Serum Phosphatidylcholine Species 32:0 as a Biomarker for Liver Cirrhosis Pre- and Post-Hepatitis C Virus Clearance
Abstract
1. Introduction
2. Results
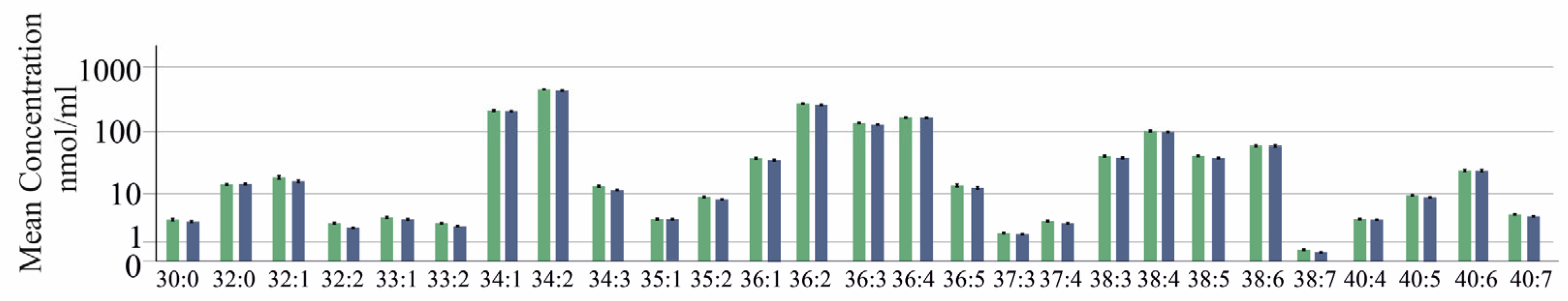
2.1. Serum PC Species Levels in Relation to Gender, Age, Body Mass Index, Liver Steatosis and Diabetes
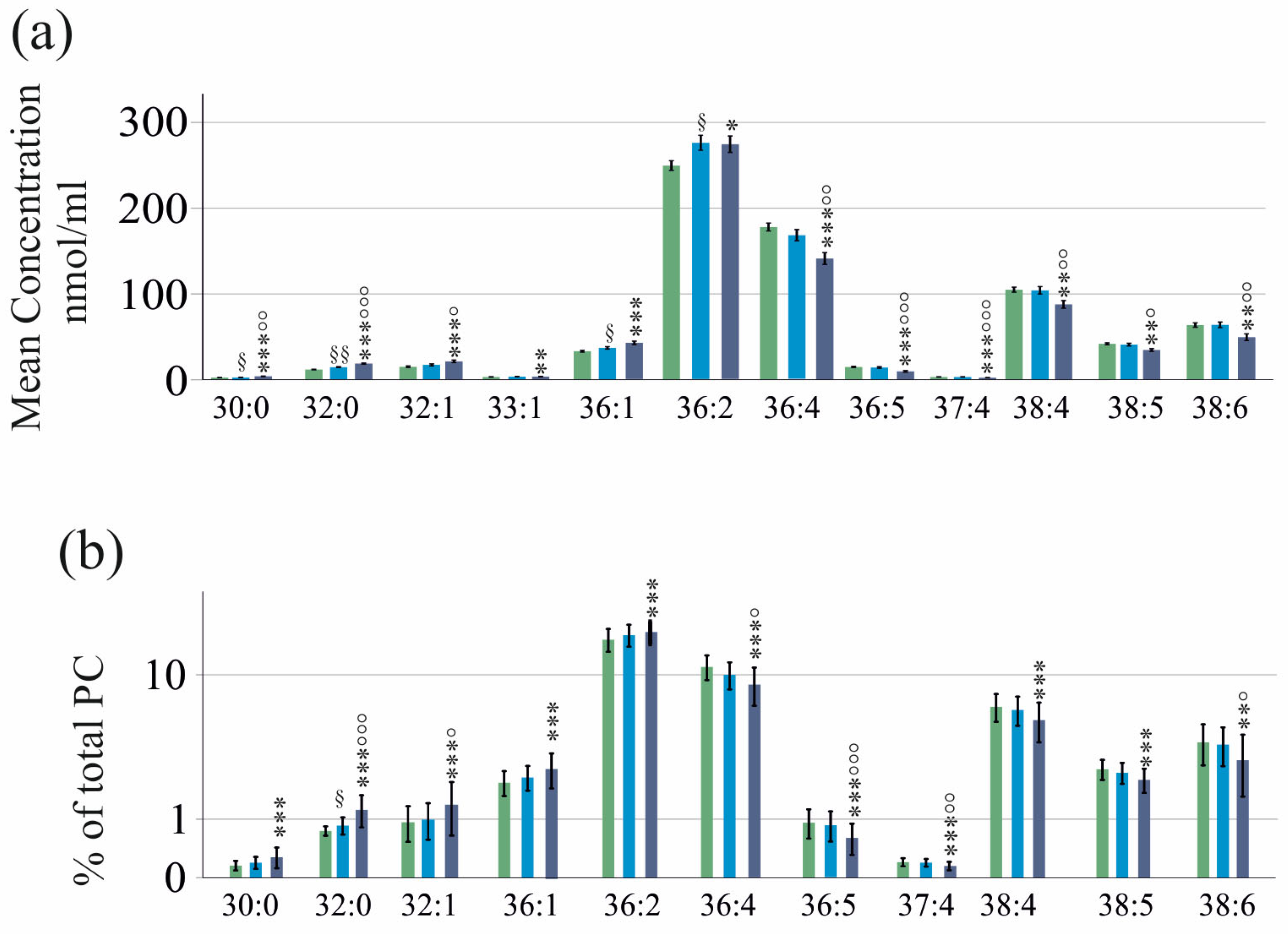
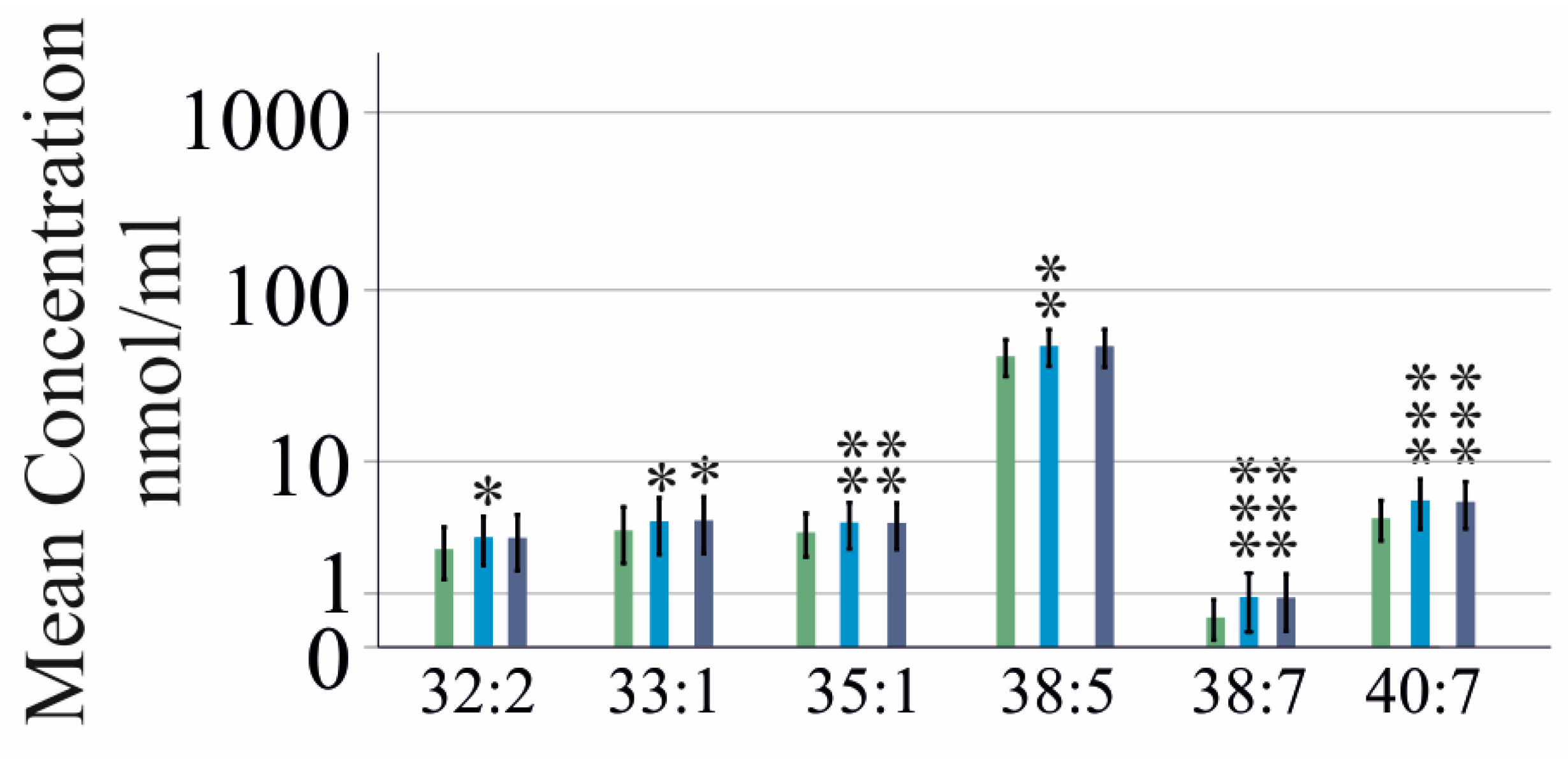
2.2. PC Species in Relation to Liver Fibrosis
2.3. PC Species in Relation to Laboratory Measures of Hepatic and Renal Function
2.4. PC Species in Relation to Markers of Inflammation and Thrombocyte Count
2.5. PC Species in Relation to Viral Load and Viral Genotype
2.6. PC Species in Relation to Viral Cure
2.7. PC Species in Relation to Laboratory Measures Post-DAA Therapy
2.8. PC Species to Discriminate Patients with and without Liver Cirrhosis
3. Discussion
4. Materials and Methods
4.1. Study Cohort
4.2. Measurement of PC Species
4.3. Analysis of Laboratory Values and Calculation of MELD Score
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casiraghi, M.A.; De Paschale, M.; Romano, L.; Biffi, R.; Assi, A.; Binelli, G.; Zanetti, A.R. Long-term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology 2004, 39, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Di Caprio, G.; Fimia, G.M.; Ippolito, G.; Tripodi, M.; Alonzi, T. Hepatitis C virus relies on lipoproteins for its life cycle. World J. Gastroenterol. 2016, 22, 1953–1965. [Google Scholar] [CrossRef]
- Sidorkiewicz, M. Hepatitis C Virus Uses Host Lipids to Its Own Advantage. Metabolites 2021, 11, 273. [Google Scholar] [CrossRef]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H.; European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Endo, D.; Satoh, K.; Shimada, N.; Hokari, A.; Aizawa, Y. Impact of interferon-free antivirus therapy on lipid profiles in patients with chronic hepatitis C genotype 1b. World J. Gastroenterol. 2017, 23, 2355–2364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashimoto, S.; Yatsuhashi, H.; Abiru, S.; Yamasaki, K.; Komori, A.; Nagaoka, S.; Saeki, A.; Uchida, S.; Bekki, S.; Kugiyama, Y.; et al. Rapid Increase in Serum Low-Density Lipoprotein Cholesterol Concentration during Hepatitis C Interferon-Free Treatment. PLoS ONE 2016, 11, e0163644. [Google Scholar] [CrossRef] [PubMed]
