Abstract
Building blocks have been identified that can be functionalised by sequential nucleophilic aromatic substitution. Some examples are reported that involve the formation of cyclic benzodioxin and phenoxathiine derivatives from 4,5-difluoro-1,2-dinitrobenzene, racemic quinoxaline thioethers, and sulfones from 2,3-dichloroquinoxaline and (2-aminophenylethane)-2,5-dithiophenyl-4-nitrobenzene from 1-(2-aminophenylethane)-2-fluoro-4,5-dinitrobenzene. Four X-ray single-crystal structure determinations are reported, two of which show short intermolecular N–O…N “π hole” contacts.
1. Introduction
Halogens activated to nucleophilic aromatic substitution are scaffold building blocks for functional materials and heterocycles with interesting properties (Figure 1). Some examples are listed here: 2,4-difluoronitrobenzene, 1, reacts with one or two different amines which can give porous organic materials with a flexible framework; [1,2,3] and references cited herein. 4,5-Difluoro-1,2-dinitrobenzene, 2, reacts with 1,2-disubstituted amines, alcohols, and thiols, forming N-heteroacenes, phenoxazines, and phenothiazines; [4] and references cited herein. 2,3-Dichloroquinoxaline reacts with different nucleophiles to give novel pharmaceutical building blocks [5,6]. 2,3-Dichlorothiadiazole, 4, is representative of a general field of research known as sulphur–nitrogen (S–N) chemistry pioneered by Charles W Rees [7,8,9,10]. 1,3-Difluoro-4,6-dinitrobenzene, 5, a building block for Marfey’s reagent, is useful in peptide chemistry [11,12]. 1,2-Dichloro-4,5-dicyanobenzene, 6, is a building block for substituted phthalocyanines [13].
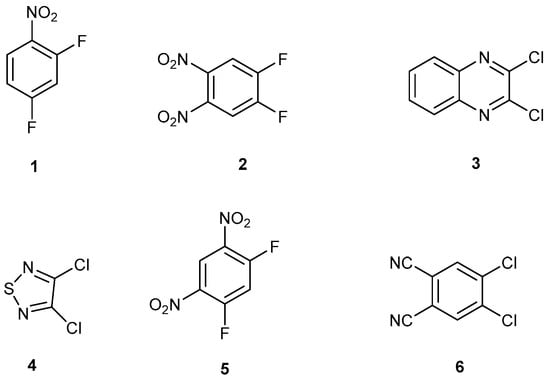
Figure 1.
Dihalogenated molecules that can undergo two sequential nucleophilic substitution reactions.
Figure 2 shows some products that can be made from compounds 5 or 6, respectively. Compound 7 is an easily made cyclophane [14], and macrocycle 8 is a precursor to an energetic substance [15]. Phthalonitrile 9 is a phthalocyanine precursor for optical limiting in polished polycarbonate discs [16].
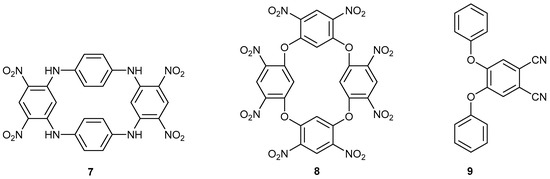
Figure 2.
Products 7 and 8, or product 9, that can be made from compounds 5 or 6, respectively.
2. Results and Discussion
New studies are being reported with building block 2 and catechol, 10, dithiocatechol, 11, and 2-hydroxythiophenol, 12, to make dioxin 13, dithiin 14, and phenoxathiin 15, respectively (Figure 3). In the supplementary section charts for proton and carbon NMR data for all new compounds are reported.
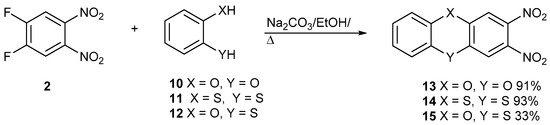
Figure 3.
The synthesis of dioxin 13, dithiin 14, and phenoxathiine 15.
The two nitro groups activate both halogens to sequential nucleophilic displacement by phenoxide and thiolate anions. The yields are good for these syntheses. The synthesis of compound 14 was reported by us previously, but it is included here for comparison with other data. Figure 4 shows the synthesis of racemic S-oxide, 16. Although the yield was good, the yield for the synthesis of the precursor heterocycle 15 was poor, which restricted the amount of material made. Further detailed studies were only carried out on compound 14.
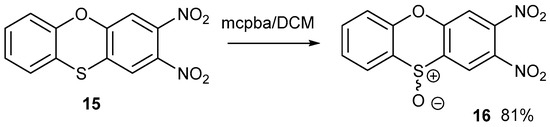
Figure 4.
The oxidation of phenoxathiin 15 to form a racemic S-oxide 16.
Compound 15 was oxidised with meta-chloroperbenzoic (mcpba) acid in DCM at room temperature to S-oxide 16 on a small scale. Owing to the racemic nature of this S-oxide, it was difficult to obtain good crystals, possibly due to the disorder of the S-oxide grouping. The oxidation of cyclic sulphide 15 to sulfoxide 16 stopped at the sulfoxide without over-oxidation to a sulfone.
X-ray single-crystal structure determinations were carried out on compounds 13–15.
Compound 13 crystallises in the triclinic space group P with one molecule in the asymmetric unit (Figure 5). The C1–C6 and C7–C12 rings are slightly puckered by 5.54 (6)°, which correlates with the fact that the dioxin ring is a very shallow boat with atoms CO1 and O2 displaced from the best plane of C4/C5/C7/C8 by –0.0722 (15) and –0.0917 (14) Å, respectively. The C5–O1–C8 and C4–O2–C7 bond angles are 115.48 (8) and 115.55 (8)°, respectively. Both nitro groups are twisted from their attached C1–C6 ring, by 54.43 (9)° for N1/O3/O4 and 27.21 (7)° for N2/O5/O6. In the extended structure of compound 13, the molecules are linked by weak C–H…O interactions, but there are no identified short contacts involving the nitro groups. In unsubstituted dibenzo-p-dioxin [17] C12H8O2, all the atoms lie on a crystallographic mirror plane, and the C–O–C bond angle is 116.4°.
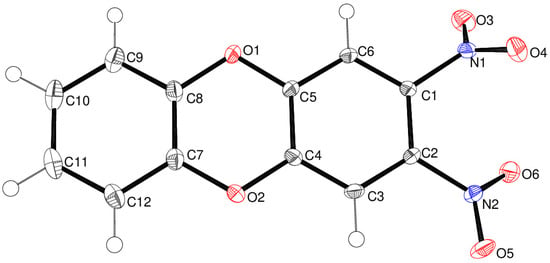
Figure 5.
The molecular structure of compound 13 showing 50% displacement ellipsoids.
