Quercetin Mitigates Lysophosphatidylcholine (LPC)-Induced Neutrophil Extracellular Traps (NETs) Formation through Inhibiting the P2X7R/P38MAPK/NOX2 Pathway
Abstract
1. Introduction
2. Result
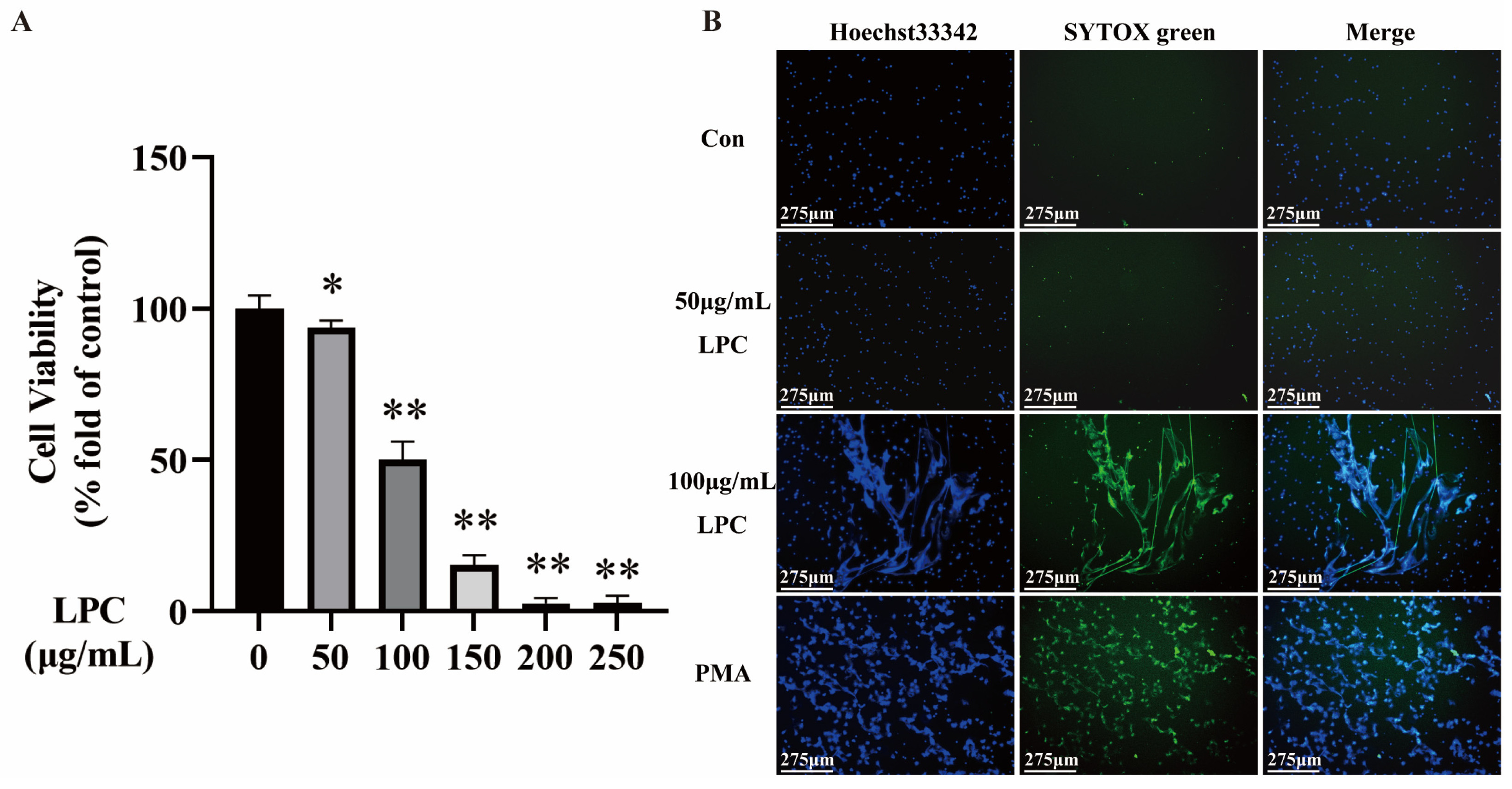
2.1. LPC Induces NETs Formation in Primary Rat Neutrophils
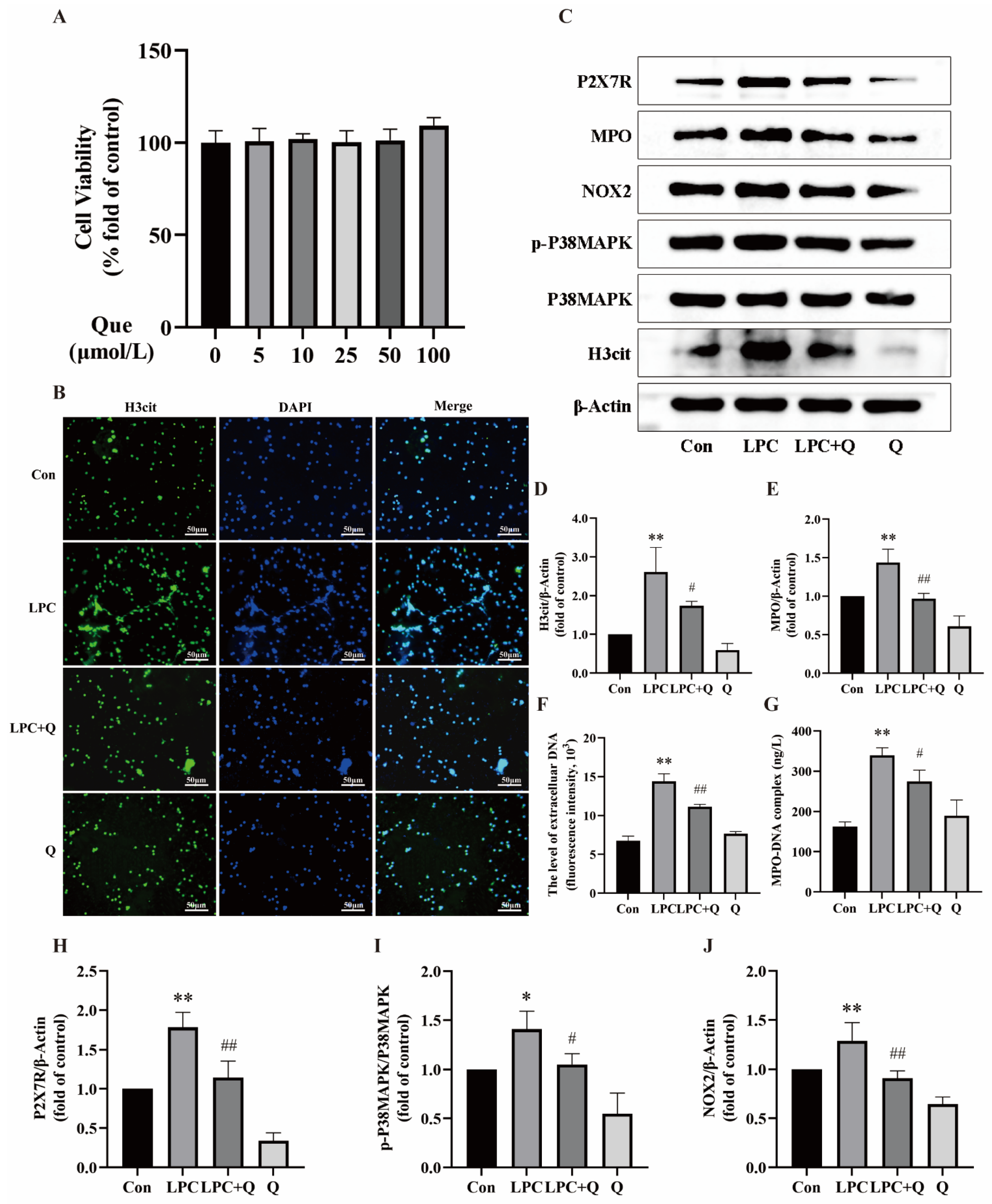
2.2. Quercetin Inhibits LPC-Induced NETs Formation
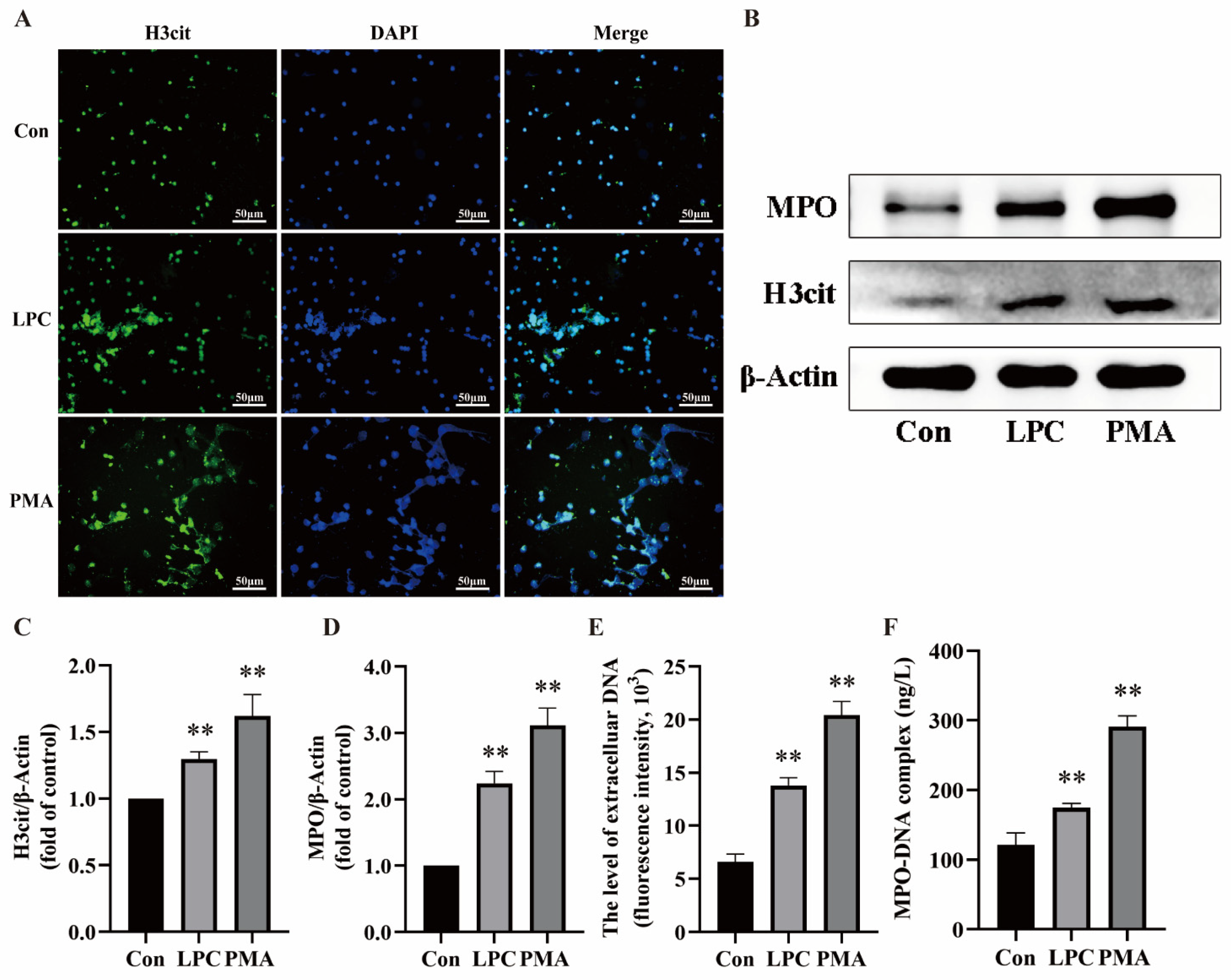
2.3. LPC-Induced NETs Formation Is Mediated by P2X7R Up-Regulation
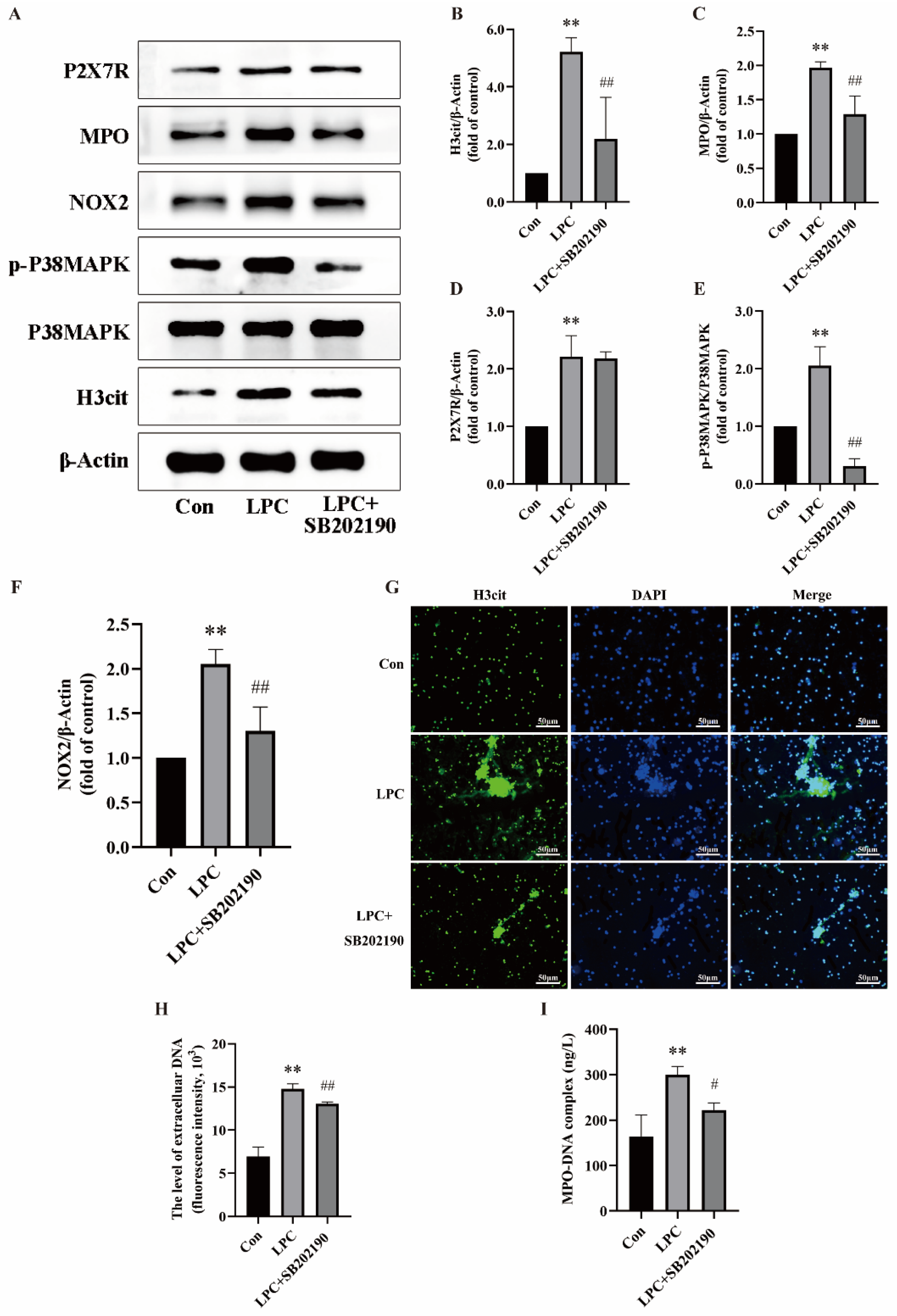
2.4. LPC-Induced NETs Formation Is Associated with the P2X7R/P38MAPK/NOX2 Pathway
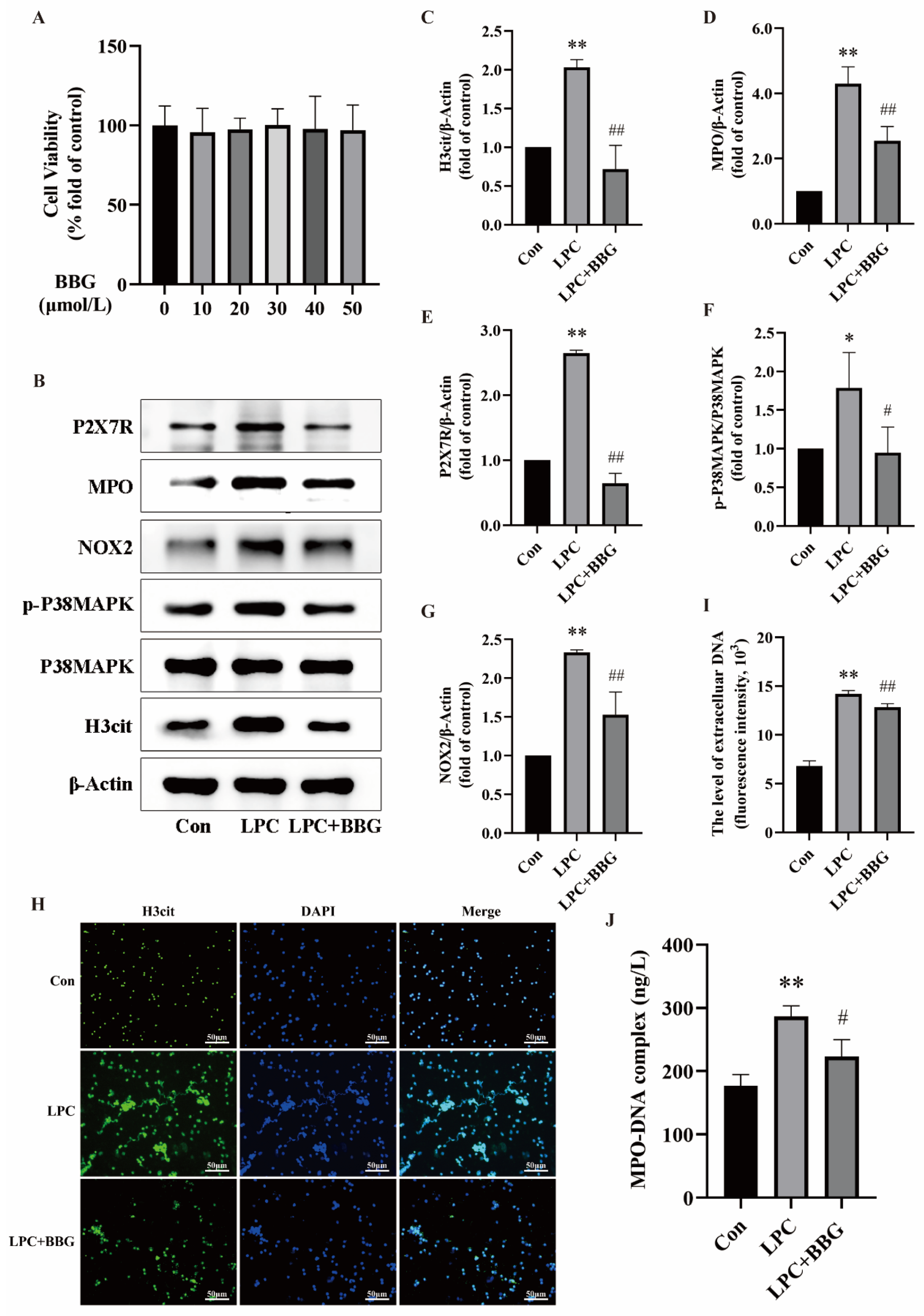
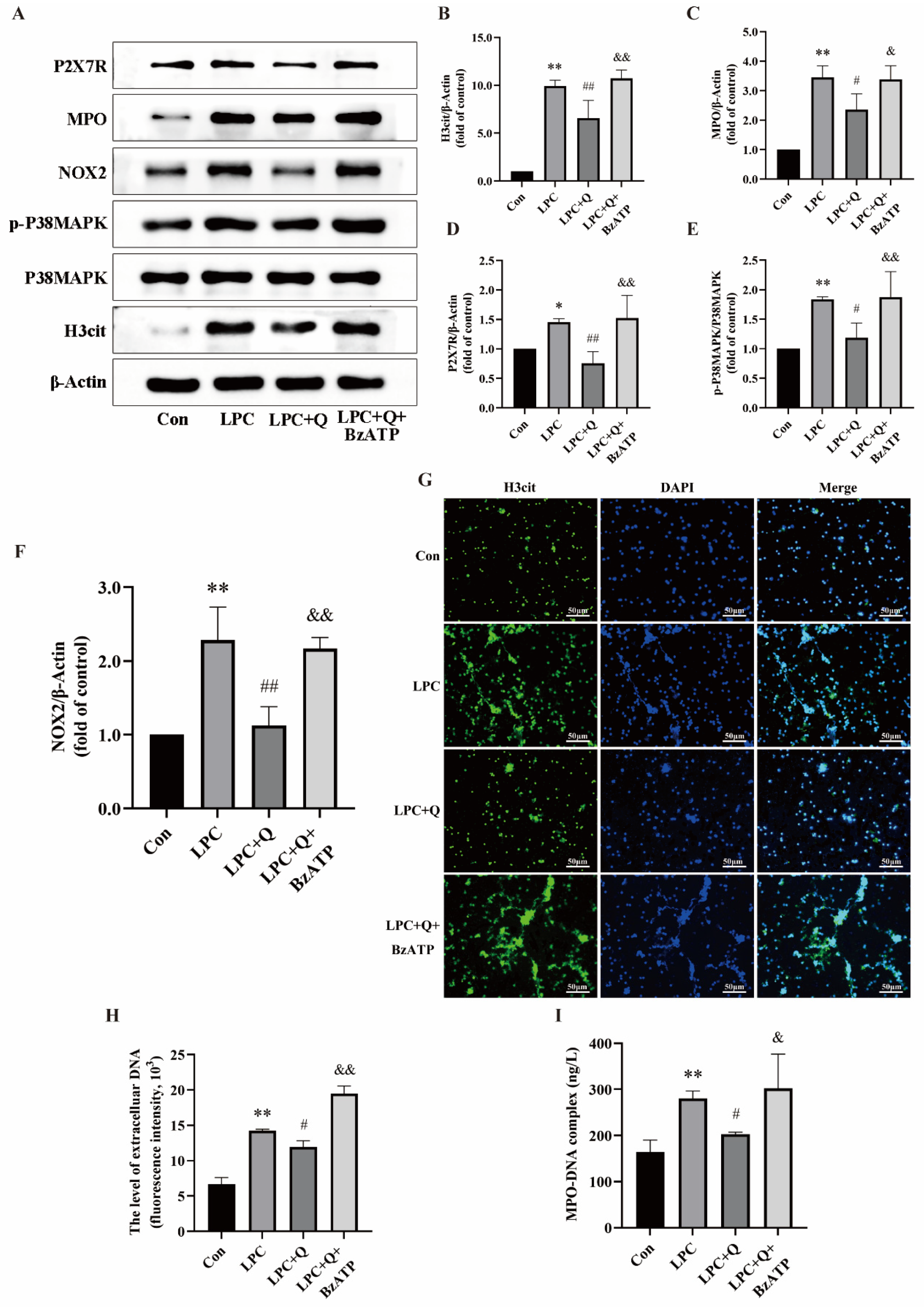
2.5. Quercetin Inhibits LPC-Induced NETs Formation by Regulating P2X7R
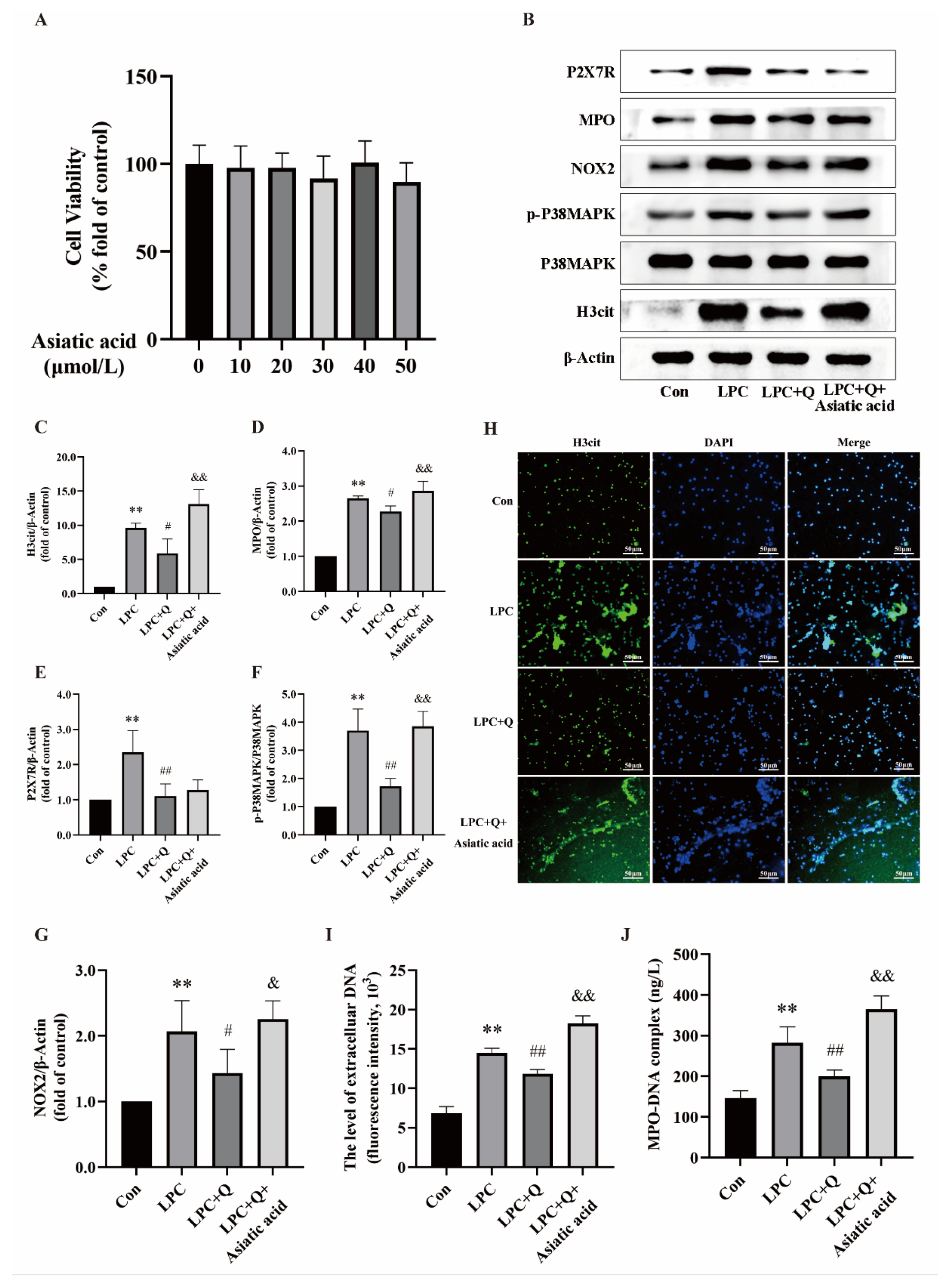
2.6. Quercetin Inhibits LPC-Induced NETs Formation by Regulating the P2X7R/P38MAPK/NOX2 Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animals
4.3. Primary Rat Neutrophils Isolation
4.4. Cell Culture and Treatment
4.5. Cell Viability Assays
4.6. Live-Cell Imaging
4.7. Immunofluorescence
4.8. Quantification of cf-DNA
4.9. MPO-DNA Complexes ELISA
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Morales-Primo, A.U.; Becker, I.; Zamora-Chimal, J. Neutrophil extracellular trap-associated molecules: A review on their immunophysiological and inflammatory roles. Int. Rev. Immunol. 2022, 41, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Hoffman, H.M.; Kubes, P.; Cassatella, M.A.; Zychlinsky, A.; Hedrick, C.C.; Catz, S.D. Neutrophils: New insights and open questions. Sci. Immunol. 2018, 3, eaat4579. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Yang, S.; Zhang, L. Neutrophil Extracellular Traps and Endothelial Dysfunction in Atherosclerosis and Thrombosis. Front. Immunol. 2017, 8, 928. [Google Scholar] [CrossRef]
- Moschonas, I.C.; Tselepis, A.D. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis 2019, 288, 9–16. [Google Scholar] [CrossRef]