- Peschel, G.; Grimm, J.; Gulow, K.; Muller, M.; Buechler, C.; Weigand, K. Chemerin Is a Valuable Biomarker in Patients with HCV Infection and Correlates with Liver Injury. Diagnostics 2020, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Villani, R.; Di Cosimo, F.; Romano, A.D.; Sangineto, M.; Serviddio, G. Serum lipid profile in HCV patients treated with direct-acting antivirals: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 13944. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef]
- Rinella, M.E.; Elias, M.S.; Smolak, R.R.; Fu, T.; Borensztajn, J.; Green, R.M. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J. Lipid Res. 2008, 49, 1068–1076. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tontonoz, P. Phospholipid Remodeling in Physiology and Disease. Annu. Rev. Physiol. 2019, 81, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Beilstein, F.; Lemasson, M.; Pene, V.; Rainteau, D.; Demignot, S.; Rosenberg, A.R. Lysophosphatidylcholine acyltransferase 1 is downregulated by hepatitis C virus: Impact on production of lipo-viro-particles. Gut 2017, 66, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Haberl, E.M.; Weiss, T.S.; Peschel, G.; Weigand, K.; Kohler, N.; Pauling, J.K.; Wenzel, J.J.; Horing, M.; Krautbauer, S.; Liebisch, G.; et al. Liver Lipids of Patients with Hepatitis B and C and Associated Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 5297. [Google Scholar] [CrossRef] [PubMed]
- Gobeil Odai, K.; O’Dwyer, C.; Steenbergen, R.; Shaw, T.A.; Renner, T.M.; Ghorbani, P.; Rezaaifar, M.; Han, S.; Langlois, M.A.; Crawley, A.M.; et al. In Vitro Hepatitis C Virus Infection and Hepatic Choline Metabolism. Viruses 2020, 12, 108. [Google Scholar] [CrossRef]
- Abomughaid, M.; Tay, E.S.E.; Pickford, R.; Malladi, C.; Read, S.A.; Coorssen, J.R.; Gloss, B.S.; George, J.; Douglas, M.W. PEMT Mediates Hepatitis C Virus-Induced Steatosis, Explains Genotype-Specific Phenotypes and Supports Virus Replication. Int. J. Mol. Sci. 2023, 24, 8781. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.T.; Chen, S.S. Human Choline Kinase-alpha Promotes Hepatitis C Virus RNA Replication through Modulation of Membranous Viral Replication Complex Formation. J. Virol. 2016, 90, 9075–9095. [Google Scholar] [CrossRef]
