Overexpression of miR-199b-5p in Colony Forming Unit-Hill’s Colonies Positively Mediates the Inflammatory Response in Subclinical Cardiovascular Disease Model: Metformin Therapy Attenuates Its Expression
Abstract
1. Introduction
2. Results
2.1. Clinical and Metabolic Characteristics of Study Participants
2.2. Comparisons of Inflammatory Markers and Vascular Health Parameters between T1DM Individuals and HCs
2.3. Expression of miR-199b-5p between Study Groups
2.4. Correlations Trends of miR-199b-5p with Inflammatory Markers and Vascular Health Parameters
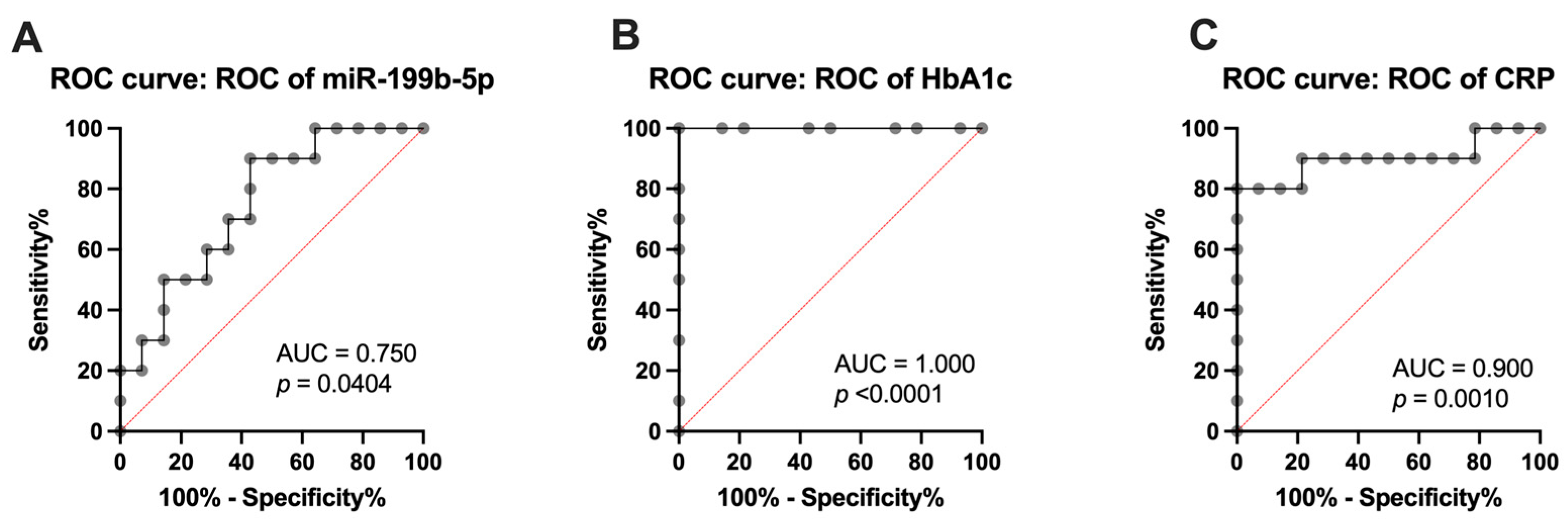
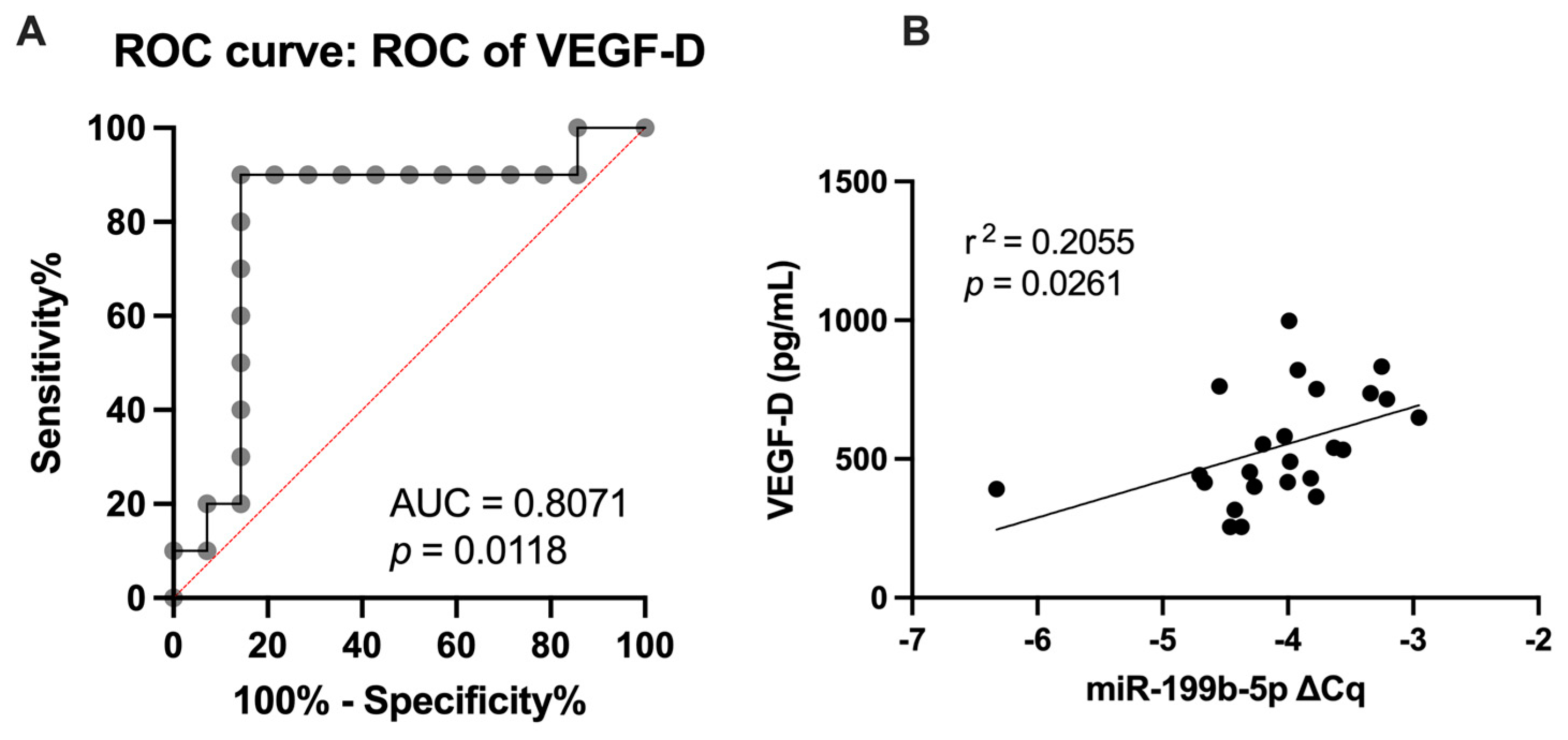
2.5. Analysis of miR-199b-5p, Inflammatory Markers, and Vascular Health Parameters as Diagnostic Markers
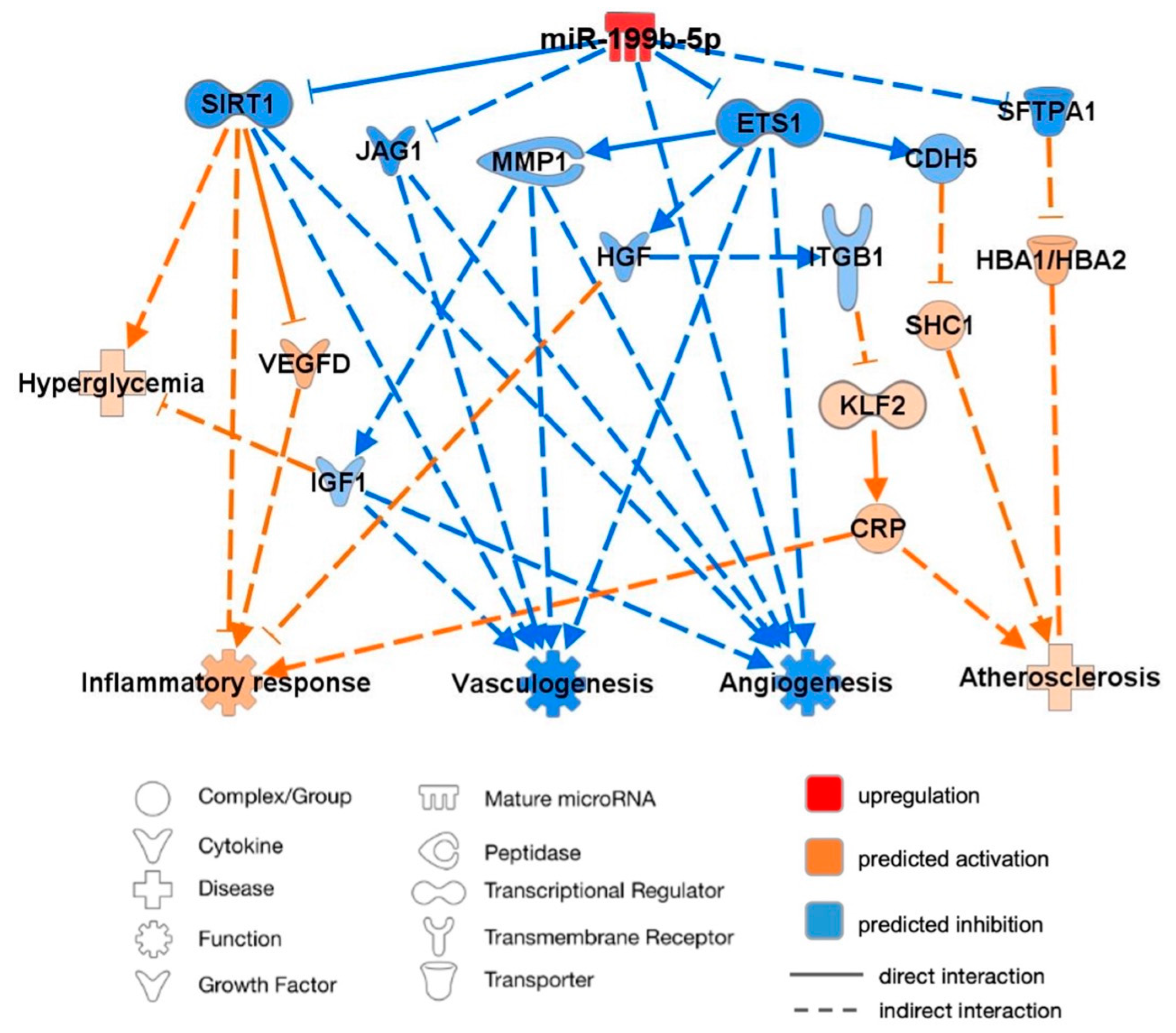
2.6. Predicted Molecular Targets and Functional Pathways of miR-199b-5p
2.7. miR-199b-5p Predicted Targets and Pathways Following Metformin Intervention
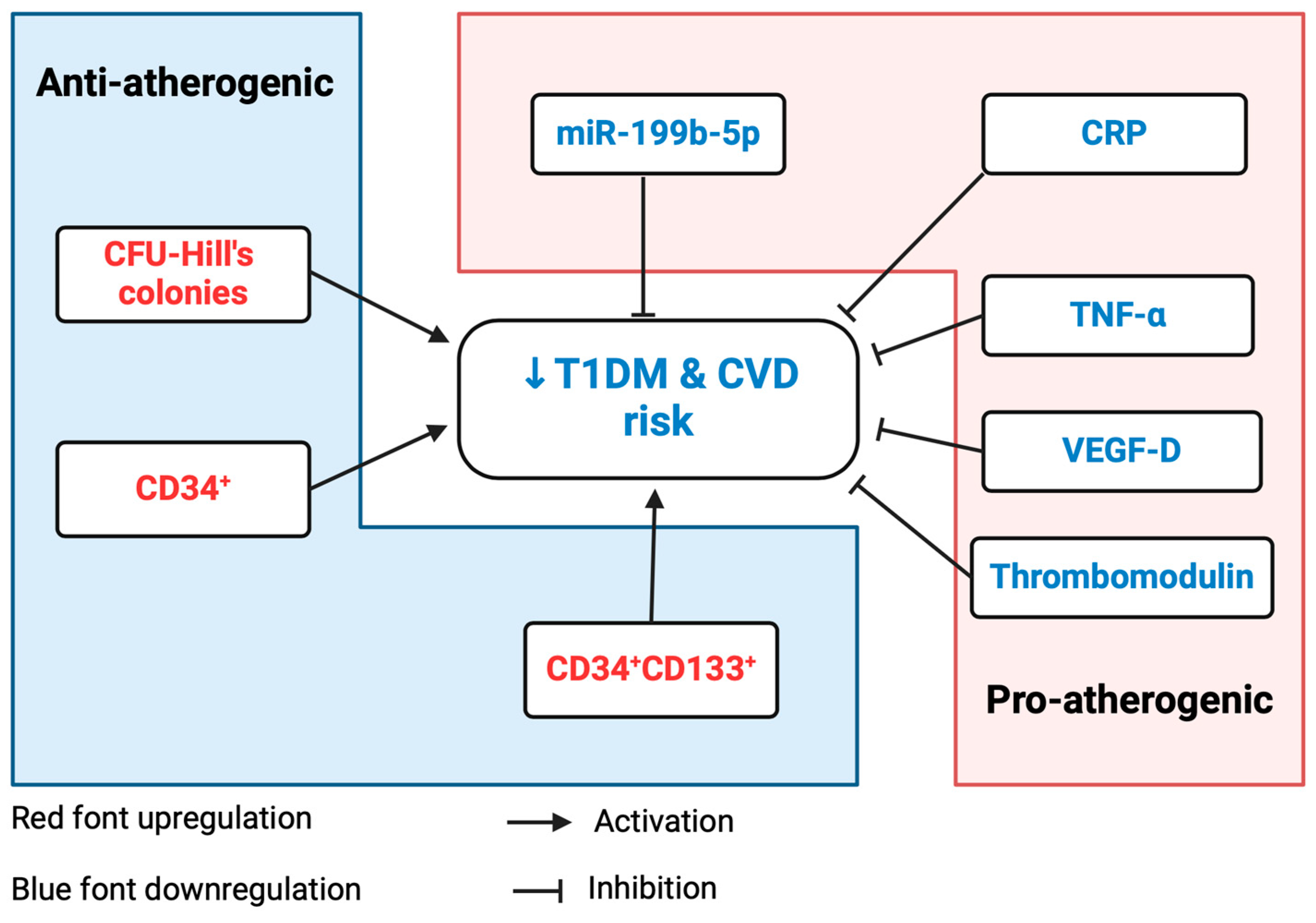
3. Discussion
3.1. Upregulation of miR-199b-5p Expression in T1DM
3.2. Association between miR-199b-5p and HbA1c
3.3. Positive Association between miR-199b-5p and Inflammatory Markers
3.3.1. CRP
3.3.2. VEGF-D
3.4. Downregulation of miR-199b-5p Expression Following Metformin Intervention
3.5. Prediction Model: Pathway Analysis of miR-199b-5p in Relation to Cardiovascular Function
3.5.1. Angiogenesis and Vasculogenesis
3.5.2. Inflammatory Response and Atherosclerosis
3.5.3. Influence of Metformin
3.6. Clinical Applications for CVD
3.6.1. miR-199b-5p
3.6.2. SIRT1
3.6.3. ETS1
3.6.4. JAG1
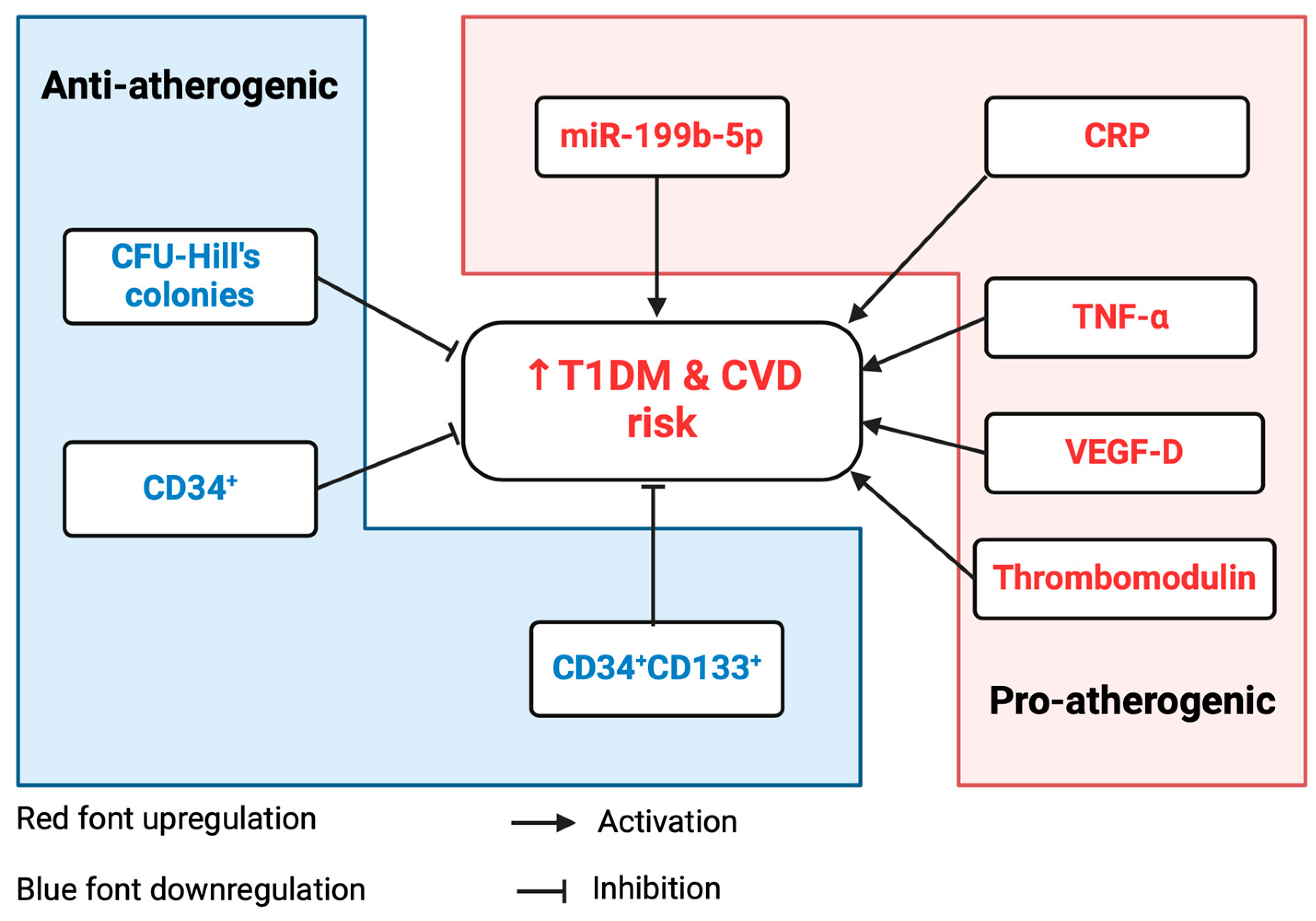
3.7. Contribution/Causation
4. Materials and Methods
4.1. Study Design
4.2. Clinical and Laboratory Methods
4.3. Meso Scale Discovery (MSD) Assay for Cytokine Analysis
4.4. IGF-1 and IGFBP-3 Enzyme-Linked Immunosorbent Assay
4.5. Flow Cytometric Evaluation of Circulating Endothelial Progenitor Cells
4.6. Culture and Quantification of CFU-Hill’s Colonies
4.7. Real-Time Quantitative Polymerase Chain Reaction (RTqPCR) for miRNA Expression
4.8. Ingenuity Pathway Analysis (IPA) of miR-199b-5p
4.9. Statistical Analysis
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, F.W.; Rider, R.; Glanville, M.; Narayanan, K.; Razvi, S.; Weaver, J.U. Metformin improves circulating endothelial cells and endothelial progenitor cells in type 1 diabetes: MERIT study. Cardiovasc. Diabetol. 2016, 15, 116. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Chen, Y.H.; Chen, Y.L.; Wu, T.C.; Chen, J.W.; Lin, S.J. Vascular endothelial function and circulating endothelial progenitor cells in patients with cardiac syndrome X. Heart 2007, 93, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.; Costacou, T.; Orchard, T. Is glycaemia or insulin dose the stronger risk factor for coronary artery disease in type 1 diabetes? Diab Vasc. Dis. Res. 2009, 6, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Zeadin, M.G.; Petlura, C.I.; Werstuck, G.H. Molecular Mechanisms Linking Diabetes to the Accelerated Development of Atherosclerosis. Can. J. Diabetes 2013, 37, 345–350. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar] [CrossRef]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus—Mechanisms, Management, and Clinical Considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef]