- Fa, P.; Ke, B.G.; Dupre, A.; Tsung, A.; Zhang, H. The implication of neutrophil extracellular traps in nonalcoholic fatty liver disease. Front. Immunol. 2023, 14, 1292679. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Gilliland, T.C.; Liu, Y.; Mohebi, R.; Miksenas, H.; Haidermota, S.; Wong, M.; Hu, X.; Cristino, J.R.; Browne, A.; Plutzky, J.; et al. Lipoprotein(a), Oxidized Phospholipids, and Coronary Artery Disease Severity and Outcomes. J. Am. Coll. Cardiol. 2023, 81, 1780–1792. [Google Scholar] [CrossRef]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef]
- Bao, L.; Qi, J.; Wang, Y.-W.; Xi, Q.; Tserennadmid, T.; Zhao, P.-F.; Qi, J.; Damirin, A. The atherogenic actions of LPC on vascular smooth muscle cells and its LPA receptor mediated mechanism. Biochem. Biophys. Res. Commun. 2018, 503, 1911–1918. [Google Scholar] [CrossRef]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.-Y.; Kang, S.-H.; Song, D.-K. Lysophosphatidylcholine induces azurophil granule translocation via Rho/Rho kinase/F-actin polymerization in human neutrophils. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2022, 26, 175–182. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, M.; Mantione, M.E.; Baccellieri, D.; Ferrara, D.; Castellano, R.; Chiesa, R.; Alfieri, O.; Foglieni, C. P2X7 receptor antagonism modulates IL-1β and MMP9 in human atherosclerotic vessels. Sci. Rep. 2017, 7, 4872. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Zhao, H.; Chen, T.; Song, Y.; Cui, Y. Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell Death Dis. 2022, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, T.; Sato, M.; Kitani, H. Lysophosphatidylcholine potentiates Ca2+ influx, pore formation and p44/42 MAP kinase phosphorylation mediated by P2X7 receptor activation in mouse microglial cells. J. Neurochem. 2007, 102, 1518–1532. [Google Scholar] [CrossRef]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat. Commun. 2016, 7, 10555. [Google Scholar] [CrossRef]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef]
