETC driving force and ROS production in cardiac mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H499–H512. [Google Scholar] [CrossRef]
- Guo, R.; Gu, J.; Zong, S.; Wu, M.; Yang, M. Structure and mechanism of mitochondrial electron transport chain. Biomed. J. 2018, 41, 9–20. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox. Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef]
- Combs, J.A.; Monk, C.H.; Harrison, M.A.A.; Norton, E.B.; Morris, C.A.; Sullivan, D.E.; Zwezdaryk, K.J. Inhibiting cytomegalo-virus replication through targeting the host electron transport chain. Antiviral. Res. 2021, 194, 105159. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Ogundele, A.; Forrest, A.; Wilton, J.; Salzwedel, K.; Doto, J.; Allaway, G.P.; Martin, D.E. Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-O-(3′,3′-dimethylsuccinyl) betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2007, 51, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, S.; Hua, T.; Wang, Y.; Wei, D.; Zhao, M.; Su, S.; Duan, J.-a. Comparative pharmacokinetics of triterpenic acids in normal and immunosuppressed rats after oral administration of Jujubae Fructus extract by UPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1077, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Du, T.; Long, F.; Yang, X.; Sun, Y.; Duan, M.; Zhang, G.; Liu, Y.; Zhou, E.M.; Chen, W.; et al. Platycodin D Suppresses Type 2 Porcine Reproductive and Respiratory Syndrome Virus in Primary and Established Cell Lines. Viruses 2018, 10, 657. [Google Scholar] [CrossRef]
- Veit, M.; Gadalla, M.R.; Zhang, M. Mass Spectrometry-Based Proteomic Analysis of Potential Host Proteins Interacting with GP5 in PRRSV-Infected PAMs. Int. J. Mol. Sci. 2024, 25, 2778. [Google Scholar] [CrossRef]
- Yang, X.; Long, F.; Jia, W.; Zhang, M.; Su, G.; Liao, M.; Zeng, Z.; Chen, W.; Chen, J. Artesunate inhibits PDE4 leading to intracellular cAMP accumulation, reduced ERK/MAPK signaling, and blockade of influenza A virus vRNP nuclear export. Antivir. Res. 2023, 215, 105635. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, F.; Su, L.; Zhang, M.; Wang, S.; Sun, Q.; Liu, J.; Chen, W.; Wang, H.; Chen, J. Betulonic Acid Inhibits Type-2 Porcine Reproductive and Respiratory Syndrome Virus Replication by Downregulating Cellular ATP Production. Int. J. Mol. Sci. 2024, 25, 10366. https://doi.org/10.3390/ijms251910366
Long F, Su L, Zhang M, Wang S, Sun Q, Liu J, Chen W, Wang H, Chen J. Betulonic Acid Inhibits Type-2 Porcine Reproductive and Respiratory Syndrome Virus Replication by Downregulating Cellular ATP Production. International Journal of Molecular Sciences. 2024; 25(19):10366. https://doi.org/10.3390/ijms251910366
Chicago/Turabian StyleLong, Feixiang, Lizhan Su, Mingxin Zhang, Shuhua Wang, Qian Sun, Jinyi Liu, Weisan Chen, Haihong Wang, and Jianxin Chen. 2024. "Betulonic Acid Inhibits Type-2 Porcine Reproductive and Respiratory Syndrome Virus Replication by Downregulating Cellular ATP Production" International Journal of Molecular Sciences 25, no. 19: 10366. https://doi.org/10.3390/ijms251910366
APA StyleLong, F., Su, L., Zhang, M., Wang, S., Sun, Q., Liu, J., Chen, W., Wang, H., & Chen, J. (2024). Betulonic Acid Inhibits Type-2 Porcine Reproductive and Respiratory Syndrome Virus Replication by Downregulating Cellular ATP Production. International Journal of Molecular Sciences, 25(19), 10366. https://doi.org/10.3390/ijms251910366