- Schofield, J.; Ho, J.; Soran, H. Cardiovascular Risk in Type 1 Diabetes Mellitus. Diabetes Ther. 2019, 10, 773–789. [Google Scholar] [CrossRef]
- Huxley, R.R.; Peters, S.A.; Mishra, G.D.; Woodward, M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 198–206. [Google Scholar] [CrossRef]
- Lind, M.; Svensson, A.M.; Kosiborod, M.; Gudbjörnsdottir, S.; Pivodic, A.; Wedel, H.; Dahlqvist, S.; Clements, M.; Rosengren, A. Glycemic control and excess mortality in type 1 diabetes. N. Engl. J. Med. 2014, 371, 1972–1982. [Google Scholar] [CrossRef]
- Taleb, S. Inflammation in atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef]
- Sibal, L.; Aldibbiat, A.; Agarwal, S.C.; Mitchell, G.; Oates, C.; Razvi, S.; Weaver, J.U.; Shaw, J.A.; Home, P.D. Circulating endothelial progenitor cells, endothelial function, carotid intima–media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia 2009, 52, 1464–1473. [Google Scholar] [CrossRef]
- Luan, Y.Y.; Yao, Y.M. The Clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Pasceri, V.; Willerson, J.T.; Yeh, E.T. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 2000, 102, 2165–2168. [Google Scholar] [CrossRef]
- Schwedler, S.B.; Filep, J.G.; Galle, J.; Wanner, C.; Potempa, L.A. C-reactive protein: A family of proteins to regulate cardiovascular function. Am. J. Kidney Dis. 2006, 47, 212–222. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science 1997, 275, 964–966. [Google Scholar] [CrossRef]
- Wesseling, M.; Sakkers, T.R.; de Jager, S.C.A.; Pasterkamp, G.; Goumans, M.J. The morphological and molecular mechanisms of epithelial/endothelial-to-mesenchymal transition and its involvement in atherosclerosis. Vasc. Pharmacol. 2018, 106, 1–8. [Google Scholar] [CrossRef]
- Loomans, C.J.; de Koning, E.J.; Staal, F.J.; Rookmaaker, M.B.; Verseyden, C.; de Boer, H.C.; Verhaar, M.C.; Braam, B.; Rabelink, T.J.; van Zonneveld, A.J. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004, 53, 195–199. [Google Scholar] [CrossRef]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and Migratory Activity of Circulating Endothelial Progenitor Cells Inversely Correlate with Risk Factors for Coronary Artery Disease. Circ. Res. 2001, 89, e1–e7. [Google Scholar] [CrossRef]
- Werner, N.; Kosiol, S.; Schiegl, T.; Ahlers, P.; Walenta, K.; Link, A.; Böhm, M.; Nickenig, G. Circulating Endothelial Progenitor Cells and Cardiovascular Outcomes. N. Engl. J. Med. 2005, 353, 999–1007. [Google Scholar] [CrossRef]
- Asicioglu, E.; Gogas Yavuz, D.; Koc, M.; Ozben, B.; Yazici, D.; Deyneli, O.; Akalin, S. Circulating endothelial cells are elevated in patients with type 1 diabetes mellitus. Eur. J. Endocrinol. 2010, 162, 711–717. [Google Scholar] [CrossRef]