- Diebold, B.A.; Smith, S.M.E.; Li, Y.; Lambeth, J.D. NOX2 As a Target for Drug Development: Indications, Possible Complications, and Progress. Antioxid. Redox Signal. 2015, 23, 375–405. [Google Scholar] [CrossRef]
- Lv, X.; Liu, Z.; Xu, L.; Song, E.; Song, Y. Tetrachlorobenzoquinone exhibits immunotoxicity by inducing neutrophil extracellular traps through a mechanism involving ROS-JNK-NOX2 positive feedback loop. Environ. Pollut. 2021, 268, 115921. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Sun, Y.; Fu, Y.; Wang, X.; Zhu, X.; Wu, Z.; Li, P.; Wang, J.; Yang, Z. Bongkrekic acid induced neutrophil extracellular traps via p38, ERK, PAD4, and P2X1-mediated signaling. Toxicol. Appl. Pharmacol. 2021, 423, 115580. [Google Scholar] [CrossRef]
- Zhou, E.; Wu, Z.; Zhu, X.; Li, P.; Wang, J.; Yang, Z. Histamine triggers the formation of neutrophil extracellular traps via NADPH oxidase, ERK and p38 pathways. Vet. Immunol. Immunopathol. 2021, 235, 110234. [Google Scholar] [CrossRef]
- Leng, B.; Li, C.; Sun, Y.; Zhao, K.; Zhang, L.; Lu, M.-L.; Wang, H.-X. Protective Effect of Astragaloside IV on High Glucose-Induced Endothelial Dysfunction via Inhibition of P2X7R Dependent P38 MAPK Signaling Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 5070415. [Google Scholar] [CrossRef]
- Hua, B.; Liu, Q.; Gao, S.; Li, W.; Li, H. Protective role of activating PPARγ in advanced glycation end products-induced impairment of coronary artery vasodilation via inhibiting p38 phosphorylation and reactive oxygen species production. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 147, 112641. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. PTR 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Yan, F.; Dong, M.; Ren, Y. Research progress of quercetin in cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1203713. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Souto, F.O.; Zarpelon, A.C.; Staurengo-Ferrari, L.; Fattori, V.; Casagrande, R.; Fonseca, M.J.V.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A. Quercetin reduces neutrophil recruitment induced by CXCL8, LTB4, and fMLP: Inhibition of actin polymerization. J. Nat. Prod. 2011, 74, 113–118. [Google Scholar] [CrossRef]
