Pro-Osteogenic Effect of the Nutraceutical BlastiMin Complex® in Women with Osteoporosis or Osteopenia: An Open Intervention Clinical Trial
Abstract
1. Introduction
2. Results
2.1. Effects of BlastiMin Complex® on Serum BTMs, CRP, and BMD Values
2.2. Analysis of Selected SNPs of COL1A1, RANKL, RANK, VDR, and CTR Genes
3. Discussion
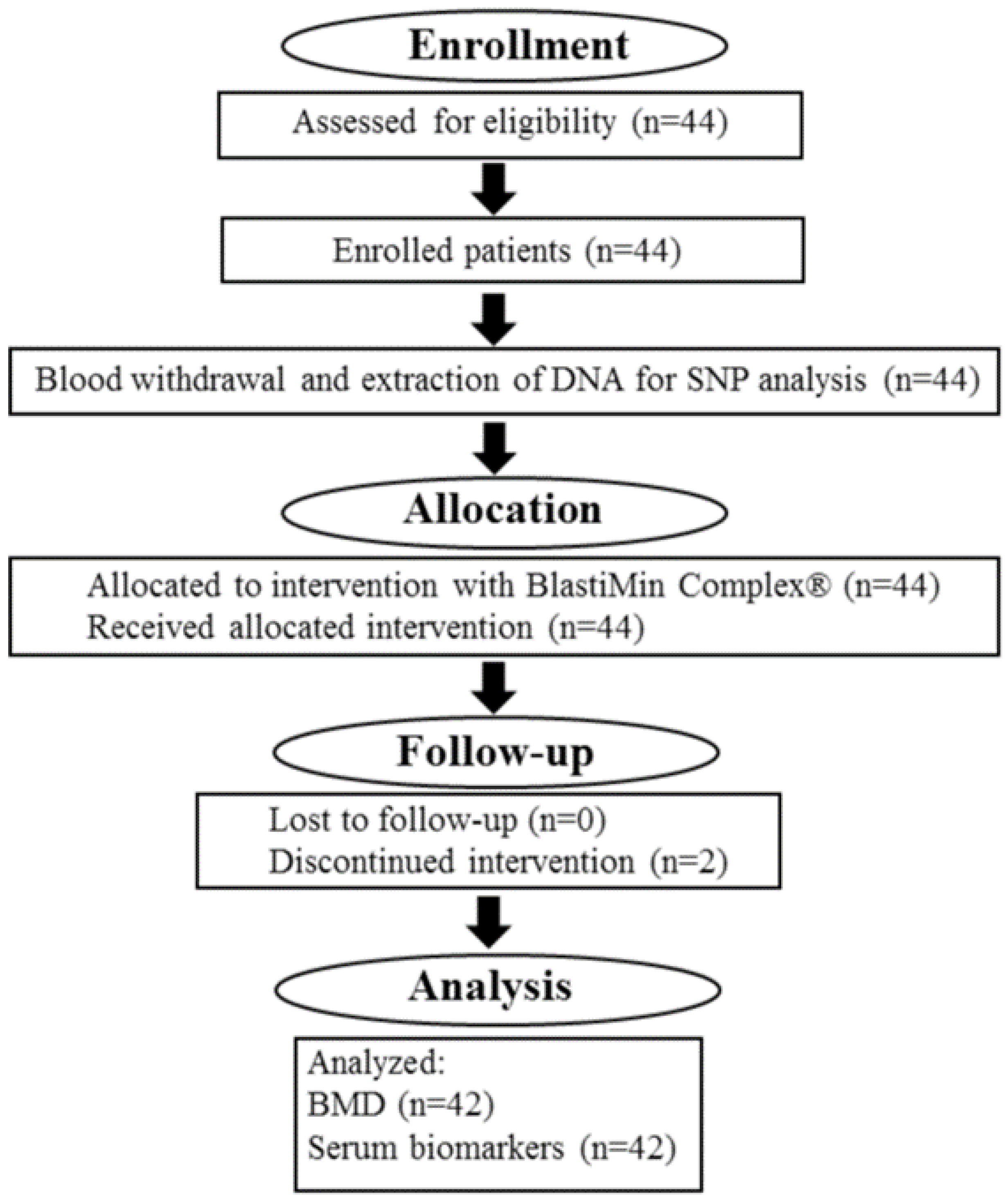
4. Materials and Methods
4.1. Study Design
4.2. Treatment
4.3. Assessments
4.4. Polymerase Chain Reaction for the SNP Analysis
4.5. BMD Measurement
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A review of treatment options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Genant, H.K.; Cooper, C.; Poor, G.; Reid, I.; Ehrlich, G.; Kanis, J.; Nordin, B.E.; Barrett-Connor, E.; Black, D.; Bonjour, J.P.; et al. Interim report and recommendations of the World Health Organization task-force for osteoporosis. Osteoporos. Int. 1999, 10, 259–264. [Google Scholar] [CrossRef]
- Kim, B.; Cho, Y.J.; Lim, W. Osteoporosis therapies and their mechanisms of action (Review). Exp. Ther. Med. 2021, 22, 1379–1392. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, C.; Lu, C.; Mo, C.; Zeng, J.; Yao, M.; Jia, B.; Liu, Z.; Yuan, P.; Xu, S. Age-related bone diseases: Role of inflammaging. J. Autoimmun. 2024, 143, 103169. [Google Scholar] [CrossRef]
- Akkawi, I.; Zmerly, H. Osteoporosis: Current Concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef]
- Szulc, P.; Delmas, P.D. Biochemical markers of bone turnover: Potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos. Int. 2008, 19, 1683–1704. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Dempster, D.W. Lessons from bone histomorphometry on the mechanisms of action of osteoporosis drugs. In Marcus and Feldman’s Osteoporosis, 5th ed.; Dempster, D., Cauley, J., Bouxsein, M., Cosman, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 76, pp. 1835–1863. [Google Scholar]
