4-Phenylbutyric Acid Treatment Reduces Low-Molecular-Weight Proteinuria in a Clcn5 Knock-in Mouse Model for Dent Disease-1
Abstract
1. Introduction
2. Results
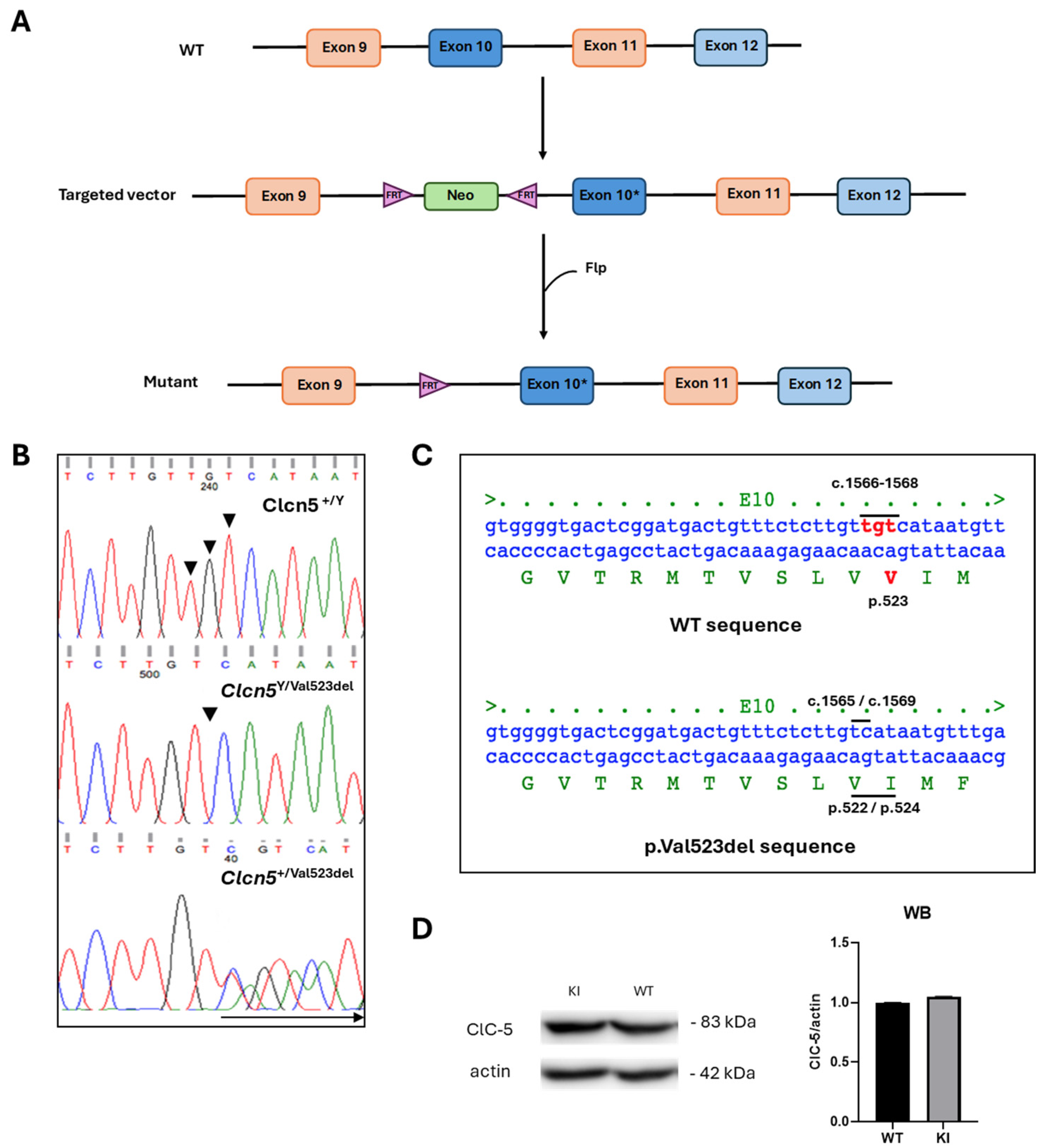
2.1. Generation of Clcn5 Val523del Knock-in Mice
2.2. Genotyping
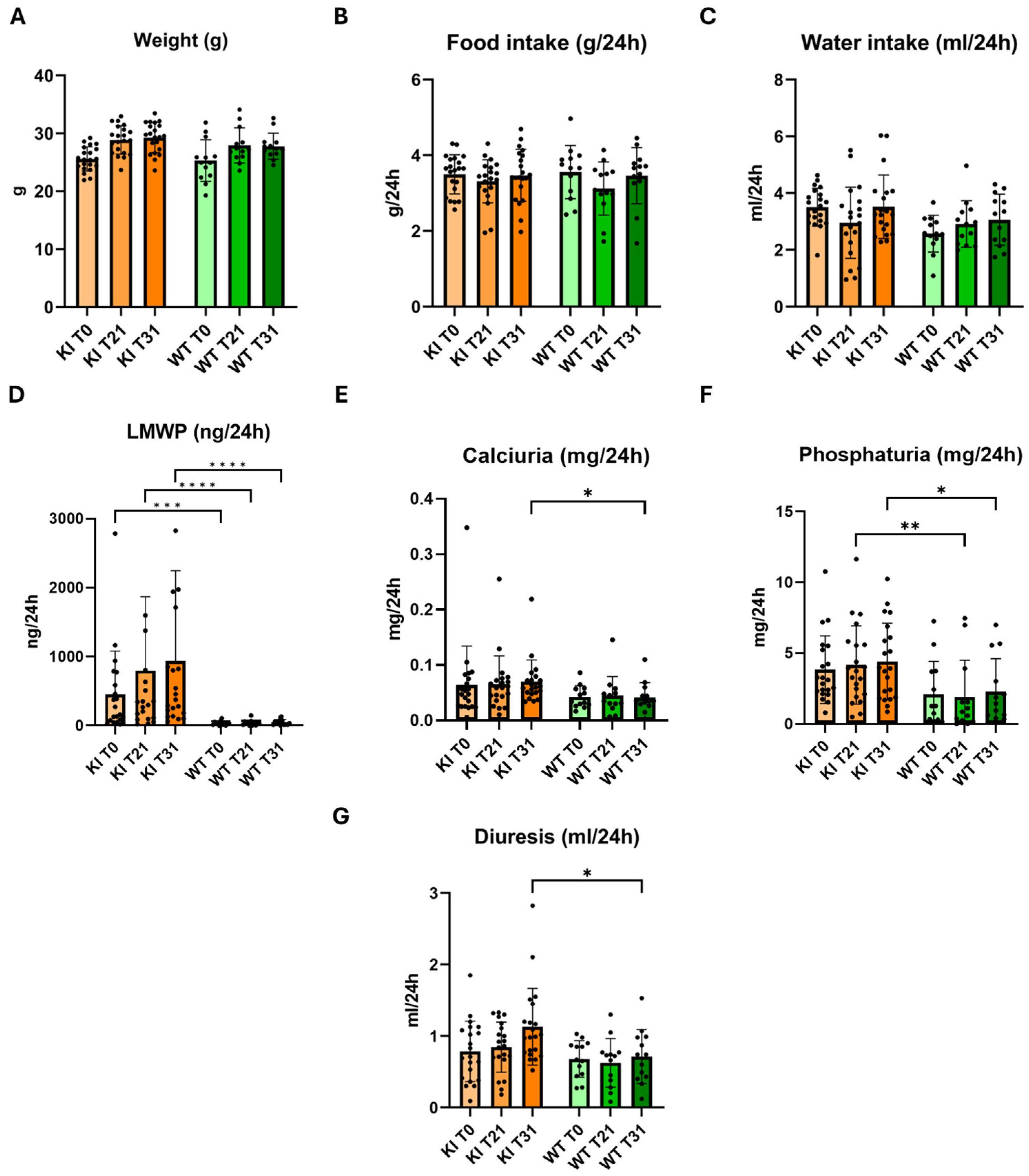
2.3. Phenotypic Characterization of Clcn5 Val523del Knock-in Mice
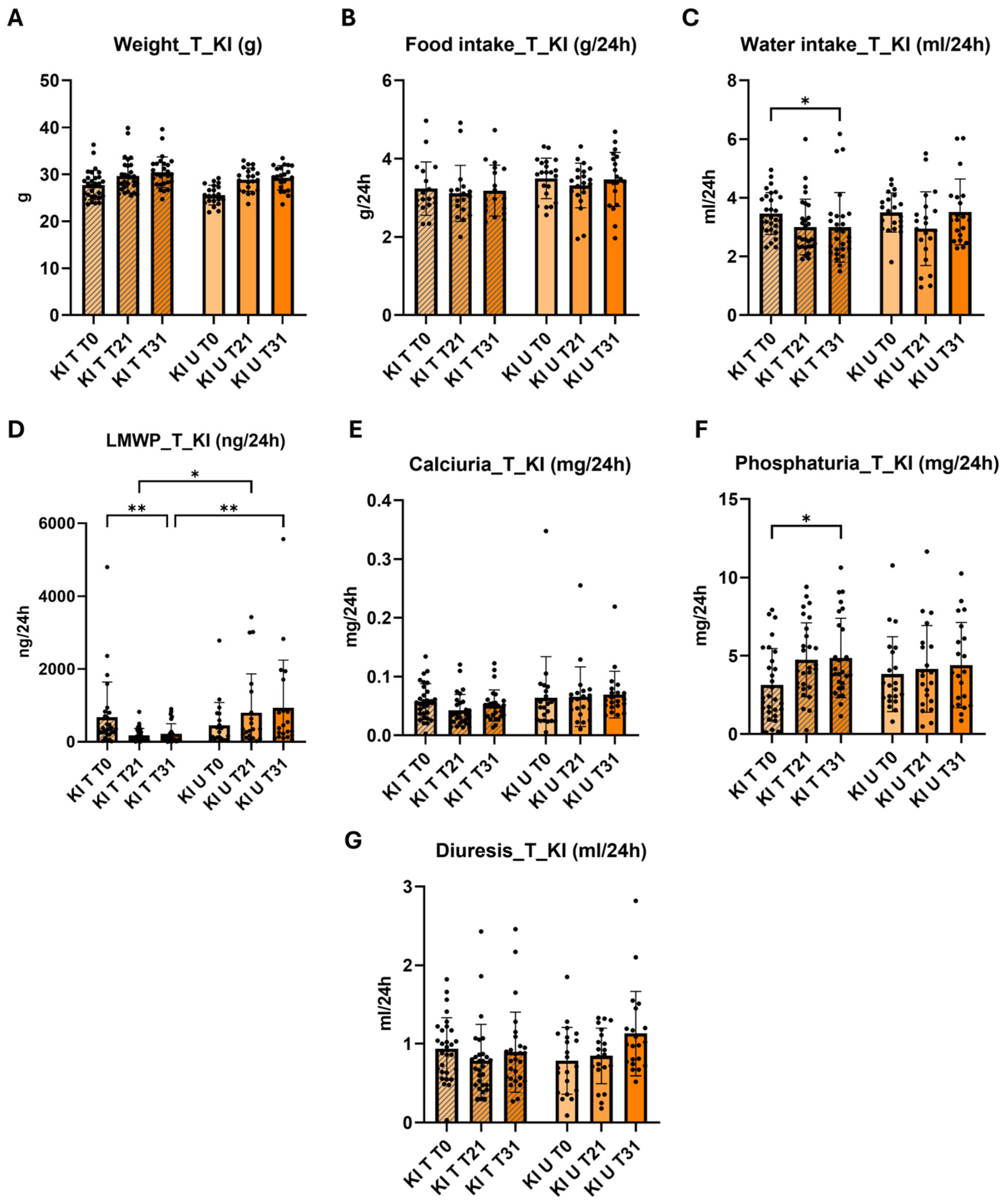
2.4. Effect of 4-PBA in the Phenotype of Knock-in Clcn5 Val523del Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of the Targeting Vector and Generation of Clcn5 Val523del Knock-in Mice
4.2. Genotyping
4.3. Western Blot
4.4. Phenotypic Characterization
4.5. Treatment with 4-PBA
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lieske, J.C.; Milliner, D.S.; Beara-Lasic, L.; Harris, P.; Cogal, A.; Abrash, E. Dent Disease. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2012; pp. 1993–2024. [Google Scholar] [PubMed]
- Claverie-Martín, F.; Ramos-Trujillo, E.; García-Nieto, V. Dent’s disease: Clinical features and molecular basis. Pediatr. Nephrol. 2010, 26, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Anglani, F.; Gianesello, L.; Beara-Lasic, L.; Lieske, J. Dent disease: A window into calcium and phosphate transport. J. Cell. Mol. Med. 2019, 23, 7132–7142. [Google Scholar] [CrossRef] [PubMed]
- Gianesello, L.; Ceol, M.; Bertoldi, L.; Terrin, L.; Priante, G.; Murer, L.; Peruzzi, L.; Giordano, M.; Paglialonga, F.; Cantaluppi, V.; et al. Genetic Analyses in Dent Disease and Characterization of CLCN5 Mutations in Kidney Biopsies. Int. J. Mol. Sci. 2020, 21, 516. [Google Scholar] [CrossRef]
- D’Ambrosio, V.; Wan, E.R.; Siew, K.; Hayes, W.; Walsh, S.B. A female patient with Dent disease due to skewed X-chromosome inactivation. Clin. Kidney J. 2024, 17, sfae092. [Google Scholar] [CrossRef]
- Lloyd, S.E.; Pearce, S.H.; Günther, W.; Kawaguchi, H.; Igarashi, T.; Jentsch, T.J.; Thakker, R.V. Idiopathic low molecular weight proteinuria associated with hypercalciuric nephrocalcinosis in Japanese children is due to mutations of the renal chloride channel (CLCN5). J. Clin. Investig. 1997, 99, 967–974. [Google Scholar] [CrossRef]
- Devuyst, O. Dent’s disease: Chloride-proton exchange controls proximal tubule endocytosis. Nephrol. Dial. Transplant. 2010, 25, 3832–3835. [Google Scholar] [CrossRef] [PubMed]