Compound 14 displays monoclinic crystal symmetry (space group P21/n) with one molecule in the asymmetric unit (Figure 6). The outer C1–C6 and C7–C12 rings are substantially puckered by 50.89 (3)°, and the dithiin ring is well described as a boat with atoms S1 and S2 displaced from the best plane of C4/C5/C7/C8 (r.m.s. deviation = 0.001 Å) by 0.6979 (13) and 0.6723 (12) Å, respectively. The C5–S1–C7 and C4–S2–C8 bond angles are 99.84 (4) and 100.31 (5)°, respectively (mean = 100.1°); these angles are much smaller than the corresponding bond angles (via O atoms) in compound 13, which is consistent with the trend that bond angles decrease for more polarisable atoms in the same group of the periodic table [18]. In the structure [19] of unsubstituted thianthrene, C12H8S2, the dihedral angle between the aromatic rings is 50.5°, and the mean C–S–C bond angle is 100.2°. Both nitro groups in compound 14 are twisted from their attached C1–C6 ring, by 53.72 (8)° for N1/O1/O2 and 27.41 (8)° for N2/O3/O4; these dihedral angles are notably similar to the corresponding values for 13.
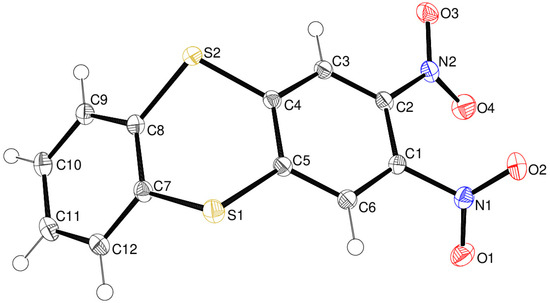
Figure 6.
The molecular structure of compound 14 showing 50% displacement ellipsoids.
In the extended structure of compound 14, the molecules are linked by weak C–H…O and C–H…S interactions as well as unusual short N–O…N “π hole” contacts [20] between symmetry-related N1-nitro groups (Figure 7) with O1…N1i = 2.8969 (13) Å and N1–O1…N1i = 145.90 (7)° (i = ½–x, y–½, ½–z), which generate [010] chains with adjacent molecules related by the 21 screw axis (the van der Waals separation of O and N is about 3.07 Å). The dihedral angle between the N1 and N1i nitro groups is 69.70 (7)°.
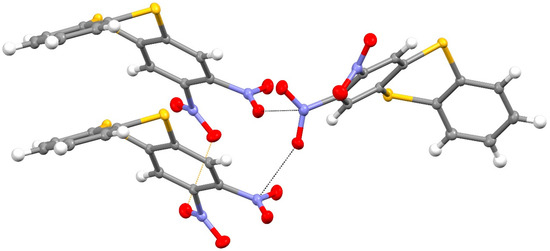
Figure 7.
Short N–O…N contacts (black dashed lines) in the extended structure of compound 14, which generate [010] chains. Yellow is sulfur, red is oxygen and blue is nitrogen.
Compound 15 crystallises with two molecules (A containing C1 and B containing C13) in the asymmetric unit (Figure 8) of space group P, both of which display disorder: in molecule A, ‘flip’ (~180° rotational) disorder about the long axis of the molecule of the O and S atoms of the oxathiine ring [occupancy of S1/O2 = 0.493 (5); occupancy of S2/O1 = 0.507 (5)] occurs as well as positional disorder of the C7–C12 ring. The B molecule also shows flip disorder [occupancy of S3/O8 = 0.180 (4); occupancy of S4/O7 = 0.820 (4)] as well as disorder of the oxygen atoms of the N3 and N4 nitro groups in 0.715 (4):0.285 (4) and 0.720 (6): 0.280 (6) ratios, respectively. The extensive disorder complicates the detailed interpretation of the ring conformations, but it may be stated that the molecules are close to planar, with dihedral angles between the C1–C6 and C7–C12 (major component) rings of 12.29 (12)° and C13–C18 and C19–C24 of 4.20 (8)°. In the extended structure of compound 15, the molecules are linked by weak C–H…O bonds, but there are no short contacts involving the nitro groups.
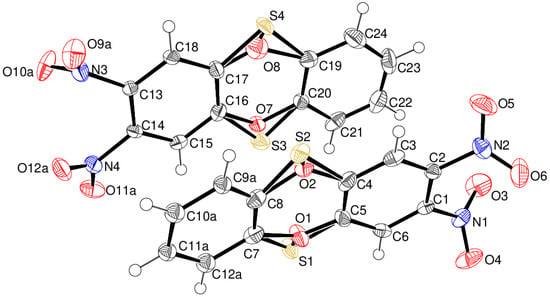
Figure 8.
The molecular structure of compound 15 showing 50% displacement ellipsoids. Both disorder components of the oxathiine rings are shown, but for clarity, only the major disorder components of the C7–C12 ring and N3 and N4 nitro groups are drawn.
The building blocks in Figure 1 are all commercially available, so more were investigated. Each chlorine atom of compound 3 is activated by a pyridine-type nitrogen atom. Compound 3 was reacted with a cheap optically pure (S) amine by a nucleophilic aromatic substitution reaction to give product 17 (Figure 9). This was reacted with a thiolate anion which is a strong nucleophile owing to its size and polarisability. Product 18 was treated with mcpba with a view to make the mono S-oxide (not drawn). Instead, the product was identified, with a clean mass spectrum, as sulfone 19 shown in Figure 9. Here, and in our previous studies, the oxidation of a cyclic sulphide stopped at the mono S-oxide, but here the acyclic sulphide smoothly converted to the sulfone. The oxidation of a cyclic sulfoxide is presumed to have a higher energy barrier. Previously, we discussed the enantiomeric fractionation of chiral sulfoxides from a silica column, which has been reported, but not for single enantiomers. The sulfoxide and sulfone have similar Rf values, which might complicate the fractionation. No X-ray single-crystal structures were obtained on compounds 17–19. The asymmetry possibly inhibits the growth of good crystals.
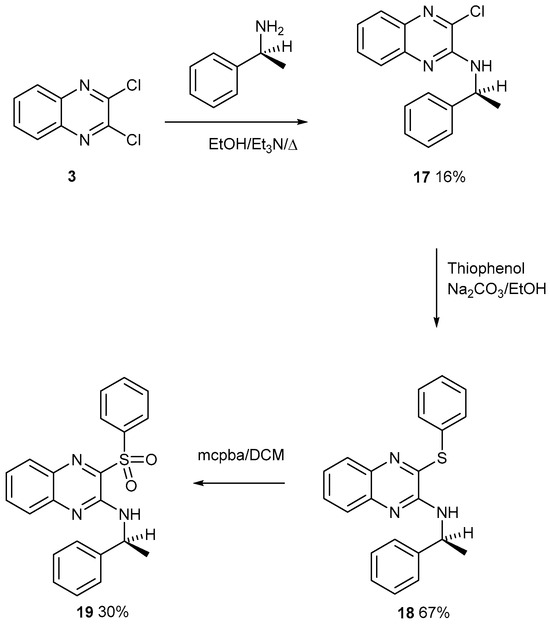
Figure 9.