- Zmuda, J.M.; Sheu, Y.T.; Moffett, S.P. The search for human osteoporosis genes. J. Musculoskelet. Neuronal Interact. 2006, 6, 3–15. [Google Scholar] [PubMed]
- Kanis, J.A.; Burlet, N.; Cooper, C.; Delmas, P.D.; Reginster, J.Y.; Borgstrom, F.; Rizzoli, R. European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO): European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2008, 19, 399–428. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Nuti, R.; Brandi, M.L.; Checchia, G.; Di Munno, O.; Dominguez, L.; Falaschi, P.; Fiore, C.E.; Iolascon, G.; Maggi, S.; Michieli, R.; et al. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019, 14, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Snow, V.; Shekelle, P.; Hopkins, R., Jr.; Forciea, M.A.; Owens, D.K. Clinical Efficacy Assessment Subcommittee of the American College of Physicians: Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: A clinical practice guidelines from the American college of physicians. Ann. Intern. Med. 2008, 149, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L. Overview of treatment approaches to osteoporosis. Br. J. Pharmacol. 2021, 178, 1891–1906. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Yang, Y.; Jung, H. Molecular mechanisms and emerging therapeutics for osteoporosis. Int. J. Mol. Sci. 2020, 21, 7623. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American association of clinical endocrinologists and American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr. Pract. 2016, 22 (Suppl. S4), S1–S42. [Google Scholar] [CrossRef] [PubMed]
- European Patent Application EP 4 205 733 A1. Available online: https://data.epo.org/publication-server/rest/v1.0/publication-dates/20230705/patents/EP4205733NWA1/document.pdf (accessed on 8 February 2024).