- Ghadir, M.R.; Riahin, A.A.; Havaspour, A.; Nooranipour, M.; Habibinejad, A.A. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat. Mon. 2010, 10, 285–288. [Google Scholar]
- Valkov, I.; Ivanova, R.; Alexiev, A.; Antonov, K.; Mateva, L. Association of Serum Lipids with Hepatic Steatosis, Stage of Liver Fibrosis and Viral Load in Chronic Hepatitis C. J. Clin. Diagn. Res. 2017, 11, OC15–OC20. [Google Scholar] [CrossRef]
- Vere, C.C.; Streba, C.T.; Streba, L.; Rogoveanu, I. Lipid serum profile in patients with viral liver cirrhosis. Med. Princ. Pract. 2012, 21, 566–568. [Google Scholar] [CrossRef]
- Castro-Narro, G.; Moctezuma-Velazquez, C.; Male-Velazquez, R.; Trejo-Estrada, R.; Bosques, F.J.; Moreno-Alcantar, R.; Rodriguez-Hernandez, H.; Bautista-Santos, A.; Cortez-Hernandez, C.; Cerda-Reyes, E.; et al. Position statement on the use of albumin in liver cirrhosis. Ann. Hepatol. 2022, 27, 100708. [Google Scholar] [CrossRef]
- Horing, M.; Peschel, G.; Grimm, J.; Krautbauer, S.; Muller, M.; Weigand, K.; Liebisch, G.; Buechler, C. Serum Ceramide Species Are Associated with Liver Cirrhosis and Viral Genotype in Patients with Hepatitis C Infection. Int. J. Mol. Sci. 2022, 23, 9806. [Google Scholar] [CrossRef]
- Peschel, G.; Krautbauer, S.; Weigand, K.; Grimm, J.; Horing, M.; Liebisch, G.; Muller, M.; Buechler, C. Rising Lysophosphatidylcholine Levels Post-Hepatitis C Clearance. Int. J. Mol. Sci. 2024, 25, 1198. [Google Scholar] [CrossRef]
- Cantoni, L.; Curri, S.B.; Andreuzzi, P.; Rocchetti, P. Plasma and red blood cell phospholipids in chronic liver diseases. Clin. Chim. Acta 1975, 60, 405–408. [Google Scholar] [CrossRef]
- Meikle, P.J.; Mundra, P.A.; Wong, G.; Rahman, K.; Huynh, K.; Barlow, C.K.; Duly, A.M.; Haber, P.S.; Whitfield, J.B.; Seth, D. Circulating Lipids Are Associated with Alcoholic Liver Cirrhosis and Represent Potential Biomarkers for Risk Assessment. PLoS ONE 2015, 10, e0130346. [Google Scholar] [CrossRef]
- McPhail, M.J.W.; Shawcross, D.L.; Lewis, M.R.; Coltart, I.; Want, E.J.; Antoniades, C.G.; Veselkov, K.; Triantafyllou, E.; Patel, V.; Pop, O.; et al. Multivariate metabotyping of plasma predicts survival in patients with decompensated cirrhosis. J. Hepatol. 2016, 64, 1058–1067. [Google Scholar] [CrossRef]