- Tóth, Š.; Jonecová, Z.; Čurgali, K.; Maretta, M.; Šoltés, J.; Švaňa, M.; Kalpadikis, T.; Caprnda, M.; Adamek, M.; Rodrigo, L.; et al. Quercetin attenuates the ischemia reperfusion induced COX-2 and MPO expression in the small intestine mucosa. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 95, 346–354. [Google Scholar] [CrossRef]
- Kanashiro, A.; Souza, J.G.; Kabeya, L.M.; Azzolini, A.E.C.S.; Lucisano-Valim, Y.M. Elastase release by stimulated neutrophils inhibited by flavonoids: Importance of the catechol group. Z. Naturforschung C J. Biosci. 2007, 62, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Lu, Y.; Li, G.; Hu, M.; Li, S.; Ju, H.; Zhang, M.; Wang, X. Mechanism of neutrophil extracellular traps generation and their role in trophoblasts apoptosis in gestational diabetes mellitus. Cell. Signal. 2021, 88, 110168. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, D.; Nagarkoti, S.; Kumar, A.; Dubey, M.; Singh, A.K.; Pathak, P.; Chandra, T.; Barthwal, M.K.; Dikshit, M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic. Biol. Med. 2016, 93, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Li, S.; Ma, Y.; Hu, D.; Xiao, F. Curcumin hinders PBDE-47-induced neutrophil extracellular traps release via Nrf2-associated ROS inhibition. Ecotoxicol. Environ. Saf. 2021, 225, 112779. [Google Scholar] [CrossRef]
- Yuan, K.; Zhu, Q.; Lu, Q.; Jiang, H.; Zhu, M.; Li, X.; Huang, G.; Xu, A. Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. J. Nutr. Biochem. 2020, 84, 108454. [Google Scholar] [CrossRef]
- Jiang, L.H.; Mackenzie, A.B.; North, R.A.; Surprenant, A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol. Pharmacol. 2000, 58, 82–88. [Google Scholar] [CrossRef]
