Flow and On-Water Synthesis and Cancer Cell Cytotoxicity of Caffeic Acid Phenethyl Amide (CAPA) Derivatives
Abstract
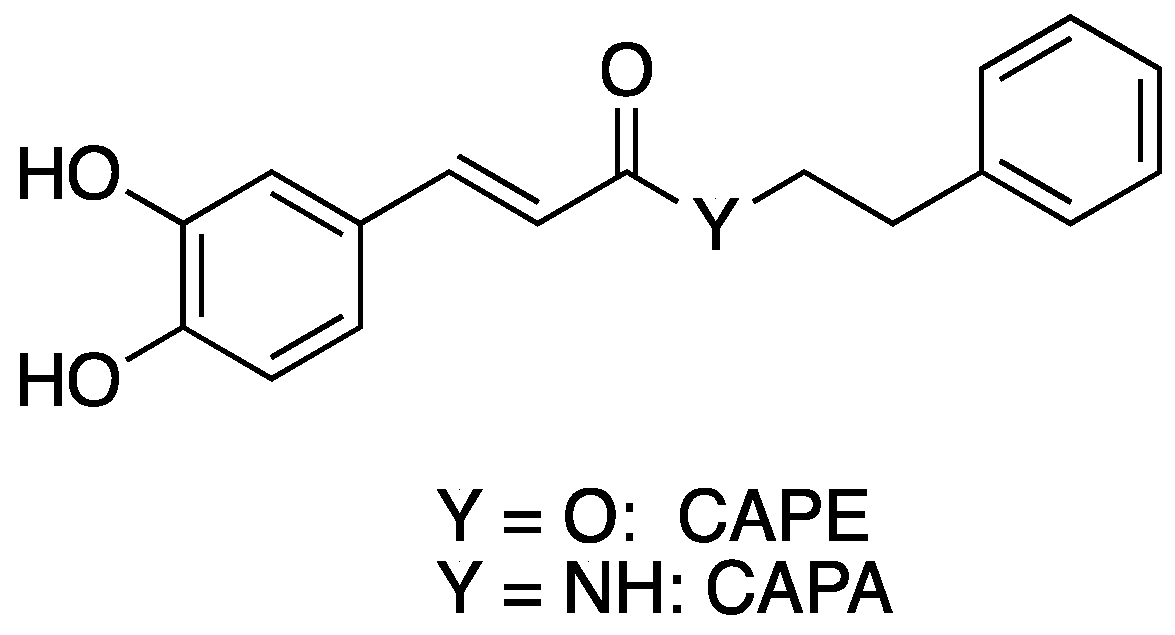
1. Introduction
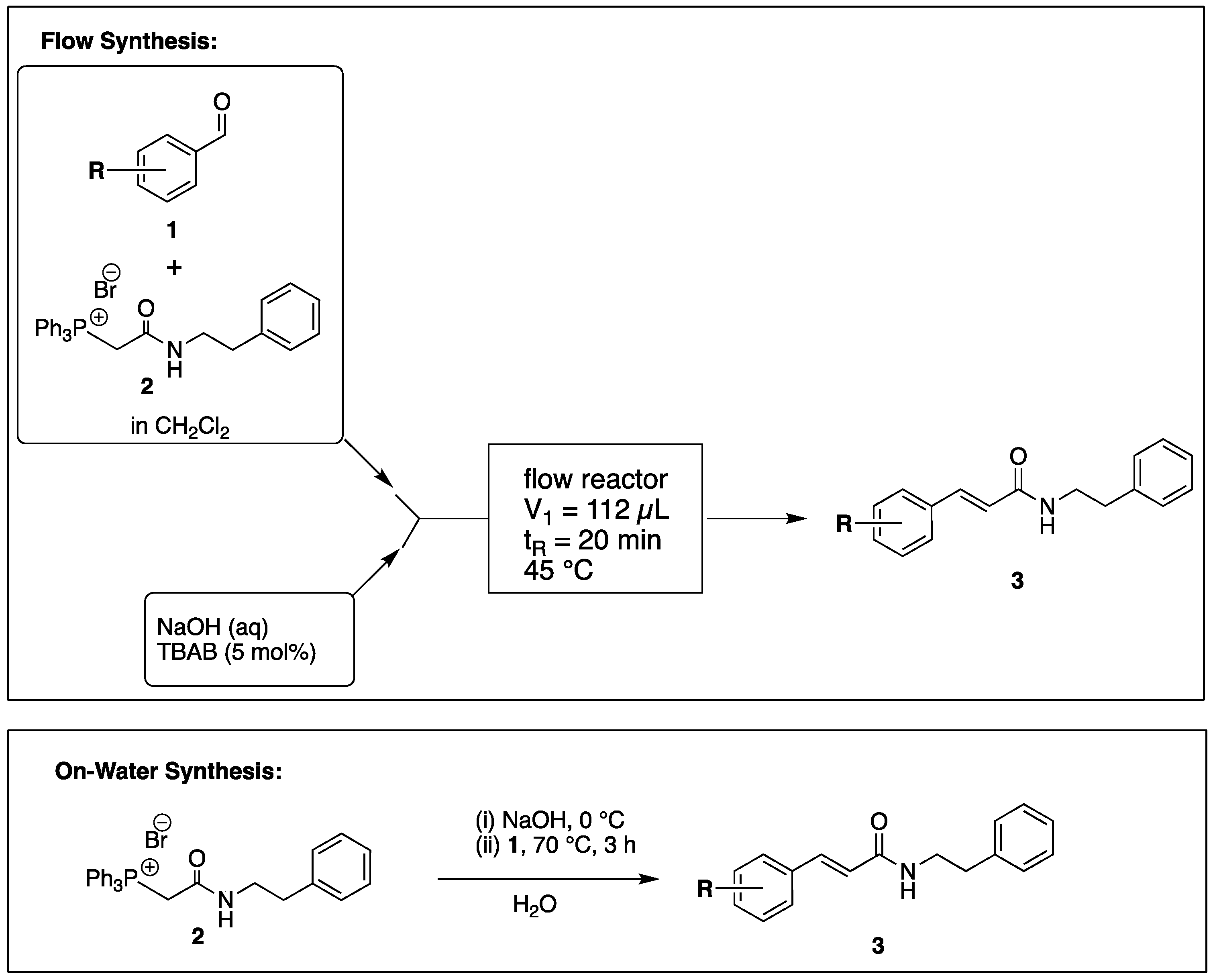
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olgierd, B.; Kamila, Z.; Anna, B.; Emilia, M. The Pluripotent Activities of Caffeic Acid Phenethyl Ester. Molecules 2021, 26, 1335. [Google Scholar] [CrossRef]
- Balaha, M.; De Filippis, B.; Cataldi, A.; di Giacomo, V. CAPE and neuroprotection: A review. Biomolecules 2021, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Erdemli, H.K.; Akyol, S.; Armutcu, F.; Akyol, O. Antiviral properties of caffeic acid phenethyl ester and its potential application. J. Intercult. Ethnopharmacol. 2015, 4, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Pittala, V.; Salerno, L.; Romeo, G.; Acquaviva, R.; Di Giacomo, C.; Sorrenti, V. Therapeutic potential of caffeic acid phenethyl ester (cape) in diabetes. Curr. Med. Chem. 2018, 25, 4827–4836. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Omar, H.A.; Azab, S.S.; Khalifa, A.E.; Abdel-Naim, A.B.; Abdel-Rahman, S.Z. Caffeic Acid Phenethyl Ester: A Review of Its Antioxidant Activity, Protective Effects against Ischemia-reperfusion Injury and Drug Adverse Reactions. Crit. Rev. Food Sci. Nutr. 2016, 56, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pang, J.; Maffucci, J.A.; Pade, D.S.; Newman, R.A.; Kerwin, S.M.; Bowman, P.D.; Stavchansky, S. Pharmacokinetics of caffeic acid phenethyl ester and its catechol-ring fluorinated derivative following intravenous administration to rats. Biopharm. Drug Dispos. 2009, 30, 221–228. [Google Scholar] [CrossRef]
- Mucsi, Z.; Chass, G.A.; Csizmadia, I.G. Amidicity Change as a Significant Driving Force and Thermodynamic Selection Rule of Transamidation Reactions. A Synergy between Experiment and Theory. J. Phys. Chem. B 2008, 112, 7885–7893. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Marriner, G.A.; Wang, X.; Bowman, P.D.; Kerwin, S.M.; Stavchansky, S. Synthesis of a Series of Caffeic Acid Phenethyl Amide (CAPA) Fluorinated Derivatives: Comparison of Cytoprotective Effects to Caffeic Acid Phenethyl Ester (CAPE). Bioorg. Med. Chem. 2010, 18, 5032–5038. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, L.H.; Maillet, J.; LeBlanc, L.M.; Jean-François, J.; Touaibia, M.; Flamand, N.; Surette, M.E. Caffeic Acid Phenethyl Ester and Its Amide Analogue Are Potent Inhibitors of Leukotriene Biosynthesis in Human Polymorphonuclear Leukocytes. PLoS ONE 2012, 7, e31833. [Google Scholar] [CrossRef]
- Dai, L.; Zang, C.; Tian, S.; Liu, W.; Tan, S.; Cai, Z.; Ni, T.; An, M.; Li, R.; Gao, Y.; et al. Design, Synthesis, and Evaluation of Caffeic Acid Amides as Synergists to Sensitize Fluconazole-Resistant Candida Albicans to Fluconazole. Bioorg. Med. Chem. Lett. 2015, 25, 34–37. [Google Scholar] [CrossRef]
- David, S.; Mandabi, A.; Uzi, S.; Aharoni, A.; Meijler, M.M. Mining Plants for Bacterial Quorum Sensing Modulators. ACS Chem. Biol. 2018, 13, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Firdaus; Soekamto, N.H.; Seniwati; Islam, M.F.; Sultan. Phenethyl Ester and Amide of Ferulic Acids: Synthesis and Bioactivity against P388 Leukemia Murine Cells. J. Phys. Conf. Ser. 2018, 979, 012016. [Google Scholar] [CrossRef]
- Beauregard, A.-P.; Harquail, J.; Lassalle-Claux, G.; Belbraouet, M.; Jean-Francois, J.; Touaibia, M.; Robichaud, G.A. CAPE Analogs Indue Growth Arrest and Apoptosis in Breast Cancer Cells. Molecules 2015, 20, 12576–12589. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Lassalle-Claux, G.; Hogan, L.; Vaillancourt, E.; Selka, A.; Luiker, K.; Kim, M.J.; Touaibia, M.; Reiman, T. Antimyeloma Potential of Caffeic Acid Phenethyl Ester and Its Analogues through Sp1 Mediated Downregulation of IKZF1-IRF4-MYC Axis. J. Nat. Prod. 2020, 83, 3526–3535. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.T.; Clabault, H.; Patton, C.; Lassalle-Claux, G.; Jean-Francois, J.; Pare, A.F.; Herbert, M.J.G.; Surette, M.E.; Touaibia, M. Antiproliferative, Antiandrogenic and Cytotoxic Effects of Novel Caffeic Acid Derivatives in LNCaP Human Androgen-dependent Prostate Cancer Cells. Bioorg. Med. Chem. 2013, 23, 7192–7193. [Google Scholar] [CrossRef] [PubMed]
- De Armas-Ricard, M.; Ruiz-Reyes, E.; Ramirez-Rodriguez, O. Caffeates and Caffeamides: Synthetic Methodologies and Their Antioxidant Properties. Int. J. Med. Chem. 2019, 2019, 2592609. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.J.; Ishitani, H.; Saito, Y.; Laroche, B.; Kobayashi, S. Reworking Organic Synthesis for the Modern Age: Synthetic Strategies Based on Continuous-Flow Addition and Condensation Reactions with Heterogeneous Catalysts. J. Org. Chem. 2020, 85, 5132–5145. [Google Scholar] [CrossRef] [PubMed]