- Chen, S.; Yin, P.; Zhao, X.; Xing, W.; Hu, C.; Zhou, L.; Xu, G. Serum lipid profiling of patients with chronic hepatitis B, cirrhosis, and hepatocellular carcinoma by ultra fast LC/IT-TOF MS. Electrophoresis 2013, 34, 2848–2856. [Google Scholar] [CrossRef]
- Virseda-Berdices, A.; Rojo, D.; Martinez, I.; Berenguer, J.; Gonzalez-Garcia, J.; Brochado-Kith, O.; Fernandez-Rodriguez, A.; Diez, C.; Hontanon, V.; Perez-Latorre, L.; et al. Metabolomic changes after DAAs therapy are related to the improvement of cirrhosis and inflammation in HIV/HCV-coinfected patients. Biomed. Pharmacother. 2022, 147, 112623. [Google Scholar] [CrossRef] [PubMed]
- Weigand, K.; Peschel, G.; Grimm, J.; Muller, M.; Horing, M.; Krautbauer, S.; Liebisch, G.; Buechler, C. HCV Infection and Liver Cirrhosis Are Associated with a Less-Favorable Serum Cholesteryl Ester Profile Which Improves through the Successful Treatment of HCV. Biomedicines 2022, 10, 3152. [Google Scholar] [CrossRef]
- Broquetas, T.; Herruzo-Pino, P.; Marino, Z.; Naranjo, D.; Vergara, M.; Morillas, R.M.; Forns, X.; Carrion, J.A. Elastography is unable to exclude cirrhosis after sustained virological response in HCV-infected patients with advanced chronic liver disease. Liver Int. 2021, 41, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Giuffre, M.; Fouraki, S.; Campigotto, M.; Colombo, A.; Visintin, A.; Buonocore, M.R.; Aversano, A.; Budel, M.; Tine, F.; Abazia, C.; et al. Alanine aminotransferase and spleno-portal dynamics affect spleen stiffness measured by point shear-wave elastography in patients with chronic hepatitis C in the absence of significant liver fibrosis. J. Ultrasound 2020, 24, 67–73. [Google Scholar] [CrossRef]
- Peschel, G.; Grimm, J.; Buechler, C.; Gunckel, M.; Pollinger, K.; Aschenbrenner, E.; Kammerer, S.; Jung, E.M.; Haimerl, M.; Werner, J.; et al. Liver stiffness assessed by shear-wave elastography declines in parallel with immunoregulatory proteins in patients with chronic HCV infection during DAA therapy. Clin. Hemorheol. Microcirc. 2021, 79, 541–555. [Google Scholar] [CrossRef]