The formation of sulfone 19 from acrylic sulphide 18 with mcpba.
Either one [10] or two chlorine atoms can be displaced from 2,3-dichloroquinoxaline, 3, with butylamine (Figure 10). Since the second Cl atom is harder to displace, and ideally requires a thiolate anion as a nucleophile, the reaction was carried out in a Parr PTFE-lined pressure vessel at a higher temperature of 150 °C. The product forms in low yield but was more polar and harder to purify. In our studies on potential porous organic materials, we found that polar compounds were harder to purify, so the polarity was reduced with butylamine substituents [1,2]. For example, if butylamine was replaced with methyl, ethyl, or propylamine, no products were isolated with smaller compounds.
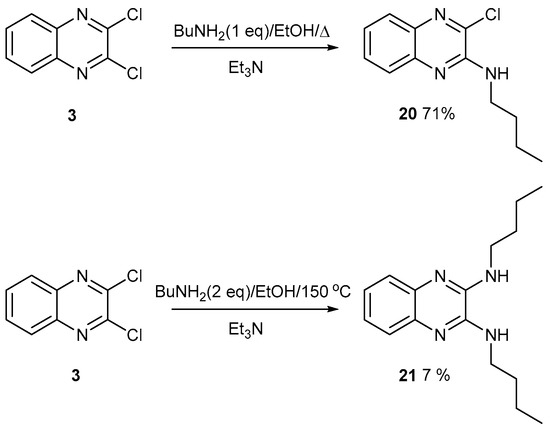
Figure 10.
Displacement of one or two chlorine atoms from 2,3-dichloroquinoxaline 3 with butylamine.
All four substituents on compound 2 are activated because each nitro group activates the other one as well as a para-fluorine atom (Figure 3) [21,22]. An X-ray single-crystal structure determination was carried out on compound 24.
Compound 24 crystallises in space group Pca21 with one molecule in the asymmetric unit (Figure 11). The morpholine ring adopts a normal chair conformation [displacements of N1 and O1 from C7 to C10 = –0.652 (4) and 0.653 (4) Å, respectively] with the exocyclic N1–C5 bond in an equatorial conformation, although the bond angle sum at N1 of 354° is suggestive of a tendency towards sp2 hybridisation. The dihedral angle between the rings (all atoms) is 24.33 (11)°. The N3/O4/O5 nitro group lies close to the plane of the C1–C6 ring [dihedral angle = 9.2 (4)°], whereas N2/O2/O3 is substantially twisted [82.57 (14)°]. This can be explained by conjugation of the N3 nitro group with atom N1 via the aromatic ring (i.e., a quinoid C=N+ resonance form); the C2–N3 bond length [1.441 (4) Å] is clearly shorter than C1–N2 [1.474 (4) Å], and the mean of C1–C6 and C3–C4 [1.368 Å] is shorter than the mean of C1–C2, C2–C3, C4–C5, and C5–C6 [1.401 Å].
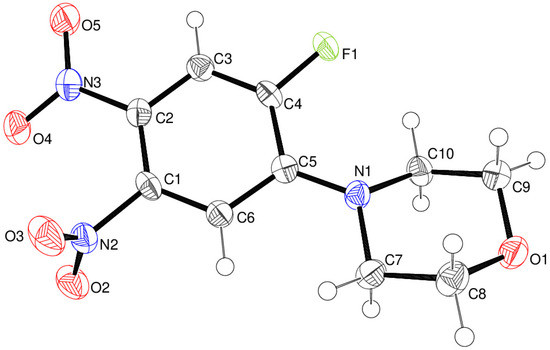
Figure 11.
The molecular structure of compound 24 showing 50% displacement ellipsoids.
The extended structure of 24 features weak C–H…O and C–H…F interactions as well as notably short N2–O2…N3ii (ii = x, 1+y, z) contacts (Figure 12), which lead to [010] chains with adjacent molecules related by translation: the O…N separation is 2.839 (3) Å, the N–O…N angle is 132.20 (19)°, and the dihedral angle between the N2 and N3ii nitro groups is 81.5 (3)°.
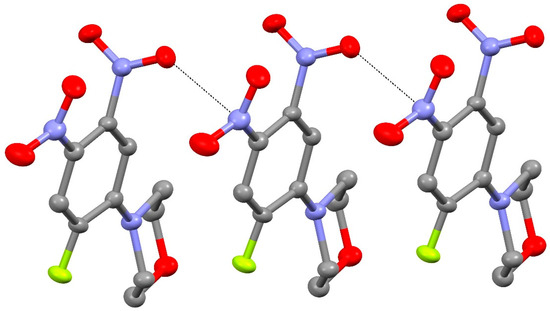
Figure 12.
Short O…N contacts (black dashed lines) in the extended structure of compound 24, which generate [010] chains.
Although these reactions are feasible because one fluorine atom is easily displaced (Figure 13), the next reaction posed considerable difficulty (Figure 14).
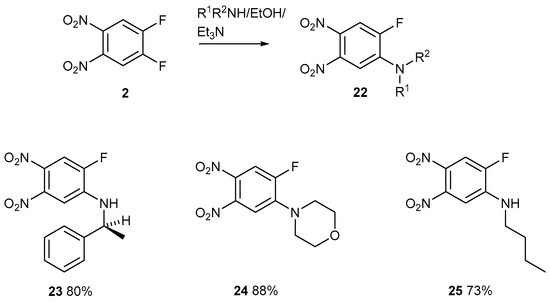
Figure 13.
The displacement of one activated fluorine atom from compound 2 with different amines.
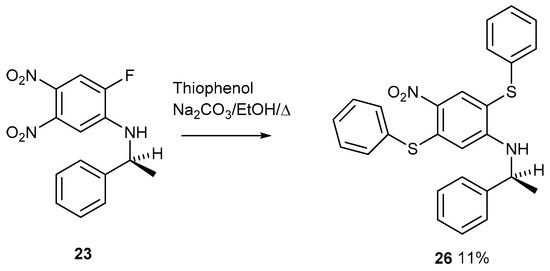
Figure 14.
The sequential displacement of an activated fluorine atom followed by an activated nitro group on compound 23.
A TLC plate run after the reaction work-up showed three spots running close together. The middle spot was eventually isolated as the pure product after running a multiple number of long (12″ × 1″) columns. The data fits for structure 26 are shown. The fluorine atom must be the first group to be displaced; otherwise, two thioethers could not be formed. The nitro group conjugated to the amine is deactivated by this conjugation. After the fluorine atom, the more reactive nitro group is displaced next. This reaction is interesting and represents a formal addition–substitution of diphenyldisulfide across the 1 and 4 positions of a benzene ring, which we believe to be a new reaction. The yield is only about 10%, but the product is pure with good data.