- Hur, J.; Yoon, C.-H.; Kim, H.-S.; Choi, J.-H.; Kang, H.-J.; Hwang, K.-K.; Oh, B.-H.; Lee, M.-M.; Park, Y.-B. Characterization of Two Types of Endothelial Progenitor Cells and Their Different Contributions to Neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef]
- Kopp, H.G.; Ramos, C.A.; Rafii, S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr. Opin. Hematol. 2006, 13, 175–181. [Google Scholar] [CrossRef]
- Boettger, T.; Braun, T.; Rooij, E.v. A New Level of Complexity. Circ. Res. 2012, 110, 1000–1013. [Google Scholar] [CrossRef]
- Du, P.; Dai, F.; Chang, Y.; Wei, C.; Yan, J.; Li, J.; Liu, X. Role of miR-199b-5p in regulating angiogenesis in mouse myocardial microvascular endothelial cells through HSF1/VEGF pathway. Environ. Toxicol. Pharmacol. 2016, 47, 142–148. [Google Scholar] [CrossRef]
- Meng, W.; Li, Y.; Chai, B.; Liu, X.; Ma, Z. miR-199a: A Tumor Suppressor with Noncoding RNA Network and Therapeutic Candidate in Lung Cancer. Int. J. Mol. Sci. 2022, 23, 8518. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.J.; Hung, Y.H.; Narisu, N.; Erdos, M.R.; Kanke, M.; Yan, T.; Grenko, C.M.; Swift, A.J.; Bonnycastle, L.L.; Sethupathy, P.; et al. Human pancreatic islet microRNAs implicated in diabetes and related traits by large-scale genetic analysis. Proc. Natl. Acad. Sci. USA 2023, 120, e2206797120. [Google Scholar] [CrossRef] [PubMed]
- Werneck-de-Castro, J.P.; Blandino-Rosano, M.; Hilfiker-Kleiner, D.; Bernal-Mizrachi, E. Glucose stimulates microRNA-199 expression in murine pancreatic beta-cells. J. Biol. Chem. 2020, 295, 1261–1270. [Google Scholar] [CrossRef]
- da Costa Martins, P.A.; Salic, K.; Gladka, M.M.; Armand, A.-S.; Leptidis, S.; el Azzouzi, H.; Hansen, A.; Coenen-de Roo, C.J.; Bierhuizen, M.F.; van der Nagel, R.; et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat. Cell Biol. 2010, 12, 1220–1227. [Google Scholar] [CrossRef]
- Dihoum, A.; Rena, G.; Pearson, E.R.; Lang, C.C.; Mordi, I.R. Metformin: Evidence from preclinical and clinical studies for potential novel applications in cardiovascular disease. Expert Opin. Investig. Drugs 2023, 32, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Zilov, A.V.; Abdelaziz, S.I.; AlShammary, A.; Al Zahrani, A.; Amir, A.; Assaad Khalil, S.H.; Brand, K.; Elkafrawy, N.; Hassoun, A.A.K.; Jahed, A.; et al. Mechanisms of action of metformin with special reference to cardiovascular protection. Diabetes Metab. Res. Rev. 2019, 35, e3173. [Google Scholar] [CrossRef]
- Petrie, J.R.; Chaturvedi, N.; Ford, I.; Brouwers, M.C.G.J.; Greenlaw, N.; Tillin, T.; Hramiak, I.; Hughes, A.D.; Jenkins, A.J.; Klein, B.E.K.; et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): A double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 597–609. [Google Scholar] [CrossRef]
- Ahmed, F.W.; Bakhashab, S.; Bastaman, I.T.; Crossland, R.E.; Glanville, M.; Weaver, J.U. Anti-Angiogenic miR-222, miR-195, and miR-21a Plasma Levels in T1DM Are Improved by Metformin Therapy, Thus Elucidating Its Cardioprotective Effect: The MERIT Study. Int. J. Mol. Sci. 2018, 19, 3242. [Google Scholar] [CrossRef]