- Giordani, C.; Matacchione, G.; Giuliani, A.; Valli, D.; Scarpa, E.S.; Antonelli, A.; Sabbatinelli, J.; Giacchetti, G.; Sabatelli, S.; Olivieri, F.; et al. Pro-Osteogenic and Anti-Inflammatory Synergistic Effect of Orthosilicic Acid, Vitamin K2, Curcumin, Polydatin and Quercetin Combination in Young and Senescent Bone Marrow-Derived Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2023, 24, 8820. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, molecular target and current status on drug development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Teitelbaum, S.L. Osteoclasts: New insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef]
- Rucci, N. Molecular biology of bone remodelling. Clin. Cases Miner. Bone Metab. 2008, 5, 49–56. [Google Scholar]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Henriksen, K.; Bollerslev, J.; Everts, V.; Karsdal, M.A. Osteoclast activity and subtypes as a function of physiology and pathology-implications for future treatments of osteoporosis. Endocr. Rev. 2011, 32, 31–63. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Infante, A.; Rodriguez, C.I. Osteogenesis and Aging: Lessons from Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 244–250. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Tsai, J.M.; Wein, M.N. Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef]
- Chau, J.F.; Leong, W.F.; Li, B. Signaling pathways governing osteoblast proliferation, differentiation and function. Histol. Histopathol. 2009, 24, 1593–1606. [Google Scholar]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef]
- Wu, J.; Shang, D.P.; Yang, S.; Fu, D.P.; Ling, H.Y.; Hou, S.S.; Lu, J.M. Association between the vitamin D receptor gene polymorphism and osteoporosis. Biomed. Rep. 2016, 5, 233–236. [Google Scholar] [CrossRef]
- SNPedia Database. Available online: https://www.snpedia.com/index.php/Rs9594759 (accessed on 5 March 2024).
- SNPedia Database. Available online: https://www.snpedia.com/index.php/Rs9594738 (accessed on 5 March 2024).
- SNPedia Database. Available online: https://www.snpedia.com/index.php/Rs17879735 (accessed on 5 March 2024).
- Moradifard, S.; Hoseinbeyki, M.; Emam, M.M.; Parchiniparchin, F.; Ebrahimi-Rad, M. Association of the Sp1 binding site and -1997 promoter variations in COL1A1 with osteoporosis risk: The application of meta-analysis and bioinformatics approaches offers a new perspective for future research. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108339. [Google Scholar] [CrossRef]
- Yunaini, L.; Suryandari, D.A.; Kusdhany, L.; Kurniati, M.; Reihannisha, I.; Auerkari, E.I. The Role of Receptor Activator of Nuclear Factor Kappa B (RANK) and Receptor Activator of Nuclear Factor Kappa B Ligand (RANKL) in Osteoporosis Risk: Gene Polymorphism and Soluble RANKL Level in Indonesia Post-Menopausal Women. OnLine J. Biol. Sci. 2018, 18, 358–364. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid intake and bone health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16–26. [Google Scholar] [CrossRef]