- Riccaboni, M.; La Porta, E.; Martorana, A.; Attanasio, R. Effect of Phase Transfer Chemistry, Segmented Fluid Flow, and Sonication on the Synthesis of Cinnamic Esters. Tetrahedron 2010, 66, 4032–4039. [Google Scholar] [CrossRef]
- Baxendale, I.R.; Griffiths-Jones, C.M.; Ley, S.V.; Tranmer, G.K. Preparation of the Neolignan Natural Product Grossamide by a Continuous-Flow Process. Synlett 2006, 2006, 427–430. [Google Scholar] [CrossRef]
- Achanta, S.; Liautard, V.; Paugh, R.; Organ, M.G. The Development of a General Strategy for the Synthesis of Tyramine-Based Natural Products by Using Continuous Flow Techniques. Chem. A Eur. J. 2010, 16, 12797–12800. [Google Scholar] [CrossRef]
- Russell, M.G.; Warren, S. Synthesis of New Water-soluble Phosphonium Salts and Their Wittig Reactions in Water. Chem. Soc. Perkin Trans. 1 2000, 4, 505–513. [Google Scholar] [CrossRef]
- Dambacher, J.; Zhao, W.; El-Batta, A.; Arness, R.; Jiang, C.; Berdgahl, M. Water is an Efficient Medium for Wittig Reactions Employing Stabilized Ylides and Aldehydes. Tetrahedron Lett. 2005, 46, 4473–4477. [Google Scholar] [CrossRef]