- Phowira, J.; Ahmed, F.W.; Bakhashab, S.; Weaver, J.U. Upregulated miR-18a-5p in Colony Forming Unit-Hill’s in Subclinical Cardiovascular Disease and Metformin Therapy; MERIT Study. Biomedicines 2022, 10, 2136. [Google Scholar] [CrossRef]
- Liang, W.J.; Zhou, S.N.; Shan, M.R.; Wang, X.Q.; Zhang, M.; Chen, Y.; Zhang, Y.; Wang, S.X.; Guo, T. AMPKalpha inactivation destabilizes atherosclerotic plaque in streptozotocin-induced diabetic mice through AP-2alpha/miRNA-124 axis. J. Mol. Med. 2018, 96, 403–412. [Google Scholar] [CrossRef]
- Tamara, A.; Coulson, D.J.; Latief, J.S.; Bakhashab, S.; Weaver, J.U. Upregulated anti-angiogenic miR-424-5p in type 1 diabetes (model of subclinical cardiovascular disease) correlates with endothelial progenitor cells, CXCR1/2 and other parameters of vascular health. Stem Cell Res. Ther. 2021, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Bakhashab, S.; O’Neill, J.; Barber, R.; Arden, C.; Weaver, J.U. Upregulation of Anti-Angiogenic miR-106b-3p Correlates Negatively with IGF-1 and Vascular Health Parameters in a Model of Subclinical Cardiovascular Disease: Study with Metformin Therapy. Biomedicines 2024, 12, 171. [Google Scholar] [CrossRef]
- Bakhashab, S.; Yuen Yeoh, M.L.; Coulson, D.J.; Steel, S.C.; Ray, S.L.; Weaver, J.U. Deciphering the Role of miR-200c-3p in Type 1 Diabetes (Subclinical Cardiovascular Disease) and Its Correlation with Inflammation and Vascular Health. Int. J. Mol. Sci. 2022, 23, 15659. [Google Scholar] [CrossRef]
- Ray, S.L.; Coulson, D.J.; Yeoh, M.L.Y.; Tamara, A.; Latief, J.S.; Bakhashab, S.; Weaver, J.U. The Role of miR-342 in Vascular Health. Study in Subclinical Cardiovascular Disease in Mononuclear Cells, Plasma, Inflammatory Cytokines and PANX2. Int. J. Mol. Sci. 2020, 21, 7217. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Marfella, R.; Sardu, C.; et al. Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence. Biomolecules 2021, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Bakhashab, S.; Ahmed, F.; Schulten, H.J.; Ahmed, F.W.; Glanville, M.; Al-Qahtani, M.H.; Weaver, J.U. Proangiogenic Effect of Metformin in Endothelial Cells Is via Upregulation of VEGFR1/2 and Their Signaling under Hyperglycemia-Hypoxia. Int. J. Mol. Sci. 2018, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Bakhashab, S.; Ahmed, F.W.; Schulten, H.J.; Bashir, A.; Karim, S.; Al-Malki, A.L.; Gari, M.A.; Abuzenadah, A.M.; Chaudhary, A.G.; Alqahtani, M.H.; et al. Metformin improves the angiogenic potential of human CD34(+) cells co-incident with downregulating CXCL10 and TIMP1 gene expression and increasing VEGFA under hyperglycemia and hypoxia within a therapeutic window for myocardial infarction. Cardiovasc. Diabetol. 