- Kurotaki, D.; Yoshida, H.; Tamura, T. Epigenetic and transcriptional regulation of osteoclast differentiation. Bone 2020, 138, 115471. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signalling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Joung, Y.H.; Darvin, P.; Kang, D.Y.; Sp, N.; Byun, H.J.; Lee, C.H.; Lee, H.K.; Yang, Y.M. Methylsulfonylmethane Inhibits RANKL-Induced Osteoclastogenesis in BMMs by Suppressing NF-κB and STAT3 Activities. PLoS ONE 2016, 11, e0159891. [Google Scholar] [CrossRef]
- Josse, A.R.; Ludwa, I.A.; Kouvelioti, R.; Calleja, M.; Falk, B.; Ward, W.E.; Klentrou, P. Dairy product intake decreases bone resorption following a 12-week diet and exercise intervention in overweight and obese adolescent girls. Pediatr. Res. 2020, 88, 910–916. [Google Scholar] [CrossRef]
- Mun, H.; Liu, B.; Pham, T.H.A.; Wu, Q. C-reactive protein and fracture risk: An updated systematic review and meta-analysis of cohort studies through the use of both frequentist and Bayesian approaches. Osteoporos. Int. 2021, 32, 425–435. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Jin, J.; Jin, D.; Cui, L.; Li, X.; Rei, Y.; Jiang, H.; Zhao, G.; Yang, G.; et al. Increased serum cathepsin K in patients with coronary artery disease. Yonsei Med. J. 2014, 55, 912–919. [Google Scholar] [CrossRef]
- Karimi, S.M.; Bayat, M.; Rahimi, R. Plant-derived natural medicines for the management of osteoporosis: A comprehensive review of clinical trials. J. Tradit. Complement. Med. 2023, 14, 1–18. [Google Scholar] [CrossRef]
- Wong, R.H.; Zaw, J.J.T.; Xian, C.J.; Howe, P.R. Regular Supplementation with Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Urano, T.; Inoue, S. Genetics of osteoporosis. Biochem. Biophys. Res. Commun. 2014, 452, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Casas-Avila, L.; Cruz-Arenas, E.; Ponce-de Leon-Suarez, V.; Sanchez-Bringas, G.; Olivares-Banuelos, B.; Chavez-Heres, T.; Valdes-Flores, M. High risk of lumbar spine osteoporosis with the RANK rs3018362 polymorphism. Gynecol. Endocrinol. 2019, 35, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Jowka, E.; Dlugolecka, B.; Cieslinski, I.; Kotowska, J. Polymorphisms in Genes Encoding VDR, CALCR and Antioxidant Enzymes as Predictors of Bone Tissue Condition in Young, Healthy Men. Int. J. Mol. Sci. 2023, 24, 3373. [Google Scholar] [CrossRef] [PubMed]
- Mansournia, M.A.; Higgings, J.P.T.; Sterne, J.A.C.; Hernan, M.A. Biases in randomized trials: A conversation between trialists and epidemiologists. Epidemiology 2017, 28, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Gioia, A.; Ceccoli, L.; Ronconi, V.; Turchi, F.; Marcheggiani, M.; Boscaro, M.; Giacchetti, G.; Balercia, G. Vitamin D levels and bone mineral density: Are LH levels involved in the pathogenesis of bone impairment in hypogonadal men? J. Endocrinol. Investig. 2014, 37, 1225–1231. [Google Scholar] [CrossRef]
- Adami, G.; Brandi, M.L.; Caffarelli, C.; Casciaro, E.; Conversano, F.; Di Paola, M.; Fassio, A.; Gatti, D.; Giusti, F.; Gonnelli, S.; et al. Bone health status evaluation in men by means of REMS technology. Aging Clin. Exp. Res. 2024, 36, 74–83. [Google Scholar]
| Parameters | t0 months | t6 months | t12 months |
|---|---|---|---|