3. Material and Methods
IR spectra were recorded on a diamond-attenuated total reflection (ATR) Fourier transform infrared (FTIR) spectrometer, Nicolet Summit Everest, (Thermo Fischer Scientific, 1 Ashley Road, Altrincham, Cheshire, England); Ultraviolet (UV) spectra were recorded using a Perkin Elmer Lambda 25 UV–Vis spectrometer with EtOH as the solvent (LAS Chalfont, Seer Green, Beaconsfield, England); The term sh means shoulder. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded at 400 and 100.5 MHz, respectively, using a Bruker 400 spectrometer (Bruker UK Ltd., Welland House, Westwood Business Park, Coventry, England); Chemical shifts, δ, are given in ppm and measured by comparison with the residual solvent. Coupling constants, J, are given in Hz. High-resolution mass spectra were obtained at the University of Wales, Swansea, using an Atmospheric Solids Analysis Probe (ASAP) (positive mode) instrument: Xevo G2-S ASAP (Waters Ltd., Stamford Avenue, Altrincham Road, Wilmslow, England); Melting points were determined on a Cole-Palmer MP-200D Stuart digital melting point microscope (CamLab, Norman Way Ind. Estate, Over, Cambridge, England).
- 2,3-Dinitrodibenzo-[1,4]-dioxin 13
4,5-Difluoro-1,2-dinitrobenzene 2 (300 mg, 1.47 mmol) in EtOH (30 mL) was mixed with catechol (162 mg, 1.47 mmol) and Na2CO3 (2.0 g) and then stirred at 75 °C for 20 h. The mixture was added to water (200 mL) and allowed to stand for 1 h. This was filtered and air-dried for 2 days to give a bright yellow precipitate of the title compound (365 mg, 91%) as yellow crystals, 205–206 mp °C (from dichloromethane/light petroleum ether). λmax (EtOH)/nm 279 (log ε 3.6) and 369 sh (3.0); νmax (diamond) (cm–1) 3062 w, 1626 w, 1594 w, 1537 s, 1505 s, 1486 s, 1340 s, 1305 s, 1290 s, 1199 s, 890 s, 873 s, 821 s, 765 s, 750 s, and 450 w; δH (400 MHz; CDCl3), 7.05–7.10 (4H, m) and 7.89 (2H, s); δC (100.1 MHz; CDCl3) 114.0, 117, 126.3, 138.5, 140.2, and 145.4; m/z (Orbitrap ASAP) 275.0300 (M+ + H, 100%) C12H6N2O6H requires 275.0304.
- 2,3-Dinitrophenoxathiine 15
4,5-Difluoro-1,2-dinitrobenzene 2 (300 mg, 1.47 mmol) in EtOH (30 mL) was mixed with 2-hydroxythiophenol (185 mg, 1.47 mmol) and Na2CO3 (2.0 g) and then stirred at 75 °C for 20 h. After cooling, the mixture was added to water (200 mL), extracted with DCM (100 mL) twice, followed by drying of the combined extracts over MgSO4. The product was purified by chromatography on silica (twice). Elution with dichloromethane/light petroleum ether (40:60) gave the title compound (145 mg, 34%) as pure red crystals, 144–145 mp °C (from dichloromethane/light petroleum ether). Alternatively, after dilution of the reaction with water (200 mL) and treatment with 5 M HCl (20 mL), the product was filtered to give the title compound (140 mg, 33%). λmax (EtOH)/nm 233 (log ε 3.6), 284 (3.6) and 400 sh (3.0); νmax (diamond) (cm–1) 3100 w, 1528 s, 1468 s, 1443 s, 1383 w, 1337 s, 1291 s, 1273 s, 1237 s, 893 s, 841 s, 749 s, 718 s, 682 w, and 447 w; δH (400 MHz; CDCl3) 7.10 (1H, d, J = 8.0), 7.18 (1H, t, J = 8.0), 7.27–7.33 (2H, m), 7.87 (1H, s) and 8.27 (1H, s); δC (100.1 MHz; CDCl3) 114.8, 116.1, 118.4, 124.6, 127.0, 127.5, 127.6, 129.9, 138.5, 142.0, 149.4, and 154.2; m/z (Orbitrap ASAP) 291.0077 (M+ + H, 100%) C12H6N2O5SH requires 291.0076.
- 2,3-Dinitrophenoxathiin-S-oxide 16
2,3-Dinitrophenoxathiin 15 (47 mg, 0.16 mmol) in DCM (30 mL) was treated with meta-chloroperbenzoic acid (mcpba) (47 mg, 0.32 mmol) for 24 h at rt. The DCM layer was diluted with more DCM (70 mL), extracted with dilute KOH) (4 pellets of KOH dissolved in 200 mL of H2O), dried over MgSO4, and then concentrated and purified by chromatography on silica. DCM eluted the title compound (40 mg, 81%) as a white powder, mp 191–192 °C (from dichloromethane/light petroleum ether). λmax (EtOH)/nm 221 (log ε 4.2) and 299 (3.8); νmax (diamond) (cm–1) 1013 w, 1591 w, 1533 s, 1463 s, 1443 s, 1395 s, 1275 s, 1232 s, 1051 s, 840 s, 762 s, and 540 s; δH (400 MHz; CDCl3) 7.59 (1H, t, J = 8.0), 7.65 (1H, J = 8.0), 7.84 (1H, J = 8.0) and 8.14 (1H, J = 8.0), 8.50 (1H, s), and 9.18 (1H, s); δC (100.1 MHz; CDCl3) 117.1, 119.2, 123.6, 127.2, 128.0, 130.4, 131.6, 135.5, 136.9, 145.8, 147.9, and 152.5; m/z (Orbitrap ASAP) 307.0021 (M+ + H, 100%) C12H6N2O6S2H requires 307.0025.
- 2-Aminophenylethane-3-chloroquinoxaline 17
2,3-Dichloroquinoxaline 3 (300 mg, 1.51 mmol), (S)-phenylethylamine (364 mg, 3.0 mmol), and Et3N (152 mg, 1.5 mmol) in EtOH (30 mL) were heated under reflux for 48 h. After cooling, the mixture was diluted with water (200 mL) and left to stand for 1 h. The white precipitate was filtered off through a sinter and the product was collected as one fibrous mat. This was purified by chromatography on flash silica. DCM eluted the title compound (67 mg, 16%) as a clear oil (from dichloromethane/light petroleum ether). λmax (EtOH)/nm 237 (log ε 4.0) and 358 (3.8); νmax (diamond) (cm–1) 3428 w, 1571 s, 1555 s, 1508 s, 1490 s, 1451 s, 1405 s, 1268 s, 1242 s, 1215 s, 1125 w, 1054 vs, 1015 w, 937 w, 829 w, 754 vs, 695 vs and 595 s; δH (400 MHz; CDCl3) 1.70 (3H, d, J = 14.0), 5.48 (1H, q, J = 8.0), 5.83 (1H, d, J = 8.0), 7.30 (1H, dd, J = 8.0 and 8.0), 7.37–7.42 (3H, m), 7.49 (2H, d, J = 8.0), 7.59 (1H, t, J = 8.0), 7.72 (1H, d, J = 8.0) and 7.80 (1H, d, J = 8.0); δC (100.1 MHz; CDCl3) 22.1, 50.5, 125.1, 126.2, 126.4, 127.4, 127.9, 128.7, 130.0, 136.5, 137.7, 141.3, 143.5 and 147.1; m/z (Orbitrap ASAP) 284.0955 (M+ + H, 100%) C16H14N3ClH requires 284.0955.