2016, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ding, B.; Shen, Y.; Yan, R.N.; Li, F.F.; Sun, R.; Jing, T.; Lee, K.O.; Ma, J.H. Rapid Changes in Serum Testosterone in Men With Newly Diagnosed Type 2 Diabetes With Intensive Insulin and Metformin. Diabetes Care 2021, 44, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Shin, Y.J.; Kim, H.; Kim, H.; Kim, J.; Min, S.A.; Kim, P.; Yu, S.D.; Park, K. Metformin-induced endocrine disruption and oxidative stress of Oryzias latipes on two-generational condition. J. Hazard. Mater. 2019, 367, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Tavlo, M.; Skakkebaek, N.E.; Mathiesen, E.R.; Kristensen, D.M.; Kjaer, K.H.; Andersson, A.M.; Lindahl-Jacobsen, R. Hypothesis: Metformin is a potential reproductive toxicant. Front. Endocrinol. 2022, 13, 1000872. [Google Scholar] [CrossRef] [PubMed]
- Elmoselhi, A.B.; Seif Allah, M.; Bouzid, A.; Ibrahim, Z.; Venkatachalam, T.; Siddiqui, R.; Khan, N.A.; Hamoudi, R.A. Circulating microRNAs as potential biomarkers of early vascular damage in vitamin D deficiency, obese, and diabetic patients. PLoS ONE 2023, 18, e0283608. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Roy, S.; Huang, Y.; Khanna, S.; Sen, C.K. The microRNA miR-199a-5p down-regulation switches on wound angiogenesis by derepressing the v-ets erythroblastosis virus E26 oncogene homolog 1-matrix metalloproteinase-1 pathway. J. Biol. Chem. 2012, 287, 41032–41043. [Google Scholar] [CrossRef] [PubMed]
- Sato-Kunisada, R.; Yoshida, N.; Nakamura, S.; Uchiyama, H.; Matsumoto, H. Enhanced Expression of miR-199b-5p Promotes Proliferation of Pancreatic β-Cells by Down-Regulation of MLK3. Microrna 2016, 5, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kozuka, K.; Kohriyama, T.; Nomura, E.; Ikeda, J.; Kajikawa, H.; Nakamura, S. Endothelial markers and adhesion molecules in acute ischemic stroke--sequential change and differences in stroke subtype. Atherosclerosis 2002, 161, 161–168. [Google Scholar] [CrossRef]
- Nakagawa, I.; Matsubara, T.; Hori, T.; Imai, S.; Ozaki, K.; Mezaki, T.; Nasuno, A.; Kubota, K.; Nakano, M.; Yamazoe, M.; et al. Significance of soluble thrombomodulin in the coronary circulation of patients with coronary artery disease. J. Cardiol. 2001, 38, 145–152. [Google Scholar] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Rutanen, J.; Leppänen, P.; Tuomisto, T.T.; Rissanen, T.T.; Hiltunen, M.O.; Vajanto, I.; Niemi, M.; Häkkinen, T.; Karkola, K.; Stacker, S.A.; et al. Vascular endothelial growth factor-D expression in human atherosclerotic lesions. Cardiovasc. Res. 2003, 59, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Suzuki, M.; Matsuda, M.; Ajiro, Y.; Shinozaki, T.; Sakagami, S.; Yonezawa, K.; Shimizu, M.; Funada, J.; Takenaka, T.; et al. Distinct Characteristics of VEGF-D and VEGF-C to Predict Mortality in Patients with Suspected or Known Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e015761. [Google Scholar] [CrossRef] [PubMed]