- Scheel, O.; Zdebik, A.A.; Lourdel, S.; Jentsch, T.J. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 2005, 436, 424–427. [Google Scholar] [CrossRef]
- Novarino, G.; Weinert, S.; Rickheit, G.; Jentsch, T.J. Endosomal Chloride-Proton Exchange Rather Than Chloride Conductance Is Crucial for Renal Endocytosis. Science 2010, 328, 1398–1401. [Google Scholar] [CrossRef]
- Bignon, Y.; Alekov, A.; Frachon, N.; Lahuna, O.; Doh-Egueli, C.J.-B.; Deschênes, G.; Vargas-Poussou, R.; Lourdel, S. A novel CLCN5 pathogenic mutation supports Dent disease with normal endosomal acidification. Hum. Mutat. 2018, 39, 1139–1149. [Google Scholar] [CrossRef]
- Piwon, N.; Günther, W.; Schwake, M.; Bösl, M.R.; Jentsch, T.J. ClC-5 Cl--channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature 2000, 408, 369–373. [Google Scholar] [CrossRef]
- Wang, S.S.; Devuyst, O.; Courtoy, P.J.; Wang, X.-T.; Wang, H.; Wang, Y.; Thakker, R.V.; Guggino, S.; Guggino, W.B. Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum. Mol. Genet. 2000, 9, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Jouret, F.; Auzanneau, C.; Courtoy, P.J. Chloride Channels and Endocytosis: New Insights from Dent’s Disease and ClC-5 Knockout Mice. Nephron Physiol. 2005, 99, p69–p73. [Google Scholar] [CrossRef] [PubMed]
- Günther, W.; Lüchow, A.; Cluzeaud, F.; Vandewalle, A.; Jentsch, T.J. ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc. Natl. Acad. Sci. USA 1998, 95, 8075–8080. [Google Scholar] [CrossRef] [PubMed]
- Moulin, P.; Igarashi, T.; Van Der Smissen, P.; Cosyns, J.-P.; Verroust, P.; Thakker, R.V.; Scheinman, S.J.; Courtoy, P.J.; Devuyst, O. Altered polarity and expression of Hþ-ATPase without ultrastructural changes in kidneys of Dent’s disease patients. Kidney Int. 2003, 63, 1285–1295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, M.-H.; Brown, M.R.; Liu, Y.; Gainullin, V.G.; Harris, P.C.; Romero, M.F.; Lieske, J.C. Cl− and H+ coupling properties and subcellular localizations of wildtype and disease-associated variants of the voltage-gated Cl−/H+ exchanger ClC-5. J. Biol. Chem. 2020, 295, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J.; Pusch, M. CLC chloride channels and transporters: Structure, function, physiology, and disease. Physiol. Rev. 2018, 98, 1493–1590. [Google Scholar] [CrossRef] [PubMed]