- Xu, M.-X.; Zhao, G.-L.; Hu, X.; Zhou, H.; Li, S.-Y.; Li, F.; Miao, Y.; Lei, B.; Wang, Z. P2X7/P2X4 Receptors Mediate Proliferation and Migration of Retinal Microglia in Experimental Glaucoma in Mice. Neurosci. Bull. 2022, 38, 901–915. [Google Scholar] [CrossRef]
- Wang, J.-J.; Wei, Z.-K.; Han, Z.; Liu, Z.-Y.; Zhang, Y.; Zhu, X.-Y.; Li, X.-W.; Wang, K.; Yang, Z.-T. Sodium fluoride exposure triggered the formation of neutrophil extracellular traps. Environ. Pollut. 2020, 257, 113583. [Google Scholar] [CrossRef]
- He, Z.; Wang, C.; Wang, J.; Zheng, K.; Ding, N.; Yu, M.; Li, W.; Tang, Y.; Li, Y.; Xiao, J.; et al. Chlamydia psittaci inhibits apoptosis of human neutrophils by activating P2X7 receptor expression. Int. J. Med. Microbiol. 2022, 312, 151571. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Xiang, L.; Xiao, L. Quercetin alleviates atherosclerosis by suppressing oxidized LDL-induced senescence in plaque macrophage via inhibiting the p38MAPK/p16 pathway. J. Nutr. Biochem. 2023, 116, 109314. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Mach, F.; Montecucco, F. Update on the role of neutrophils in atherosclerotic plaque vulnerability. Curr. Drug Targets 2015, 16, 321–333. [Google Scholar] [CrossRef] [PubMed]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Varley, P.R.; Goswami, J.; Yazdani, H.O.; Tohme, S.; Loughran, P.; O’Doherty, R.M.; Minervini, M.I.; et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatol. Baltim. Md 2018, 68, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G.-T. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 321–333. [Google Scholar] [CrossRef]
- Döring, Y.; Libby, P.; Soehnlein, O. Neutrophil Extracellular Traps Participate in Cardiovascular Diseases: Recent Experimental and Clinical Insights. Circ. Res. 2020, 126, 1228–1241. [Google Scholar] [CrossRef]
- Ohinata, H.; Obama, T.; Makiyama, T.; Watanabe, Y.; Itabe, H. High-Density Lipoprotein Suppresses Neutrophil Extracellular Traps Enhanced by Oxidized Low-Density Lipoprotein or Oxidized Phospholipids. Int. J. Mol. Sci. 2022, 23, 13992. [Google Scholar] [CrossRef]