- Berntsson, J.; Smith, J.G.; Johnson, L.S.B.; Soderholm, M.; Borne, Y.; Melander, O.; Orho-Melander, M.; Nilsson, J.; Engstrom, G. Increased vascular endothelial growth factor D is associated with atrial fibrillation and ischaemic stroke. Heart 2019, 105, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Bhardwaj, S.; Babu, M.; Kokina, I.; Uotila, S.; Ahtialansaari, T.; Laitinen, T.; Hakumaki, J.; Laakso, M.; Herzig, K.-H.; et al. VEGF-A, VEGF-D, VEGF receptor-1, VEGF receptor-2, NF-KB, and RAGE in atherosclerotic lesions of diabetic Watanabe heritable hyperlipidemic rabbits. FASEB J. 2006, 20, 2159–2161. [Google Scholar] [CrossRef] [PubMed]
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [CrossRef]
- Rutledge, C.A.; Lagranha, C.; Chiba, T.; Redding, K.; Stolz, D.B.; Goetzman, E.; Sims-Lucas, S.; Kaufman, B.A. Metformin preconditioning protects against myocardial stunning and preserves protein translation in a mouse model of cardiac arrest. J. Mol. Cell Cardiol. Plus 2023, 4, 100034. [Google Scholar] [CrossRef]
- Zhang, C.-X.; Pan, S.-N.; Meng, R.-S.; Peng, C.-Q.; Xiong, Z.-J.; Chen, B.-L.; Chen, G.-Q.; Yao, F.-J.; Chen, Y.-L.; Ma, Y.-D.; et al. Metformin attenuates ventricular hypertrophy by activating the AMP-activated protein kinase–endothelial nitric oxide synthase pathway in rats. Clin. Exp. Pharmacol. Physiol. 2011, 38, 55–62. [Google Scholar] [CrossRef]
- Tseng, H.W.; Li, S.C.; Tsai, K.W. Metformin Treatment Suppresses Melanoma Cell Growth and Motility Through Modulation of microRNA Expression. Cancers 2019, 11, 209. [Google Scholar] [CrossRef]
- George, M.; Ayuso, E.; Casellas, A.; Costa, C.; Devedjian, J.C.; Bosch, F. Beta cell expression of IGF-I leads to recovery from type 1 diabetes. J. Clin. Investig. 2002, 109, 1153–1163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, D.-C.; Romero, R.; Kim, C.J.; Chaiworapongsa, T.; Tarca, A.L.; Lee, J.; Suh, Y.-L.; Mazaki-Tovi, S.; Vaisbuch, E.; Mittal, P.; et al. Surfactant Protein-A as an Anti-Inflammatory Component in the Amnion: Implications for Human Pregnancy. J. Immunol. 2010, 184, 6479–6491. [Google Scholar] [CrossRef]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef]
- Ou, X.; Chae, H.D.; Wang, R.H.; Shelley, W.C.; Cooper, S.; Taylor, T.; Kim, Y.J.; Deng, C.X.; Yoder, M.C.; Broxmeyer, H.E. SIRT1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood 2011, 117, 440–450. [Google Scholar] [CrossRef]
- Tomita, N.; Morishita, R.; Taniyama, Y.; Koike, H.; Aoki, M.; Shimizu, H.; Matsumoto, K.; Nakamura, T.; Kaneda, Y.; Ogihara, T. Angiogenic property of hepatocyte growth factor is dependent on upregulation of essential transcription factor for angiogenesis, ets-1. Circulation 2003, 107, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Alsaigh, T.; Shaked, H.; Altshuler, A.E.; Pocock, E.S.; Kistler, E.B.; Karin, M.; Schmid-Schönbein, G.W. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J. Biol. Chem. 2013, 288, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, R.; Yue, Q.; Peng, H. MicroRNA-29 regulates myocardial microvascular endothelial cells proliferation and migration in association with IGF1 in type 2 diabetes. Biochem. Biophys. Res. Commun. 2017, 487, 15–21. [Google Scholar] [CrossRef]