- Shipman, K.E.; Weisz, O.A. Making a Dent in Dent Disease. Function 2020, 1, zqaa017. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Morales, M.M.; Sousa-Menzes, J.; Ornellas, D.; Sipes, J.; Cui, Y.; Cui, I.; Hulamm, P.; Cebotaru, V.; Cebotaru, L.; et al. Transcriptional adaptation to Clcn5 knockout in proximal tubules of mouse kidney. Physiol. Genom. 2008, 33, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Yoo, K.W.; Atala, A.; Lu, B. Lentiviral vector mediated gene therapy for type I Dent disease ameliorates Dent disease-like phenotypes for three months in ClC-5 null mice. Mol. Ther. Methods Clin. Dev. 2022, 27, 149–166. [Google Scholar] [CrossRef]
- Wu, F.; Reed, A.A.; Williams, S.E.; Loh, N.Y.; Lippiat, J.D.; Christie, P.T.; Large, O.; Bettinelli, A.; Dillon, M.J.; Goldraich, N.P.; et al. Mutational Analysis of CLC-5, Cofilin and CLC-4 in Patients with Dent’s Disease. Nephron Physiol. 2009, 112, p53–p62. [Google Scholar] [CrossRef]
- Ramos-Trujillo, E.; Claverie-Martin, F.; Garcia-Nieto, V.; Ariceta, G.; Vara, J.; Gonzalez-Acosta, H.; Garcia-Ramirez, M.; Fons, J.; Cordoba-Lanus, E.; Gonzalez-Paredes, J.; et al. Dent’s disease: Identification of seven new pathogenic mutations in the CLCN5 gene. J. Pediatr. Genet. 2013, 2, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Komoda, F.; Miura, K.; Takita, J.; Shimadzu, M.; Matsuyama, T.; Ashida, A.; Igarashi, T. Japanese Dent disease has a wider clinical spectrum than Dent disease in Europe/USA: Genetic and clinical studies of 86 unrelated patients with low-molecular-weight proteinuria. Nephrol. Dial. Transplant. 2013, 29, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Choi, H.J.; Lee, J.M.; Ahn, Y.H.; Kang, H.G.; Choi, Y.M.; Park, S.J.; Cho, H.Y.; Park, Y.-H.; Lee, S.J.; et al. Muscle involvement in Dent disease 2. Pediatr. Nephrol. 