- Kim, S.-W.; Davaanyam, D.; Seol, S.-I.; Lee, H.-K.; Lee, H.; Lee, J.-K. Adenosine Triphosphate Accumulated Following Cerebral Ischemia Induces Neutrophil Extracellular Trap Formation. Int. J. Mol. Sci. 2020, 21, 7668. [Google Scholar] [CrossRef]
- Zhu, C.-L.; Xie, J.; Zhao, Z.-Z.; Li, P.; Liu, Q.; Guo, Y.; Meng, Y.; Wan, X.-J.; Bian, J.-J.; Deng, X.-M.; et al. PD-L1 maintains neutrophil extracellular traps release by inhibiting neutrophil autophagy in endotoxin-induced lung injury. Front. Immunol. 2022, 13, 949217. [Google Scholar] [CrossRef]
- Wei, J.-Y.; Hu, M.-Y.; Chen, X.-Q.; Wei, J.-S.; Chen, J.; Qin, X.-K.; Lei, F.-Y.; Zou, J.-S.; Zhu, S.-Q.; Qin, Y.-H. Hypobaric Hypoxia Aggravates Renal Injury by Inducing the Formation of Neutrophil Extracellular Traps through the NF-κB Signaling Pathway. Curr. Med. Sci. 2023, 43, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, Y.; Sun, R.; Hu, H.; Liu, Y.; Herrmann, M.; Zhao, Y.; Muñoz, L.E. Receptor-Mediated NETosis on Neutrophils. Front. Immunol. 2021, 12, 775267. [Google Scholar] [CrossRef]
- Singel, K.L.; Segal, B.H. NOX2-dependent regulation of inflammation. Clin. Sci. 2016, 130, 479–490. [Google Scholar] [CrossRef]
- Liu, F.-C.; Yu, H.-P.; Liao, C.-C.; Chou, A.-H.; Lee, H.-C. Corilagin Inhibits Neutrophil Extracellular Trap Formation and Protects against Hydrochloric Acid/Lipopolysaccharide-Induced Acute Lung Injury in Mice by Suppressing the STAT3 and NOX2 Signaling Pathways. Antioxidants 2024, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.A.; Jones, H.M.; Kaldor, C.D.; Hampton, M.B.; Winterbourn, C.C. Neutrophil NET Formation with Microbial Stimuli Requires Late Stage NADPH Oxidase Activity. Antioxidants 2021, 10, 1791. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, H.; Yang, Z.-T.; Mao, E.-Q.; Chen, Y.; Chen, E.-Z. Free fatty acids-induced neutrophil extracellular traps lead to dendritic cells activation and T cell differentiation in acute lung injury. Aging 2021, 13, 26148–26160. [Google Scholar] [CrossRef]