- 2-Aminophenylethane-3-thiophenylquinoxaline 18
2-Aminophenylethane-3-chloroquinoxaline 17 (40 mg, 0.14 mmol) and thiophenol (64 mg, 0.58 mmol) in EtOH (30 mL) with Na2CO3 (0.5 g) was heated under reflux with stirring for 18 h. Upon cooling, it was diluted in water (200 mL), extracted with DCM (100 mL), dried over MgSO4, and filtered. The product was purified by chromatography on flash silica. The column was made up with light petroleum ether 40–60. DCM eluted front by-products and then the title compound (34 mg, 67%) as a colourless oil (from dichloromethane/light petroleum ether). λmax (EtOH)/nm 226 (log ε 3.9) and 362 (3.7); νmax (diamond) (cm–1) 3407 w, 1542 s, 1505 s, 1439 s, 1405 w, 1266 w, 1240 w, 1214 w, 1124 w, 1054 s, 1022 w, 756 s, 742 s, 696 s, 686 s, 608 w, 596 s and 537 w; δH (400 MHz; CDCl3) 1.60 (3H, d, J = 8.0), 5.47 (1H, q, J = 8.0), 5.68 (1H, d, J = 8.0), 7.25–7.29 (1H, m), 7.31–7.36 (5H, m), 7.40–7.41(3H, m), 7.50–7.54 (3H, m), 7.69 (1H, d, J = 8.0) and 7.75 (1H, d, J = 8.0); δC (100.1 MHz; CDCl3) 22.3, 50.4, 124.5, 126.1, 126.3, 127.2, 128.3, 128.6, 129.5, 130.1, 132.7, 137.4, 141.1, 143.9, 144.2 and 148.8 (two peaks are overlapping; peak at 129.5 is asymmetric); m/z (Orbitrap ASAP) 358.1374 (M+ + H, 100%) C22H19N3SH requires 358.1378.
- 2-Aminophenylethane-3-thiophenylquinoxaline sulfone 19
2-Aminophenylethane-3-thiophenylquinoxaline 18 (35 mg, 0.098 mmol) in DCM (30 mL) was treated with mcpba (34 mg, 0.197 mmol) and stirred for 18 h at rt. The DCM layer was extracted with water (100 mL) and made basic with 5 pellets of KOH. It was then extracted with water (100 mL), dried with MgSO4, and filtered. The product was purified by chromatography on a silica column 12 inches in length and a 1-inch-wide outer diameter. DCM eluted the title compound (11 mg, 30%) as a yellow solid, mp 111–113 °C (from dichloromethane/light petroleum ether). λmax (EtOH)/nm 254 (log ε 4.0), 305 (3.1) and 400 (3.1); νmax (diamond) (cm–1) 3394 w, 3378 w, 1542 s, 1523 s, 1444 s, 1360 s, 1315 s, 1301 s, 11,250 s, 1137 s, 1087 s, 1056 s, 752 s, 732 s, 714 s, 700 s, 684 s, 631 s, 620 s, 565 s, 549 s, 475 s and 429 s; δH (400 MHz; CDCl3) 1.71 (3H, d, J = 8.0), 5.49 (1H, q, J = 8.0), 7.28 (1H, t, J = 8.0 and 8.0), 7.37 (3H, t, J = 8.0 and 8.0), 7.46 (2H, d, J = 8.0), 7.57 (2H, t, J = 8.0 and 8.0), 7.61–7.63 (2H, m), 7.67 (2H, t, J = 8.0 and 8.0), 7.82 (1H, d, J = 8.0), 8.10 (2H, d, J = 8.0); δC (100.1 MHz; CDCl3) 22.5, 50.2, 125.4, 126.2, 126.3, 127.2, 128.7, 128.8, 129.2, 129.8, 132.9, 134.3, 135.5, 138.6, 141.5, 143.6, 143.8 and 147.5; m/z (Orbitrap ASAP) 390.1279 (M+ + H, 100%) C22H19N3SO2H requires 390.1276.
- 2-Butylamino-3-chloroquinoxaline 20
2,3-Dichloroquinoxaline 3 (200 mg, 1.0 mmol) in EtOH (30 mL) was treated with butylamine (147 mg, 2.0 mmol) and triethylamine (203 mg, 2.0 mmol). The mixture was heated at 75 °C for 18 h. After cooling, the mixture was diluted with water (200 mL), extracted with DCM (100 mL), back-extracted with water (100 mL), dried with MgSO4, and evaporated to dryness. The product was purified by flash chromatography on silica. Elution with DCM gave the title compound (167 mg, 71%) as colourless crystals, mp 44–45 °C (from dichloromethane/light petroleum ether). λmax (EtOH)/nm 347 (log ε 3.5); νmax (diamond) (cm–1) 3433 s, 2962 s, 2926 s, 2858 s, 1575 s, 1555 s, 1510 s, 1460 s, 1405 s, 1291 s, 1085 s, 1048 s, 829 s, 765 s, 756 s and 587 s; δH (400 MHz; CDCl3) 0.90 (3H, t, J = 8.0), 1.38 (2H, s, J = 8.0), 1.61 (2H, q, J = 8.0), 3.50 (2H, q, J = 8.0), 5.44 (1H, s, br), 7.28 (1H, t, J = 8.0 and 8.0), 7.47(1H, t, J = 8.0 and 8.0), 7.62 (1H, d, J = 8.0) and 7.70 (1H, d, J = 8.0); δC (100.1 MHz; CDCl3) 13.7, 20.1, 31.0, 41.1, 124.7, 125.8, 127.9, 129.9, 136.3, 138.0, 141.2 and 148.3; m/z (Orbitrap ASAP) 236.0956 (M+ + H, 100%) C12H14N3ClH requires 236.0954.