2014, 29, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Hureaux, M.; Ashton, E.; Dahan, K.; Houillier, P.; Blanchard, A.; Cormier, C.; Koumakis, E.; Iancu, D.; Belge, H.; Hilbert, P.; et al. High-throughput sequencing contributes to the diagnosis of tubulopathies and familial hypercalcemia hypocalciuria in adults. Kidney Int. 2019, 96, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Dutzler, R.; Campbell, E.B.; Cadene, M.; Chait, B.T.; MacKinnon, R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 2002, 415, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Roche, P.; Christie, P.T.; Loh, N.Y.; Reed, A.A.; Esnouf, R.M.; Thakker, R.V. Modeling study of human renal chloride channel (hCLC-5) mutations suggests a structural-functional relationship. Kidney Int. 2003, 63, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Durán, M.; Burballa, C.; Cantero-Recasens, G.; Butnaru, C.M.; Malhotra, V.; Ariceta, G.; Sarró, E.; Meseguer, A. Novel Dent disease 1 cellular models reveal biological processes underlying ClC-5 loss-of-function. Hum. Mol. Genet. 2021, 30, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nakaki, T. Reduced renal ClC-5 Cl− channel expression in spontaneously hypertensive rats with microalbuminuria. Eur. J. Pharmacol. 2004, 501, 185–189. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Ulrich Hartl, F. Molecular Chaperone Functions in Protein Folding and Proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef]
- Carlisle, R.E.; Brimble, E.; Werner, K.E.; Cruz, G.L.; Ask, K.; Ingram, A.J.; Dickhout, J.G. 4-Phenylbutyrate Inhibits Tunicamycin-Induced Acute Kidney Injury via CHOP/GADD153 Repression. PLoS ONE 2014, 9, e84663. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Di, X.-J.; Mu, T.-W. Using pharmacological chaperones to restore proteostasis. Pharmacol. Res. 2014, 83, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Mohammed-Ali, Z.; Lu, C.; Marway, M.K.; Carlisle, R.E.; Ask, K.; Lukic, D.; Krepinsky, J.C.; Dickhout, J.G. Endoplasmic reticulum stress inhibition attenuates hypertensive chronic kidney disease through reduction in proteinuria. Sci. Rep. 2017, 7, srep41572. [Google Scholar] [CrossRef]
- Yue, J.; Sun, X.; Duan, X.; Sun, C.; Chen, H.; Sun, H.; Zhang, L. Triphenyl phosphate proved more potent than its metabolite diphenyl phosphate in inducing hepatic insulin resistance through endoplasmic reticulum stress. Environ. Int. 2023, 172, 107749. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-K.; Hsu, S.-P.; Wu, C.-T.; Huang, J.-W.; Cheng, H.