- Buechler, C.; Aslanidis, C. Role of lipids in pathophysiology, diagnosis and therapy of hepatocellular carcinoma. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158658. [Google Scholar] [CrossRef]
- Boursier, J.; Anty, R.; Carette, C.; Cariou, B.; Castera, L.; Caussy, C.; Fontaine, H.; Garioud, A.; Gourdy, P.; Guerci, B.; et al. Management of diabetes mellitus in patients with cirrhosis: An overview and joint statement. Diabetes Metab. 2021, 47, 101272. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Patel, K.; Sebastiani, G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020, 2, 100067. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, H.D. The Evolution of the MELD Score and Its Implications in Liver Transplant Allocation: A Beginner’s Guide for Trainees. ACG Case Rep. J. 2022, 9, e00763. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Andrade, V.G.; Yamashiro, F.D.S.; Oliveira, C.V.; Kurozawa, L.L.; Moreira, A.; Silva, G.F. Increase of Lipids during Hcv Treatment: Virus Action or Medication? Arq. Gastroenterol. 2018, 55, 184–187. [Google Scholar] [CrossRef]
- Keikha, M.; Eslami, M.; Yousefi, B.; Ali-Hassanzadeh, M.; Kamali, A.; Yousefi, M.; Karbalaei, M. HCV genotypes and their determinative role in hepatitis C treatment. Virusdisease 2020, 31, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, D.A.; Shawa, I.T.; Thomas, E.L.; Felmlee, D.J.; Bridge, S.H.; Neely, D.; Cobbold, J.F.; Holmes, E.; Bassendine, M.F.; Taylor-Robinson, S.D. Infection with the hepatitis C virus causes viral genotype-specific differences in cholesterol metabolism and hepatic steatosis. Sci. Rep. 2022, 12, 5562. [Google Scholar] [CrossRef] [PubMed]
- Peschel, G.; Grimm, J.; Muller, M.; Horing, M.; Krautbauer, S.; Weigand, K.; Liebisch, G.; Buechler, C. Sex-specific changes in triglyceride profiles in liver cirrhosis and hepatitis C virus infection. Lipids Health Dis. 2022, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Kumarage, T.; Morris, N.B.; Ashkar, R. The effects of molecular and nanoscopic additives on phospholipid membranes. Front. Phys. 2023, 11, 1251146. [Google Scholar] [CrossRef]