- Pereira, G.S.; Percebom, I.; Mendes, S.; Souza, P.S.S.; Diniz, L.F.A.; Costa, M.F.; Lopes, B.R.P.; Toledo, K.A. Quercetin inhibits neutrophil extracellular traps release and their cytotoxic effects on A549 cells, as well the release and enzymatic activity of elastase and myeloperoxidase. Braz. J. Biol. Rev. Brasleira Biol. 2022, 84, e252936. [Google Scholar]
- Zhao, X.; Gong, L.; Wang, C.; Liu, M.; Hu, N.; Dai, X.; Peng, C.; Li, Y. Quercetin mitigates ethanol-induced hepatic steatosis in zebrafish via P2X7R-mediated PI3K/ Keap1/Nrf2 signaling pathway. J. Ethnopharmacol. 2021, 268, 113569. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Zhang, C.; Xuan, J.; Su, C.; Wang, Y. Quercetin attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Gene 2016, 577, 275–280. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, X.; Chen, Q.; Wang, X.; Wu, Y.; Zhao, X. Quercetin inhibits the NOX2/ROS-mediated NF-κB/TXNIP signaling pathway to ameliorate pyroptosis of cardiomyocytes to relieve sepsis-induced cardiomyopathy. Toxicol. Appl. Pharmacol. 2023, 477, 116672. [Google Scholar] [CrossRef]
- Li, J.-X.; Tian, R.; Lu, N. Quercetin Attenuates Vascular Endothelial Dysfunction in Atherosclerotic Mice by Inhibiting Myeloperoxidase and NADPH Oxidase Function. Chem. Res. Toxicol. 2023, 36, 260–269. [Google Scholar] [CrossRef]
- Tan, S.-A.; Yam, H.C.; Cheong, S.L.; Chow, Y.C.; Bok, C.Y.; Ho, J.M.; Lee, P.Y.; Gunasekaran, B. Inhibition of Porphyromonas gingivalis peptidyl arginine deiminase, a virulence factor, by antioxidant-rich Cratoxylum cochinchinense: In vitro and in silico evaluation. Saudi J. Biol. Sci. 2022, 29, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-H.; Han, J.-R.; Ren, P.-P.; Xie, Y.; Jiang, D.