-T.; Chang, Y.-W.; Hung, K.-Y.; Wu, K.-D.; Liu, S.-H. Endoplasmic Reticulum Stress Implicated in the Development of Renal Fibrosis. Mol. Med. 2011, 17, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ke, Z.; Luo, J. Thiamine Deficiency and Neurodegeneration: The Interplay Among Oxidative Stress, Endoplasmic Reticulum Stress, and Autophagy. Mol. Neurobiol. 2016, 54, 5440–5448. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, K.; Hou, L.; Liao, J.; Zhang, H.; Han, Q.; Guo, J.; Li, Y.; Hu, L.; Pan, J.; et al. Endoplasmic reticulum stress contributes to pyroptosis through NF-κB/NLRP3 pathway in diabetic nephropathy. Life Sci. 2023, 322, 121656. [Google Scholar] [CrossRef]
- Ni, Y.-H.; Deng, H.-F.; Zhou, L.; Huang, C.-S.; Wang, N.-N.; Yue, L.-X.; Li, G.-F.; Yu, H.-J.; Zhou, W.; Gao, Y. Ginsenoside Rb1 Ameliorated Bavachin-Induced Renal Fibrosis via Suppressing Bip/eIF2α/CHOP Signaling-Mediated EMT. Front. Pharmacol. 2022, 13, 872474. [Google Scholar] [CrossRef]
- Gianesello, L.; Del Prete, D.; Ceol, M.; Priante, G.; Calò, L.A.; Anglani, F. From protein uptake to Dent disease: An overview of the CLCN5 gene. Gene 2020, 747, 144662. [Google Scholar] [CrossRef]
- van Berkel, Y.; Ludwig, M.; van Wijk, J.A.E.; Bökenkamp, A. Proteinuria in Dent disease: A review of the literature. Pediatr. Nephrol. 2016, 32, 1851–1859. [Google Scholar] [CrossRef]
- Ishola, D.A.; van der Giezen, D.M.; Hahnel, B.; Goldschmeding, R.; Kriz, W.; Koomans, H.A.; Joles, J.A.; Hegarty, J.; Chiu, D.Y.Y.; Middleton, R.J.; et al. In mice, proteinuria and renal inflammatory responses to albumin overload are strain-dependent. Nephrol. Dial. Transplant. 2005, 21, 591–597. [Google Scholar] [CrossRef]
- Mansour-Hendili, L.; Blanchard, A.; Le Pottier, N.; Roncelin, I.; Lourdel, S.; Treard, C.; González, W.; Vergara-Jaque, A.; Morin, G.; Colin, E.; et al. Mutation Update of the CLCN5 Gene Responsible for Dent Disease 1. Hum. Mutat. 2015, 36, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Stechman, M.J.; Ahmad, B.N.; Loh, N.Y.; Reed, A.A.C.; Stewart, M.; Wells, S.; Hough, T.; Bentley, L.; Cox, R.D.; Brown, S.D.M.; et al. Establishing normal plasma and 24-hour urinary biochemistry ranges in C3H, BALB/c and C57BL/6J mice following acclimatization in metabolic cages. Lab. Anim. 2010, 44, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.; Zammit, T.; Azar, T.; Lawson, D. Stress-like responses to common procedures in individually and group-housed female rats. J. Am. Assoc. Lab. Anim. Sci. 2003, 42, 9–18. [Google Scholar]