- Alem, S.A.; Abdellatif, Z.; Mabrouk, M.; Zayed, N.; Elsharkawy, A.; Khairy, M.; Musa, S.; Anwar, I.; Yosry, A. Diagnostic accuracy of acoustic radiation force impulse elastography (ARFI) in comparison to other non-invasive modalities in staging of liver fibrosis in chronic HCV patients: Single-center experience. Abdom. Radiol. 2019, 44, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Q.; Hu, C.; Zhang, Y.; Wang, Q.; Zhao, X.; Liu, X.; Wang, C.; Jia, W.; Xu, G. Serum lipidomics profiles reveal potential lipid markers for prediabetes and type 2 diabetes in patients from multiple communities. Front. Endocrinol. 2022, 13, 966823. [Google Scholar] [CrossRef] [PubMed]
- Deterding, K.; Honer Zu Siederdissen, C.; Port, K.; Solbach, P.; Sollik, L.; Kirschner, J.; Mix, C.; Cornberg, J.; Worzala, D.; Mix, H.; et al. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment. Pharmacol. Ther. 2015, 42, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Herzer, K.; Welzel, T.M.; Spengler, U.; Hinrichsen, H.; Klinker, H.; Berg, T.; Ferenci, P.; Peck-Radosavljevic, M.; Inderson, A.; Zhao, Y.; et al. Real-world experience with daclatasvir plus sofosbuvir +/- ribavirin for post-liver transplant HCV recurrence and severe liver disease. Transpl. Int. 2017, 30, 243–255. [Google Scholar] [CrossRef]
- Freeman, R.B., Jr.; Wiesner, R.H.; Roberts, J.P.; McDiarmid, S.; Dykstra, D.M.; Merion, R.M. Improving liver allocation: MELD and PELD. Am. J. Transplant. 2004, 4 (Suppl. S9), 114–131. [Google Scholar] [CrossRef]
- Joseph, J. Serum Marker Panels for Predicting Liver Fibrosis—An Update. Clin. Biochem. Rev. 2020, 41, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Su, C.W.; He, Y.J.; Lee, Y.H.; Huang, Y.H.; Hou, M.C.; Huo, T.I. Current noninvasive liver reserve models do not predict histological fibrosis severity in hepatocellular carcinoma. Sci. Rep. 2018, 8, 15074. [Google Scholar] [CrossRef]
- Savendahl, L.; Mar, M.H.; Underwood, L.E.; Zeisel, S.H. Prolonged fasting in humans results in diminished plasma choline concentrations but does not cause liver dysfunction. Am. J. Clin. Nutr. 1997, 66, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Sirikwanpong, S.; Dahlan, W.; Ngamukote, S.; Sangsuthum, S.; Adisakwattana, S.; Nopponpunth, V.; Himathongkam, T. The Alterations of Erythrocyte Phospholipids in Type 2 