- Boscolo, E.; Stewart, C.L.; Greenberger, S.; Wu, J.K.; Durham, J.T.; Herman, I.M.; Mulliken, J.B.; Kitajewski, J.; Bischoff, J. JAGGED1 signaling regulates hemangioma stem cell-to-pericyte/vascular smooth muscle cell differentiation. Arter. Thromb. Vasc. Biol. 2011, 31, 2181–2192. [Google Scholar] [CrossRef]
- High, F.A.; Jain, R.; Stoller, J.Z.; Antonucci, N.B.; Lu, M.M.; Loomes, K.M.; Kaestner, K.H.; Pear, W.S.; Epstein, J.A. Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J. Clin. Investig. 2009, 119, 1986–1996. [Google Scholar] [CrossRef]
- High, F.A.; Lu, M.M.; Pear, W.S.; Loomes, K.M.; Kaestner, K.H.; Epstein, J.A. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc. Natl. Acad. Sci. USA 2008, 105, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Xu, Q.; Gao, H.; Peres-da-Silva, A.; Draper, D.W.; Fessler, M.B.; Purushotham, A.; Li, X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell Biol. 2010, 30, 4712–4721. [Google Scholar] [CrossRef] [PubMed]
- Detoraki, A.; Staiano, R.I.; Granata, F.; Giannattasio, G.; Prevete, N.; de Paulis, A.; Ribatti, D.; Genovese, A.; Triggiani, M.; Marone, G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J. Allergy Clin. Immunol. 2009, 123, 1142–1149.e1145. [Google Scholar] [CrossRef] [PubMed]
- Coudriet, G.M.; He, J.; Trucco, M.; Mars, W.M.; Piganelli, J.D. Hepatocyte growth factor modulates interleukin-6 production in bone marrow derived macrophages: Implications for inflammatory mediated diseases. PLoS ONE 2010, 5, e15384. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Martin-Padura, I.; de Nigris, F.; Giorgio, M.; Mansueto, G.; Somma, P.; Condorelli, M.; Sica, G.; De Rosa, G.; Pelicci, P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc. Natl. Acad. Sci. USA 2003, 100, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, Y.Q.; Zheng, Y.Y.; Wang, W.; Wang, P.; Liang, J.; Zhao, W.; Tao, T.; Sun, J.; Wei, L.; et al. The intragenic microRNA miR199A1 in the dynamin 2 gene contributes to the pathology of X-linked centronuclear myopathy. J. Biol. Chem. 2020, 295, 8656–8667. [Google Scholar] [CrossRef] [PubMed]
- Duygu, B.; Poels, E.M.; Juni, R.; Bitsch, N.; Ottaviani, L.; Olieslagers, S.; de Windt, L.J.; da Costa Martins, P.A. miR-199b-5p is a regulator of left ventricular remodeling following myocardial infarction. Noncoding RNA Res. 2017, 2, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, I.; Kumarswamy, R.; Pfaff, N.; Fiedler, J.; Dangwal, S.; Holzmann, A.; Batkai, S.; Geffers, R.; Lother, A.; Hein, L.; et al. MicroRNA-mediated epigenetic silencing of sirtuin1 contributes to impaired angiogenic responses. Circ. Res. 2013, 113, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Liu, L.; Lee, M.Y.; Xu, C.; Liang, Y.; Man, R.Y.; Vanhoutte, P.M.; Wang, Y. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ. Res. 2010, 106, 1384–1393. [Google Scholar] [CrossRef]