-Y. Exploration of the mechanism of Zisheng Shenqi decoction against gout arthritis using network pharmacology. Comput. Biol. Chem. 2021, 90, 107358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Qin, H.; Chen, W.; Xiang, J.; Jiang, M.; Zhang, L.; Zhou, K.; Hu, Y. Utilizing network pharmacology, molecular docking, and animal models to explore the therapeutic potential of the WenYang FuYuan recipe for cerebral ischemia-reperfusion injury through AGE-RAGE and NF-κB/p38MAPK signaling pathway modulation. Exp. Gerontol. 2024, 191, 112448. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Tian, R.; Yang, Z.; Peng, Y.-Y.; Lu, N. Quercetin suppressed NADPH oxidase-derived oxidative stress via heme oxygenase-1 induction in macrophages. Arch. Biochem. Biophys. 2019, 671, 69–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, Y.; Ying, L.; Li, H.; Zhang, K.; Liang, N.; Luo, G.; Xiao, L. Quercetin Mitigates Lysophosphatidylcholine (LPC)-Induced Neutrophil Extracellular Traps (NETs) Formation through Inhibiting the P2X7R/P38MAPK/NOX2 Pathway. Int. J. Mol. Sci. 2024, 25, 9411. https://doi.org/10.3390/ijms25179411
Liu S, Wang Y, Ying L, Li H, Zhang K, Liang N, Luo G, Xiao L. Quercetin Mitigates Lysophosphatidylcholine (LPC)-Induced Neutrophil Extracellular Traps (NETs) Formation through Inhibiting the P2X7R/P38MAPK/NOX2 Pathway. International Journal of Molecular Sciences. 2024; 25(17):9411. https://doi.org/10.3390/ijms25179411
Chicago/Turabian StyleLiu, Si, Yan Wang, Linyao Ying, Hao Li, Keyi Zhang, Na Liang, Gang Luo, and Lin Xiao. 2024. "Quercetin Mitigates Lysophosphatidylcholine (LPC)-Induced Neutrophil Extracellular Traps (NETs) Formation through Inhibiting the P2X7R/P38MAPK/NOX2 Pathway" International Journal of Molecular Sciences 25, no. 17: 9411. https://doi.org/10.3390/ijms25179411
APA StyleLiu, S., Wang, Y., Ying, L., Li, H., Zhang, K., Liang, N., Luo, G., & Xiao, L. (2024). Quercetin Mitigates Lysophosphatidylcholine (LPC)-Induced Neutrophil Extracellular Traps (NETs) Formation through Inhibiting the P2X7R/P38MAPK/NOX2 Pathway. International Journal of Molecular Sciences, 25(17), 9411. https://doi.org/10.3390/ijms25179411