- Späni, D.; Arras, M.; König, B.; Rülicke, T. Higher heart rate of laboratory mice housed individually vs in pairs. Lab. Anim. 2003, 37, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Champy, M.; Selloum, M.; Piard, L.; Zeitler, V.; Caradec, C.; Chambon, P.; Auwerx, J. Mouse functional genomics requires standardization of mouse handling and housing conditions. Mamm. Genome 2004, 15, 768–783. [Google Scholar] [CrossRef]
- Van Loo, P.L.P.; Van de Weerd, H.A.; Van Zutphen, L.F.M.; Baumans, V. Preference for social contact versus environmental enrichment in male laboratory mice. Lab. Anim. 2004, 38, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, P.L.P.; Van der Meer, E.; Kruitwagen, C.L.J.J.; Koolhaas, J.M.; Van Zutphen, L.F.M.; Baumans, V. Long-term effects of husbandry procedures on stress-related parameters in male mice of two strains. Lab. Anim. 2004, 38, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.V.; Blaisdell, C.J.; Guggino, S.E.; Guggino, W.B.; Devuyst, O.; Pham, P.-T.; Matsumoto, N.; Shih, R.N.G.; Jo, O.D.; Yanagawa, N.; et al. PTH regulates expression of ClC-5 chloride channel in the kidney. Am. J. Physiol. Physiol. 2000, 278, F238–F245. [Google Scholar] [CrossRef] [PubMed]
- Maritzen, T.; Rickheit, G.; Schmitt, A.; Jentsch, T. Kidney-specific upregulation of vitamin D3 target genes in ClC-5 KO mice. Kidney Int. 2006, 70, 79–87. [Google Scholar] [CrossRef]
- Gekle, M.; Völker, K.; Mildenberger, S.; Freudinger, R.; Shull, G.E.; Wiemann, M. NHE3 Na+/H+ exchanger supports proximal tubular protein reabsorption in vivo. Am. J. Physiol. Physiol. 2004, 287, F469–F473. [Google Scholar] [CrossRef]
- Günther, W.; Piwon, N.; Jentsch, T.J. The ClC-5 chloride channel knock-out mouse–an animal model for Dent’s disease. Pflügers Arch. 2003, 445, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Carretero-Colomer, M. Fenilbutirato de sodio. Offarm 2004, 23, 132–134. [Google Scholar]