Diabetes Observed after Oral High-Fat Meal Loading: The FTIR Spectroscopic and Mass Spectrometric Studies. J. Clin. Biochem. Nutr. 2010, 47, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kiser, J.J.; Burton, J.R.; Anderson, P.L.; Everson, G.T. Review and management of drug interactions with boceprevir and telaprevir. Hepatology 2012, 55, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Wong, G.; Tan, R.; Giral, P.; Robillard, P.; Orsoni, A.; Hounslow, N.; Magliano, D.J.; Shaw, J.E.; Curran, J.E.; et al. Statin action favors normalization of the plasma lipidome in the atherogenic mixed dyslipidemia of MetS: Potential relevance to statin-associated dysglycemia. J. Lipid Res. 2015, 56, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Matsui, O.; Kobayashi, S.; Minami, T.; Kitao, A.; Gabata, T. Morphometric changes in liver cirrhosis: Aetiological differences correlated with progression. Br. J. Radiol. 2016, 89, 20150896. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef]
- Horing, M.; Ejsing, C.S.; Krautbauer, S.; Ertl, V.M.; Burkhardt, R.; Liebisch, G. Accurate quantification of lipid species affected by isobaric overlap in Fourier-Transform mass spectrometry. J. Lipid Res. 2021, 62, 100050. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Horing, M.; Ekroos, K.; Baker, P.R.S.; Connell, L.; Stadler, S.C.; Burkhardt, R.; Liebisch, G. Correction of Isobaric Overlap Resulting from Sodiated Ions in Lipidomics. Anal. Chem. 2020, 92, 10966–10970. [Google Scholar] [CrossRef]
- Husen, P.; Tarasov, K.; Katafiasz, M.; Sokol, E.; Vogt, J.; Baumgart, J.; Nitsch, R.; Ekroos, K.; Ejsing, C.S. Analysis of lipid experiments (ALEX): A software framework for analysis of high-resolution shotgun lipidomics data. PLoS ONE 2013, 8, e79736. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, R.A.; Pawlik, T.M. Staging and Prognostic Models for Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729235. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Chahar, D.S.; Lal, R.; Sharma, S. Review Article on Parametric and Nonparametric Test. World J. Pharm. Med. Res. 2024, 10, 72–75. [Google Scholar]



| Parameter | Baseline (178 Patients) | 12 Weeks of Therapy (176 Patients) | p-Value |