- 2,3-bis(Butylamino)quinoxaline 21
2,3-Dichloroquinoxaline 3 (200 mg, 1.0 mmol) in EtOH (10 mL) was treated with butylamine (147 mg, 2.0 mmol) and triethylamine (203 mg, 2.0 mmol) in a 23 mL PTFE-lined Parr Pressure Vessel. The vessel was heated at 150 °C for 18 h and then cooled. The mixture was diluted with water (200 mL), extracted with DCM (100 mL), back-extracted with water (100 mL), dried with MgSO4, and evaporated to dryness. The product was purified by flash chromatography on silica. Elution with Et2O/DCM (10:90) gave the title compound (18 mg, 7%) as colourless crystals, mp 111–112 °C (from dichloromethane/light petroleum ether). λmax (EtOH)/nm 350 (log ε 3.8); νmax (diamond) (cm–1) 3337 s, 2957 s, 2928 s, 2860 s, 1595 s, 1555 s, 1502 s, 1455 s, 1322 s, 1203 s, 1143 s, 747 vs, 604 s, 462 s; δH (400 MHz; CDCl3) 0.97 (6H, t, J = 8.0), 1.47 (4H, s, J = 8.0), 1.69 (4H, q, J = 8.0), 3.58 (4H, t, J = 8.0), 4.62 (2H, s br), 7.32 (2H, dd, J = 4.0 and 4.0) and 7.67 (2H, dd, J = 4.0); δC (100.1 MHz; CDCl3) 13.6, 20.3, 31.3, 41.6, 124.5, 125.5, 136.7 and 144.3; m/z (Orbitrap ASAP) 273.2078 (M+ + H, 100%) C16H24N4H requires 273.2079.
- 1-(2-Aminophenylethane)-2-fluoro-4,5-dinitrobenzene 23
4,5-Difluoro-1,2-dinitrobenzene 2 (300 mg, 1.47 mmol) and 2-aminophenylethane (178 mg, 1.47 mmol) in EtOH (30 mL) were treated with Et3N (149 mg, 1.47 mmol) and refluxed for 24 h. Upon cooling, the mixture was diluted with water (200 mL), extracted with DCM (100 mL), separated, and dried over MgSO4. After filtration and evaporation, the mixture was purified by chromatography on silica. DCM eluted the title compound (360 mg, 80%) as a yellow solid, mp 143–144 °C (from dichloromethane/light petroleum ether). λmax (EtOH)/nm 228 (log ε 3.5) and 378 (3.4); νmax (diamond) (cm–1) 3373 w, 1617 s, 1541 s, 1508 s, 1475 s, 1450 s, 1386 s, 1314 s, 1292 s, 1247 s, 1209 s, 1184 s, 1119 s, 1040 s, 882 s, 845 s, 820 s, 801 s, 746 s, 697 s, 657 s, 615 s, 592 s, 533 s and 452 s; δH (400 MHz; CDCl3) 1.67 (3H, d, J = 8.0), 4.63 (1H, q, J = 8.0), 5.26 (1H, s), 6.63 (1H, d, J = 4.0), 7.30–7.43 (5H, m) and 7.79 (1H, d, J = 8.0); δC (100.1 MHz; CDCl3) 24.3, 53.4, 106.5, 112.2 (1C, d, J = 20), 125.5, 128.2, 129.4, 141.0 (1C, d, J = 5), 141.4, 142.9 and 148.8 (1CF, d, J = 220); m/z (Orbitrap ASAP) 306.0890 (M+ + H, 100%) C14H12N3O4FH requires 306.0890.
- 1-Fluoro-2-morpholino-4,5-dinitrobenzene 24
4,5-Difluoro-1,2-dinitrobenzene 2 (500 mg, 2.45 mmol), morpholine (213 mg, 2.45 mmol), and Et3N (248 mg, 2.45 mmol) in EtOH (30 mL) were refluxed for 18 h. After cooling, the reaction was diluted with water (200 mL), left standing for 5 min, and filtered, which worked well. The filtrate was yellow. The product was a single pure spot by TLC. It was air-dried to give the title compound (587 mg, 88%) as a yellow solid, mp 167–168 °C (from dichloromethane/light petroleum ether). λmax (EtOH)/nm 380 (log ε 3.2); νmax (diamond) (cm–1) 1609 s, 1526 s, 1504 s, 1368 s, 1320 s, 1304 s, 1250 s, 1206 s, 1111 s, 1066 w, 1011 s, 922 s, 870 s, 841 s, 804 s, 750 s, 719 s, 658 s, 631 s, 596 s, 559 s and 424 w; δH (400 MHz; CDCl3) 3.38 (4H, t, J = 5.0), 3.90 (4H, t, J = 5.0), 7.16 (1H, d, J = 4.0) and 7.77 (1H, d, J = 8.0); δC (151.0 MHz; CDCl3) 49.7, 66.3, 112.9, 114.5 (1C, d, J C-F = 24.0), 132.9, 141.6, 144.2 (1C, d, J-C = 24.0) and 153.0 (1C, d, J C-F = 172.0) ; m/z (Orbitrap ASAP) 272.0679 (M+ + H, 100%) C10H10N3O5FH requires 272.0683.
- 1-Fluoro-2-butylamino-4,5-dinitrobenzene 25
4,5-Difluoro-1,2-dinitrobenzene 2 (500 mg, 2.45 mmol), butylamine (179 mg, 2.45 mmol), and Et3N (248 mg, 2.45 mmol) in EtOH (30 mL) were refluxed for 18 h. After cooling, the reaction was diluted with water (200 mL), treated with cHCl (2 mL), left standing for 2 h, and filtered, which worked well until the end. It was air-dried to give the title compound (458 mg, 73%) as a yellow solid, mp 95–96 °C (from dichloromethane/light petroleum ether). λmax (EtOH)/nm 382 (log ε 3.4); νmax (diamond) (cm–1) 3341 s, 1617 s, 1542 s, 1504 s, 1435 w, 1392 w, 1369 w, 1297 s, 1190 s, 1077 s, 882 s, 806 s, 7703 s, 655 s and 587 s; δH (400 MHz; CDCl3) 1.01 (3H, d, J = 7.0), 1.47 (2H, sextet, J = 7.0), 1.69 (2H, quintet, J = 7.0), 3.3 (2H, m), 4.9 (1H, s, br NH), 6.81 (1H, d, J-C = 12.0) and 7.76 (1H, d, J-C = 12.0); δC (100.1 MHz; CDCl3) 13.6, 20.1, 30.7, 42.8, 105.1 (1C, dJ-C = 4.0), 112.2 (1C, dJ-C = 23), 128.0 (1C, dJ-C = 10.0), 142.4 (1C, dJ-C = 10.0), 143.5, 148.7 (1C, dJ-C = 240.0); m/z 258.0889 (Orbitrap ASAP) (M+ + H, 100%) C10H12N3O4FH requires 258.0890.