- Iannitti, T.; Palmieri, B. Clinical and Experimental Applications of Sodium Phenylbutyrate. Drugs R D 2011, 11, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, D.H. Chemical chaperones: A pharmacological strategy for disorders of protein folding and trafficking. Pediatr. Res. 2002, 52, 832–836. [Google Scholar] [CrossRef]
- Ryu, H.; Smith, K.; Camelo, S.I.; Carreras, I.; Lee, J.; Iglesias, A.H.; Dangond, F.; Cormier, K.A.; Cudkowicz, M.E.; Brown, R.H.; et al. Sodium phenylbutyrate prolongs survival and regulates expression of antiapoptotic genes in transgenic amyotrophic lateral sclerosis mice. J. Neurochem. 2005, 93, 1087–1098. [Google Scholar] [CrossRef]
- Singh, O.V.; Vij, N.; Mogayzel, P.J.; Jozwik, C.; Pollard, H.B.; Zeitlin, P.L. Pharmacoproteomics of 4-phenylbutyrate-treated IB3-1 cystic fibrosis bronchial epithelial cells. J. Proteome Res. 2006, 5, 562–571. [Google Scholar] [CrossRef]
- Villani, S.; Dematteis, G.; Tapella, L.; Gagliardi, M.; Lim, D.; Corazzari, M.; Aprile, S.; Del Grosso, E. Quantification of the Chemical Chaperone 4-Phenylbutyric Acid (4-PBA) in Cell Culture Media via LC-HRMS: Applications in Fields of Neurodegeneration and Cancer. Pharmaceuticals 2023, 16, 298. [Google Scholar] [CrossRef]
- Özcan, U.; Yilmaz, E.; Özcan, L.; Furuhashi, M.; Vaillancourt, E.; Smith, R.O.; Görgün, C.Z.; Hotamisligil, G.S. Chemical Chaperones Reduce ER Stress and Restore Glucose Homeostasis in a Mouse Model of Type 2 Diabetes. Science 2006, 313, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Hosoi, T.; Okuma, Y.; Kaneko, M.; Nomura, Y. Sodium 4-Phenylbutyrate Protects Against Cerebral Ischemic Injury. Mol. Pharmacol. 2004, 66, 899–908. [Google Scholar] [CrossRef]
- Ricobaraza, A.; Cuadrado-Tejedor, M.; Marco, S.; Pérez-Otaño, I.; García-Osta, A. Phenylbutyrate Rescues Dendritic Spine Loss Associated with Memory Deficits in a Mouse Model of Alzheimer Disease. Hippocampus 2010, 22, 1040–1050. [Google Scholar] [CrossRef]
- Zeng, M.; Sang, W.; Chen, S.; Chen, R.; Zhang, H.; Xue, F.; Li, Z.; Liu, Y.; Gong, Y.; Zhang, H.; et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol. Lett. 2017, 271, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Butchbach, M.E.; Lumpkin, C.J.; Harris, A.W.; Saieva, L.; Edwards, J.D.; Workman, E.; Simard, L.R.; Pellizzoni, L.; Burghes, A.H. Protective effects of butyrate-based compounds on a mouse model for spinal muscular atrophy. Exp. Neurol. 