- Javaherian, M.; Movaheditabar, P. On-water Biphasic Organic Synthesis. J. Iran. Chem. Soc. 2023, 20, 2103–2125. [Google Scholar] [CrossRef]
- Sidoryk, K.; Jaromin, A.; Filipczak, N.; Cmoch, P.; Cybulski, M. Synthesis and Antioxidant Activity of Caffeic Acid Derivatives. Molecules 2018, 23, 2199. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-T.; Ma, W.; Yen, P.; Xie, J.-G.; Han, J.; Frenkel, K.; Grunberger, D.; Conney, A.H. Inhibitory effects of caffeic acid phenethyl ester (CAPE) on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin and the synthesis of DNA, RNA, and protein in HeLa cells. Carcinogenesis 1996, 17, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.H.; Chu, C.C.; Hung, M.W.; Lee, H.J.; Hsu, H.J.; Chang, T.C. Caffeic acid phenethyl ester induces E2F-1-mediated growth inhibition and cell-cycle arrest in human cervical cancer cells. FEBS J. 2013, 280, 2581–2593. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nesterenko, V.; Putt, K.S.; Hergenrother, P.J. Identification from a Combinatorial Library of a Small Molecule that Selectively Induces Apoptosis in Cancer Cells. J. Am. Chem. Soc. 2003, 125, 14672–14673. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wu, P.