- 1-(2-Aminophenylethane)-2,5-dithiophenyl-4-nitrobenzene 26
1-(2-Aminophenylethane)-2-fluoro-4,5-dinitrobenzene 23 (100 mg, 0.327 mmol), thiophenol (36 mg, 0.327 mmol), and Na2CO3 (500 mg) in EtOH (30 mL) were heated under reflux with stirring for 24 h. After cooling, the reaction was diluted with water (200 mL), extracted with DCM (100 mL), dried over MgSO4, and filtered. After evaporation, the mixture was purified by chromatography on silica. DCM eluted the title compound (16 mg, 11%) as a yellow solid, mp 59–60 °C (from dichloromethane/light petroleum ether). Multiple numbers of columns were required to obtain pure product owing to close running spots thought to be diphenyldisulfide and starting material. λmax (EtOH)/nm 240 (log ε 4.0), 297 (3.95) and 373 (3.85); νmax (diamond) (cm–1) 1575 s, 1544 s, 1509 s, 1473 s, 1438 s, 1294 vs, 1247 vs, 1118 s, 1073 s, 1021 s, 999 s, 967 s, 907 s, 831 s, 737 vs, 687 vs, 551 s and 429 s; δH (400 MHz; CDCl3) 1.27 (3H, d, J = 8.0), 3.92 (1H, q, J = 8.0), 6.51 (2H, d, J = 8.0), 7.12 (2H, t, J = 8.0 and 8.0), 7.17–7.21 (3H, m), 7.26 (1H, d, J = 8.0), 7.29–7.30 (4H, m), 7.42–7.43 (3H, m), 7.52–7.55 (1H, m) and 8.57 (1H, s); δC (100.1 MHz; CDCl3) 24.3, 52.8, 107.9, 112.0, 125.3, 126.7, 127.2, 127.6, 128.7, 129.3, 129.8, 130.1, 130.6, 134.1, 134.7, 135.6, 136.2, 142.3, 145.2 and 150.6; m/z (Orbitrap ASAP) 459.1199 (M+ + H, 100%) C26H22N2O2S2H requires 459.1201; 369.1071 (M+ + H, 100%) C20H17N2O2SF requires 369.1062
Crystal Structure Determinations
The crystal structures of 13, 14, 15, and 24 (all recrystallised from the mixed solvents of dichloromethane/light petroleum ether) were established using intensity data collected at 100 K on a Rigaku CCD diffractometer using either Mo Kα radiation (13, 14, and 15) or Cu Kα radiation (24). The structures were routinely solved by dual-space methods using SHELXT [23], and the structural models were completed and optimised by refinement against |F|2 with SHELXL-2019 [24]. Extensive disorder was found for 15. The H atoms were placed in idealised locations (C–H = 0.95–0.98 Å) and refined as riding atoms with Uiso(H) = 1.2Ueq(carrier). Full details of the structures and refinements are available in the deposited cifs.
- Crystal data for compound 13 C12H6N2O6, yellow plate, 0.19 × 0.09 × 0.03 mm, Mr = 274.19, triclinic, space group P (No. 2), a = 7.8050 (2) Å, b = 8.1532 (3) Å, c = 8.9461 (2) Å, α = 97.688 (2)°, β = 99.428 (2)°, γ = 94.297 (2)°, V = 553.82 (3) Å3, Z = 2, Mo Kα radiation (λ = 0.71073 Å),T = 100 K, μ = 0.136 mm–1, ρcalc = 1.644 g cm–3, 26,948 reflections measured (4.7 ≤ 2θ ≤ 61.0°), 2919 unique (RInt = 0.040), R(F) = 0.039 [2919 reflections with I > 2σ(I)], wR(F2) = 0.113 (all data), Δρmin,max (e Å–3) = –0.29, +0.47, CCDC deposition number 2362025.
- Crystal data for compound 14 C12H6N2O4S2, yellow plate, 0.12 × 0.07 ×0.01 mm, Mr = 306.31, monoclinic, space group P21/n (No. 14), a = 16.4666 (3) Å, b = 4.17460 (10) Å, c = 17.5585 (4) Å, β = 93.833 (2)°, V = 1204.30 (5) Å3, Z = 4, Mo Kα radiation (λ = 0.71073 Å), T = 100 K, μ = 0.457 mm–1, ρcalc = 1.689 g cm–3, 52,290 reflections measured (4.7 ≤ 2θ ≤ 76.2°), 6238 unique (RInt = 0.035), R(F) = 0.037 [4630 reflections with I > 2σ(I)], wR(F2) = 0.098 (all data), Δρmin,max (e Å–3) = –0.24, +0.61, CCDC deposition number 2362026.
- Crystal data for compound 15 C12H6N2O5S, orange rod, 0.44 × 0.15 × 0.07 mm, Mr = 290.25, triclinic, space group P (No. 2), a = 7.89759 (13) Å, b = 11.40431 (17) Å, c = 14.0329 (2) Å, α = 70.1769 (13)°, β = 87.4953 (12)°, γ = 79.3130 (13)°, V = 1168.13 (3) Å3, Z = 4, Mo Kα radiation (λ = 0.71073 Å), T = 100 K, μ = 0.300 mm–1, ρcalc = 1.650 g cm–3, 113,652 reflections measured (3.1 ≤ 2θ ≤ 61.0°), 7132 unique (RInt = 0.056), R(F) = 0.078 [6713 reflections with I > 2σ(I)], wR(F2) = 0.166 (all data), Δρmin,max (e Å–3) = –0.74, +0.89, CCDC deposition number 2362027.
- Crystal data for compound 24 C10H10FN3O5, yellow slab, 0.05 × 0.04 × 0.02 mm, Mr = 271.21, orthorhombic, space group Pca21 (No. 29), a = 18.1738 (6) Å, b = 4.60825 (14) Å, c = 13.3823 (5) Å, V = 1120.76 (7) Å3, Z = 4, Cu Kα radiation (λ = 1.54184 Å), T = 100 K, μ = 1.229 mm–1, ρcalc = 1.607 g cm–3, 8299 reflections measured (9.7 ≤ 2θ ≤ 140.2°), 1970 unique (RInt = 0.038), R(F) = 0.031 [1804 reflections with I > 2σ(I)], wR(F2) = 0.078 (all data), flack absolute structure parameter 0.08 (11), Δρmin,max (e Å–3) = –0.17, +0.17, CCDC deposition number 2362028.
4. Conclusions
Further chemistry is developed for substrates with two halogens activated for nucleophilic displacement by strong electron withdrawing groups. The use of compound 2 gave dinitrated heterocyclic benzodioxin 13, dithiin 14, and phenoxathiine 15. All three heterocycles gave satisfactory X-ray single-crystal structure data, but phenoxathiine 15 was disordered. The cyclic thioether of compound 15 was readily oxidised with mcpba to racemic sulfoxide 16 without over-oxidation to the sulfone occurring. The chemistry of this intermediate was not developed [21] any further because of the low yield for the formation of heterocycle 15. The yield was lower than for the two symmetrical systems 13 and 14. Presumably, there is a molecular strain in the heterocycle because it is not planar but rather folded with a butterfly shape. As expected, one or two fluorine atoms are easily displaced with compound 2 [3] New aromatic derivatives 23, 24, and 25 are reported here. This leaves a further activated fluorine atom and two nitro groups, which both activate each other. A thiol was chosen to react with compound 23 because it is polarisable owing to its large size, making it a good nucleophile. The F atom was displaced, followed by the most reactive nitro group. This gave an interesting 1,4-bis(thiophenyl)benzene derivative 26, which might be a new reaction not requiring metallic catalysis such as Pd, Pd(II), or Cu(II).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25158162/s1.