2016, 279, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, G.; Labelle-Dumais, C.; Gould, D.B. Use of sodium 4-phenylbutyrate to define therapeutic parameters for reducing intracerebral hemorrhage and myopathy in Col4a1 mutant mice. Dis. Model. Mech. 2018, 11, dmm034157. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
| T0 | T21 | T31 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | Low | Medium | High | |
| Untreated | 23.8% | 52.4% | 23.8% | 14.3% | 57.1% | 28.6% | 4.8% | 57.1% | 38.1% |
| Treated | 24.1% | 51.7% | 24.1% | 68.9% | 27.6% | 3.4% | 75.9% | 13.8% | 10.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdomo-Ramírez, A.; Ramos-Trujillo, E.; Machado, J.D.; García-Nieto, V.; Mura-Escorche, G.; Claverie-Martin, F., on behalf of the RenalTube Group. 4-Phenylbutyric Acid Treatment Reduces Low-Molecular-Weight Proteinuria in a Clcn5 Knock-in Mouse Model for Dent Disease-1. Int. J. Mol. Sci. 2024, 25, 8110. https://doi.org/10.3390/ijms25158110
Perdomo-Ramírez A, Ramos-Trujillo E, Machado JD, García-Nieto V, Mura-Escorche G, Claverie-Martin F on behalf of the RenalTube Group. 4-Phenylbutyric Acid Treatment Reduces Low-Molecular-Weight Proteinuria in a Clcn5 Knock-in Mouse Model for Dent Disease-1. International Journal of Molecular Sciences. 2024; 25(15):8110. https://doi.org/10.3390/ijms25158110
Chicago/Turabian StylePerdomo-Ramírez, Ana, Elena Ramos-Trujillo, Jose David Machado, Victor García-Nieto, Glorián Mura-Escorche, and Félix Claverie-Martin on behalf of the RenalTube Group. 2024. "4-Phenylbutyric Acid Treatment Reduces Low-Molecular-Weight Proteinuria in a Clcn5 Knock-in Mouse Model for Dent Disease-1" International Journal of Molecular Sciences 25, no. 15: 8110. https://doi.org/10.3390/ijms25158110
APA StylePerdomo-Ramírez, A., Ramos-Trujillo, E., Machado, J. D., García-Nieto, V., Mura-Escorche, G., & Claverie-Martin, F., on behalf of the RenalTube Group. (2024). 4-Phenylbutyric Acid Treatment Reduces Low-Molecular-Weight Proteinuria in a Clcn5 Knock-in Mouse Model for Dent Disease-1. International Journal of Molecular Sciences, 25(15), 8110. https://doi.org/10.3390/ijms25158110