|---|---|---|---|
| Age years | 54 (24–82) | 54 (24–82) | ns |
| Female/male | 74/104 | 74/102 | ns |
| BMI kg/m2 | 25.6 (17.6–41.6) | 25.6 (17.6–41.6) | ns |
| MELD Score | 7 (6–21) | 7 (6–21) | ns |
| Ferritin ng/mL | 128.6 (5.6–2309) | 94.2 (2.9–1161) | 0.004 |
| ALT U/L | 61 (2–305) | 26 (6–388) | <0.001 |
| AST U/L | 47 (7–1230) | 22 (6–836) | <0.001 |
| Bilirubin mg/dL | 1.0 (1.0–4.3) | 1.0 (1.0–7.5) | ns |
| INR | 1.05 (1.00–2.44) | 1.04 (1.00–2.22) | ns |
| Creatinine mg/dL | 0.78 (0.14–14.00) | 0.76 (0.14–14.7) | ns |
| Thrombocytes n × 109/L | 195 (38–402) | 206 (37–407) | ns |
| Leukocytes n × 109/L | 6.5 (2.2–72.4) | 6.8 (2.4–62.9) | ns |
| C-reactive protein mg/L | 2.9 (1.0–55.0) | 2.9 (2.9–20.3) | ns |
| Albumin g/L | 38 (2–50) | 39 (16–93) | ns |
| HDL mg/dL | 52 (19–111) | 50 (13–96) | ns |
| LDL mg/dL | 95 (23–296) | 119 (33–251) | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigand, K.; Peschel, G.; Grimm, J.; Höring, M.; Krautbauer, S.; Liebisch, G.; Müller, M.; Buechler, C. Serum Phosphatidylcholine Species 32:0 as a Biomarker for Liver Cirrhosis Pre- and Post-Hepatitis C Virus Clearance. Int. J. Mol. Sci. 2024, 25, 8161. https://doi.org/10.3390/ijms25158161
Weigand K, Peschel G, Grimm J, Höring M, Krautbauer S, Liebisch G, Müller M, Buechler C. Serum Phosphatidylcholine Species 32:0 as a Biomarker for Liver Cirrhosis Pre- and Post-Hepatitis C Virus Clearance. International Journal of Molecular Sciences. 2024; 25(15):8161. https://doi.org/10.3390/ijms25158161
Chicago/Turabian StyleWeigand, Kilian, Georg Peschel, Jonathan Grimm, Marcus Höring, Sabrina Krautbauer, Gerhard Liebisch, Martina Müller, and Christa Buechler. 2024. "Serum Phosphatidylcholine Species 32:0 as a Biomarker for Liver Cirrhosis Pre- and Post-Hepatitis C Virus Clearance" International Journal of Molecular Sciences 25, no. 15: 8161. https://doi.org/10.3390/ijms25158161
APA StyleWeigand, K., Peschel, G., Grimm, J., Höring, M., Krautbauer, S., Liebisch, G., Müller, M., & Buechler, C. (2024). Serum Phosphatidylcholine Species 32:0 as a Biomarker for Liver Cirrhosis Pre- and Post-Hepatitis C Virus Clearance. International Journal of Molecular Sciences, 25(15), 8161. https://doi.org/10.3390/ijms25158161