| Vitamin D2 + D3 (ng/mL) | 33.62 ± 12.87 | 33.91 ± 9.47 | 32.60 ± 8.31 |
| P1NP (μg/L) | 63.64 ± 17.80 | 71.43 ± 26.49 * | 74.30 ± 24.57 *** |
| CTX (pg/mL) | 381.67 ± 152.14 | 430.88 ± 130.18 * | 406.60 ± 160.42 |
| OCN (ng/mL) | 21.59 ± 5.46 | 20.35 ± 4.20 | 22.41 ± 5.92 |
| bALP (ng/mL) | 14.56 ± 6.44 | 14.72 ± 4.61 | 15.03 ± 6.02 |
| CRP (mg/dL) | 0.26 ± 0.05 | 0.22 ± 0.07 | 0.12 ± 0.09 *** |
| BTI | 0.167 | 0.166 | 0.183 |
| BMD L1-L4 (g/cm2) | 0.831 ± 0.08 | 0.834 ± 0.07 | 0.829 ± 0.08 |
| BMD FN (g/cm2) | 0.640 ± 0.08 | 0.640 ± 0.07 | 0.627 ± 0.08 |
| Parameters | t0 months | t12 months |
|---|---|---|
| DXA L1-L4 T-score | −1.512 ± 1.029 | −1.551 ± 1.014 |
| DXA FN T-score | −1.514 ± 0.607 | −1.515 ± 0.687 |
| REMS L1-L4 T-score | −1.96 ± 0.68 | −2.00 ± 0.71 |
| REMS FN T-score | −1.88 ± 0.74 | −1.98 ± 0.74 |
| Gene | SNP | Homozygous wt | Heterozygous | Homozygous Mut |
|---|---|---|---|---|
| CTR | rs1801197 T>C | 56.8% | 40.9% | 2.3% |
| VDR | rs17879735 G>T | 29.6% | 45.4% | 25.0% |
| VDR | rs1544410 G>A | 43.2% | 43.2% | 13.6% |
| VDR | rs11568820 G>A | 50.0% | 43.2% | 6.8% |
| VDR | rs2228570 T>C | 50.0% | 40.9% | 9.1% |
| VDR | rs731236 T>C | 59.1% | 47.7% | 11.4% |
| COL1A1 | rs1800012 G>T | 68.2% | 27.3% | 4.5% |
| RANK | rs3018362 A>G | 65.9% | 59.1% | 6.8% |
| RANKL | rs9594759 C>T | 18.2% | 54.5% | 27.3% |
| RANKL | rs9594738 C>T | 22.7% | 59.1% | 18.2% |
| Characteristics | |
|---|---|
| Sex, no. (%) | |
| Male | 0% |
| Female | 100% |
| Age, y | 62.4 ± 6.1 |
| Weight, kg | 63.5 ± 9.8 |
| BMI | 24.1 ± 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatelli, S.; Scarpa, E.-S.; Giuliani, A.; Giordani, C.; Sabbatinelli, J.; Rippo, M.R.; Cabodi, S.; Petrini, B.; Balercia, G.; Giacchetti, G. Pro-Osteogenic Effect of the Nutraceutical BlastiMin Complex® in Women with Osteoporosis or Osteopenia: An Open Intervention Clinical Trial. Int. J. Mol. Sci. 2024, 25, 8565. https://doi.org/10.3390/ijms25168565
Sabatelli S, Scarpa E-S, Giuliani A, Giordani C, Sabbatinelli J, Rippo MR, Cabodi S, Petrini B, Balercia G, Giacchetti G. Pro-Osteogenic Effect of the Nutraceutical BlastiMin Complex® in Women with Osteoporosis or Osteopenia: An Open Intervention Clinical Trial. International Journal of Molecular Sciences. 2024; 25(16):8565. https://doi.org/10.3390/ijms25168565
Chicago/Turabian StyleSabatelli, Sofia, Emanuele-Salvatore Scarpa, Angelica Giuliani, Chiara Giordani, Jacopo Sabbatinelli, Maria Rita Rippo, Sara Cabodi, Barbara Petrini, Giancarlo Balercia, and Gilberta Giacchetti. 2024. "Pro-Osteogenic Effect of the Nutraceutical BlastiMin Complex® in Women with Osteoporosis or Osteopenia: An Open Intervention Clinical Trial" International Journal of Molecular Sciences 25, no. 16: 8565. https://doi.org/10.3390/ijms25168565
APA StyleSabatelli, S., Scarpa, E.-S., Giuliani, A., Giordani, C., Sabbatinelli, J., Rippo, M. R., Cabodi, S., Petrini, B., Balercia, G., & Giacchetti, G. (2024). Pro-Osteogenic Effect of the Nutraceutical BlastiMin Complex® in Women with Osteoporosis or Osteopenia: An Open Intervention Clinical Trial. International Journal of Molecular Sciences, 25(16), 8565. https://doi.org/10.3390/ijms25168565