Author Contributions
Conceptualisation, M.J.P.; methodology, M.J.P.; software, W.T.A.H.; validation, M.J.P.; formal analysis, M.J.P. and W.T.A.H.; investigation, M.J.P.; resources, M.J.P.; data curation, W.T.A.H.; writing-review and editing, M.J.P. and W.T.A.H.; writing draft preparation, M.J.P.; supervision, M.J.P.; visualisation, M.J.P.; project administration, M.J.P.; funding acquisition, M.J.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Aberdeen University Library.
Acknowledgments
We thank the UK EPSRC National Mass Spectrometry Service Centre for mass spectrometric data and the UK National Crystallography Centre (University of Southampton) for the X-ray data collections. Datasets were obtained free of charge from the National Crystallography Centre, Southampton University.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Plater, M.J.; Harrison, W.T.A. An Organic Zeolite with 10 Å Diameter Pores Assembles From a Soluble and Flexible Building Block by Non-Covalent Interactions. ChemistryOpen 2019, 8, 457–463. [Google Scholar] [CrossRef]
- Plater, M.J.; Esslemont, A.J.; Harrison, W.T.A. Porous and Close Packed Supramolecular Assemblies from 2,4-Difluoronitrobenzene with Three Different Linkers and an n-Butylamine Cap. Int. J. Mol. Sci. 2023, 24, 14683. [Google Scholar] [CrossRef]
- Plater, M.J.; Harrison, W.T.A. Reactions of 4,5-difluoro-1,2-dinitrobenzene with amines in DMF or EtOH. J. Chem. Res. 2023, 47, 1–8. [Google Scholar] [CrossRef]
- Plater, M.J.; Harrison, W.T.A. New funtionalised phenoxazines and phenothiazines. ACS Omega 2023, 8, 44163–44171. [Google Scholar] [CrossRef]
- Li, Y.; Lou, Z.; Li, H.; Yang, H.; Zhao, Y.; Fu, H. Bioorthogonal Ligation and Cleavage by Reactions of Chloroquinoxalines with ortho-Dithiophenols. Angew. Chem. Int. Ed. 2020, 59, 3671–3677. [Google Scholar] [CrossRef]
- Holzhauer, L.; Liagre, C.; Fuhr, O.; Jung, N.; Bräse, S. Scope of tetrazolo [1,5-a]quinoxalines in CuAAC reactions for the synthesis of triazoloquinoxalines, imidazoloquinoxalines, and rhenium complexes thereof. Beilstein J. Org. Chem. 2022, 18, 1088–1099. [Google Scholar] [CrossRef]
- Weinstock, L.M.; Davis, P.; Handelsman, B.; Tull, R.J. General synthetic system for 1,2,5-thiadiazoles. J. Org. Chem. 1967, 32, 2823–2829. [Google Scholar] [CrossRef]
- Plater, M.J.; Rees, C.W.; Slawin, A.M.Z.; Williams, D.J. Aminotrithiadiazepines. J. Chem. Soc. Chem. Commun. 1990, 1315–1317. [Google Scholar] [CrossRef]
- Plater, M.J.; Rees, C.W. Trithiadiazepyne. J. Chem. Soc. Chem. Commun. 1990, 1317–1319. [Google Scholar] [CrossRef]
- Cava, M.P.; Lakshmikantham, M.V.; Hoffmann, R.; Williams, R.M.R.B. Woodward’s unfinished symphony: Designing organic superconductors (1975–79). Tetrahedron 2011, 67, 6771–6797. [Google Scholar] [CrossRef]
- Bhushan, R.; Bruckner, H. Marfey’s reagent for chiral amino acid analysis: A review. Amino Acids 2004, 27, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.-I.; Shimizu, Y.; Fujii, K. A chiral anisotropic reagent for determination of the absolute configuration of a primary amino compound. Tetrahedron Lett. 1998, 39, 6245–6248. [Google Scholar] [CrossRef]
- Wohrle, D.; Eskes, M.; Shigehara, K.; Yamada, A. A Simple Synthesis of 4,5-Disubstituted 1,2-Dicyanobenzenes and 2,3,9,10,16,17,23,24-Octasubstituted Phthalocyanines. Synthesis 1993, 194–196. [Google Scholar] [CrossRef]
- Touil, M.; Raimundo, J.M.; Lachkar, M.; Marsal, P.; Siri, O. Unprecedented N(H)-bridged tetraaza [1.1.1.1]m,p,m,p-cyclophanes. Tetrahedron 2010, 66, 4377–4382. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, H.; Yang, H.; Cheng, G. Synthesis and Detonation Properties of 5-Amino-2,4,6-trinitro-1,3-dihydroxy-benzene. ChemistryOpen 2017, 6, 447–45121. [Google Scholar] [CrossRef]
- Plater, M.J.; Jeremiah, A.; Bourhill, G. Synthesis of soluble halogenated aryloxy substituted indium phthalocyanines. J. Chem. Soc. Perkin. Trans. 1 2002, 91–96. [Google Scholar]
- Singh, P.; McKinney, J.D. Dibenzo-p-dioxin: A refinement. Acta Cryst. 1978, B34, 2956–2957. [Google Scholar] [CrossRef]
- Linker, G.-J.; van Duijnen, P.T.; Broer, R. Understanding trends in molecular bond angles. J. Phys. Chem. A 2020, 124, 1306–1311. [Google Scholar] [CrossRef]
- Larson, S.B.; Simonsen, S.H.; Martin, G.E.; Smith, K.; Puig-Torres, S. Structures of Thianthrene (I), C12H6S2, (Redeterminations at 163 K and 295 K) and l-Azathianthrene (II), C11H7NS2, (at 163 K). Acta Cryst. 1984, C40, 103–106. [Google Scholar]
- Bauzá, A.; Sharko, A.V.; Senchyk, G.A.; Rusanov, E.B.; Frontera, A.; Domasevitch, K.V. π–hole interactions at work: Crystal engineering with nitro-derivatives. CrystEngComm 2017, 19, 1933–1937. [Google Scholar] [CrossRef]
- Plater, M.J.; Harrison, W.T.A. Chiral Thianthrenes. Int. J. Mol. Sci. 2024, 25, 4311. [Google Scholar] [CrossRef] [PubMed]
- Chiacchiera, S.M.; Singh, J.O.; Anunziata, J.D.; Silber, J.J. Kinetics of the reactions between 1,2-dinitrobenzene and aliphatic primary amines in benzene. A probable mechanism for the observed mild acceleration. J. Chem. Soc. Perkin Trans. 1988, 11, 1585–1589. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).