- Li, P.; Wei, J.; Li, X.; Cheng, Y.; Chen, W.; Cui, Y.; Simoncini, T.; Gu, Z.; Yang, J.; Fu, X. 17β-Estradiol Enhances Vascular Endothelial Ets-1/miR-126-3p Expression: The Possible Mechanism for Attenuation of Atherosclerosis. J. Clin. Endocrinol. Metab. 2016, 102, 594–603. [Google Scholar] [CrossRef]
- Sharma, V.; Dogra, N.; Saikia, U.N.; Khullar, M. Transcriptional regulation of endothelial-to-mesenchymal transition in cardiac fibrosis: Role of myocardin-related transcription factor A and activating transcription factor 3. Can. J. Physiol. Pharmacol. 2017, 95, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Oliver, N.S.; Choudhary, P.; Levy, J.C.; Hindmarsh, P.; Matthews, D.R. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol. Ther. 2011, 13, 921–928. [Google Scholar] [CrossRef] [PubMed]


| Target Gene | Representative Transcript | Gene Name | Transcript Position | Predicted Consequential Pairing of Target Region Transcript (Top) and miRNA (Bottom) | Site Type |
|---|---|---|---|---|---|
| SIRT1 | ENST00000212015.6 | Sirtuin 1 | 507–513 3′UTR | 5′CACAAUUAUUU-UUAAACACUGGC 3′CUUGUCCAUCA-GACUUGUGACCC | 7mer-m8 |
| ETS1 | ENST00000531611.1 | ETS Proto-Oncogene 1, Transcription Factor | 2886–2893 3′UTR | 5′UGGUGGGUGGU-UUAUACACUGGA 3′CUUGUCUAUCA-GAUUUGUGACCC | 8mer |
| JAG1 | ENST00000254958.5 | Jagged Canonical Notch Ligand 1 | 135–141 3′UTR | 5′UUGACAAGCUG-GCUUACACUGGC 3′CUUGUCUAUCA-GAUUUGUGACCC | 7mer-m8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhashab, S.; Barber, R.; O’Neill, J.; Arden, C.; Weaver, J.U. Overexpression of miR-199b-5p in Colony Forming Unit-Hill’s Colonies Positively Mediates the Inflammatory Response in Subclinical Cardiovascular Disease Model: Metformin Therapy Attenuates Its Expression. Int. J. Mol. Sci. 2024, 25, 8087. https://doi.org/10.3390/ijms25158087
Bakhashab S, Barber R, O’Neill J, Arden C, Weaver JU. Overexpression of miR-199b-5p in Colony Forming Unit-Hill’s Colonies Positively Mediates the Inflammatory Response in Subclinical Cardiovascular Disease Model: Metformin Therapy Attenuates Its Expression. International Journal of Molecular Sciences. 2024; 25(15):8087. https://doi.org/10.3390/ijms25158087
Chicago/Turabian StyleBakhashab, Sherin, Rosie Barber, Josie O’Neill, Catherine Arden, and Jolanta U. Weaver. 2024. "Overexpression of miR-199b-5p in Colony Forming Unit-Hill’s Colonies Positively Mediates the Inflammatory Response in Subclinical Cardiovascular Disease Model: Metformin Therapy Attenuates Its Expression" International Journal of Molecular Sciences 25, no. 15: 8087. https://doi.org/10.3390/ijms25158087
APA StyleBakhashab, S., Barber, R., O’Neill, J., Arden, C., & Weaver, J. U. (2024). Overexpression of miR-199b-5p in Colony Forming Unit-Hill’s Colonies Positively Mediates the Inflammatory Response in Subclinical Cardiovascular Disease Model: Metformin Therapy Attenuates Its Expression. International Journal of Molecular Sciences, 25(15), 8087. https://doi.org/10.3390/ijms25158087