-Y.W.; Chen, C.-W.; Lyu, J.-L.; Liu, Y.-J.; Wen, K.-C.; Lin, C.-Y.; Kuo, Y.-H.; Chiang, H.-M. Protective Effects and Mechanisms of N-Phenethyl Caffeamide from UVA-Induced Skin Damage in Human Epidermal Keratinocytes through Nrf2/HO-1 Regulation. Int. J. Mol. Sci. 2019, 20, 164. [Google Scholar] [CrossRef]
- Chen, L.; Jin, Y.; Chen, H.; Sun, C.; Fu, W.; Zheng, L.; Lu, M.; Chen, P.; Chen, G.; Zhang, Y.; et al. Discovery of caffeic acid phenethyl ester derivatives as novel myeloid differentiation protein 2 inhibitors for treatment of acute lung injury. Eur. J. Med. Chem. 2018, 143, 361–375. [Google Scholar] [CrossRef]
- Khaldoun, K.; Safer, A.; Saidi-Besbes, A.; Carboni, B.; Le Gueverl, R.; Car-reaux, F. An Efficient Solvent-Free Microwave-Assisted Synthesis of Cinnamamides by Amidation Reaction Using Phenylboronic Acid/Lewis Base Co-catalytic Systems. Synthesis 2019, 51, 3891–3900. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
| Aldehyde (1) | Product (3) | Method | Yield |
|---|---|---|---|
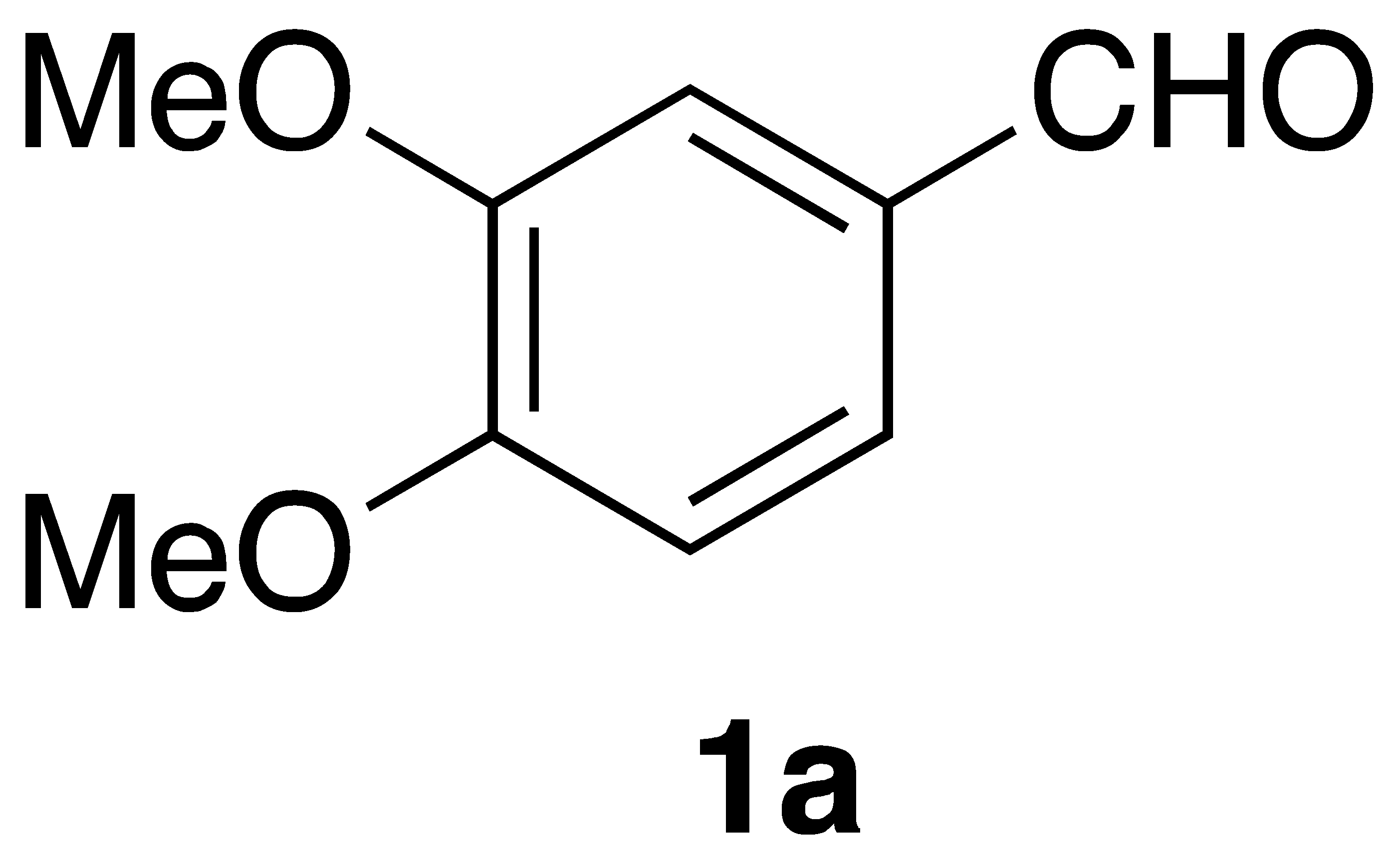 |  | Flow | 24% |
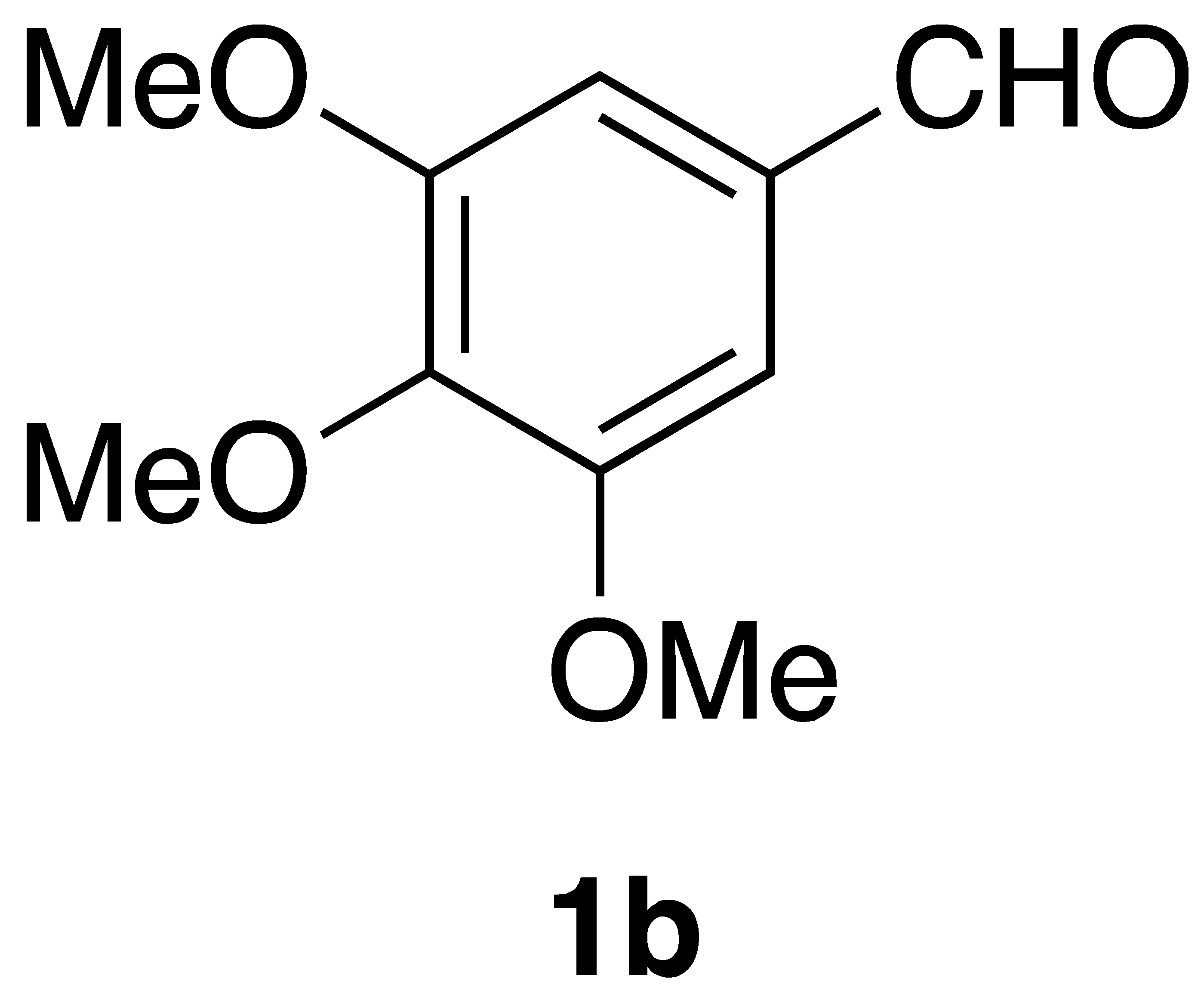 | 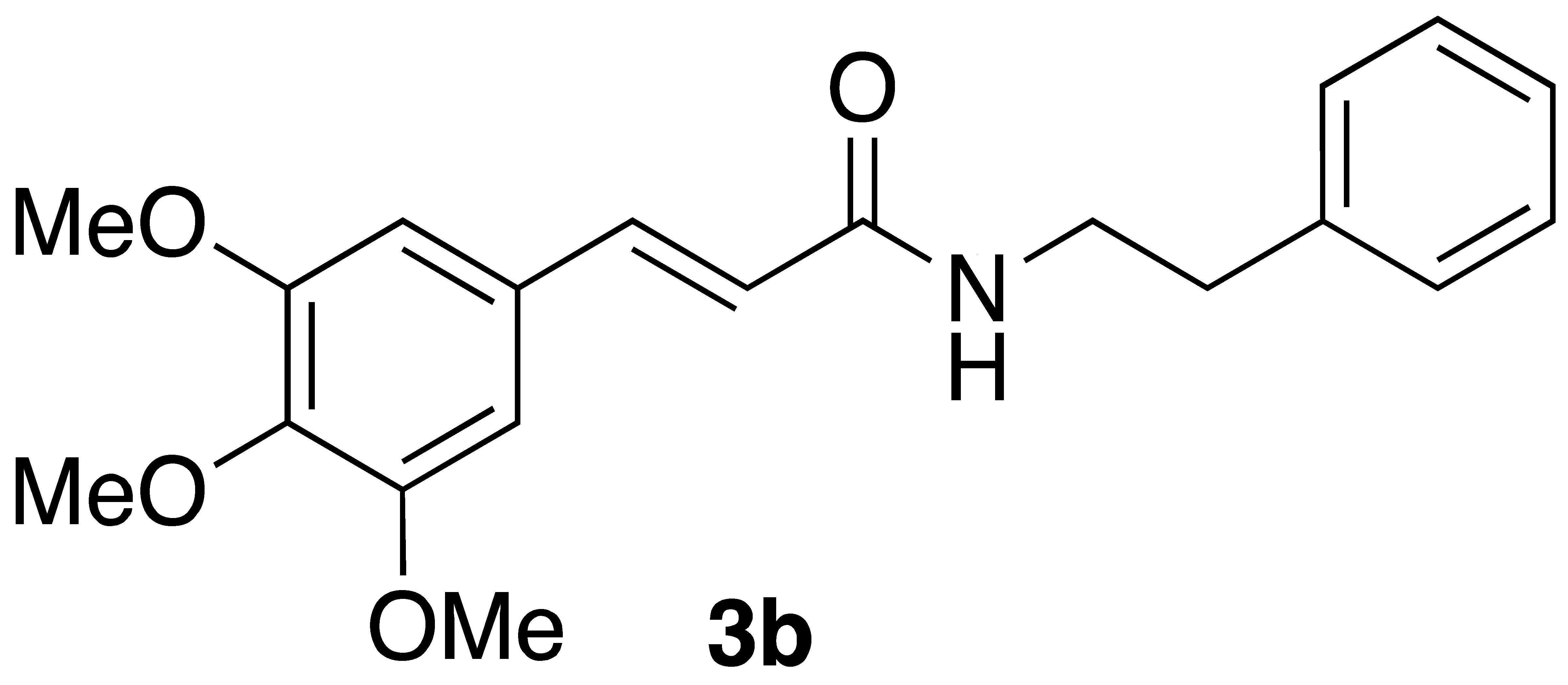 | Flow | 52% |
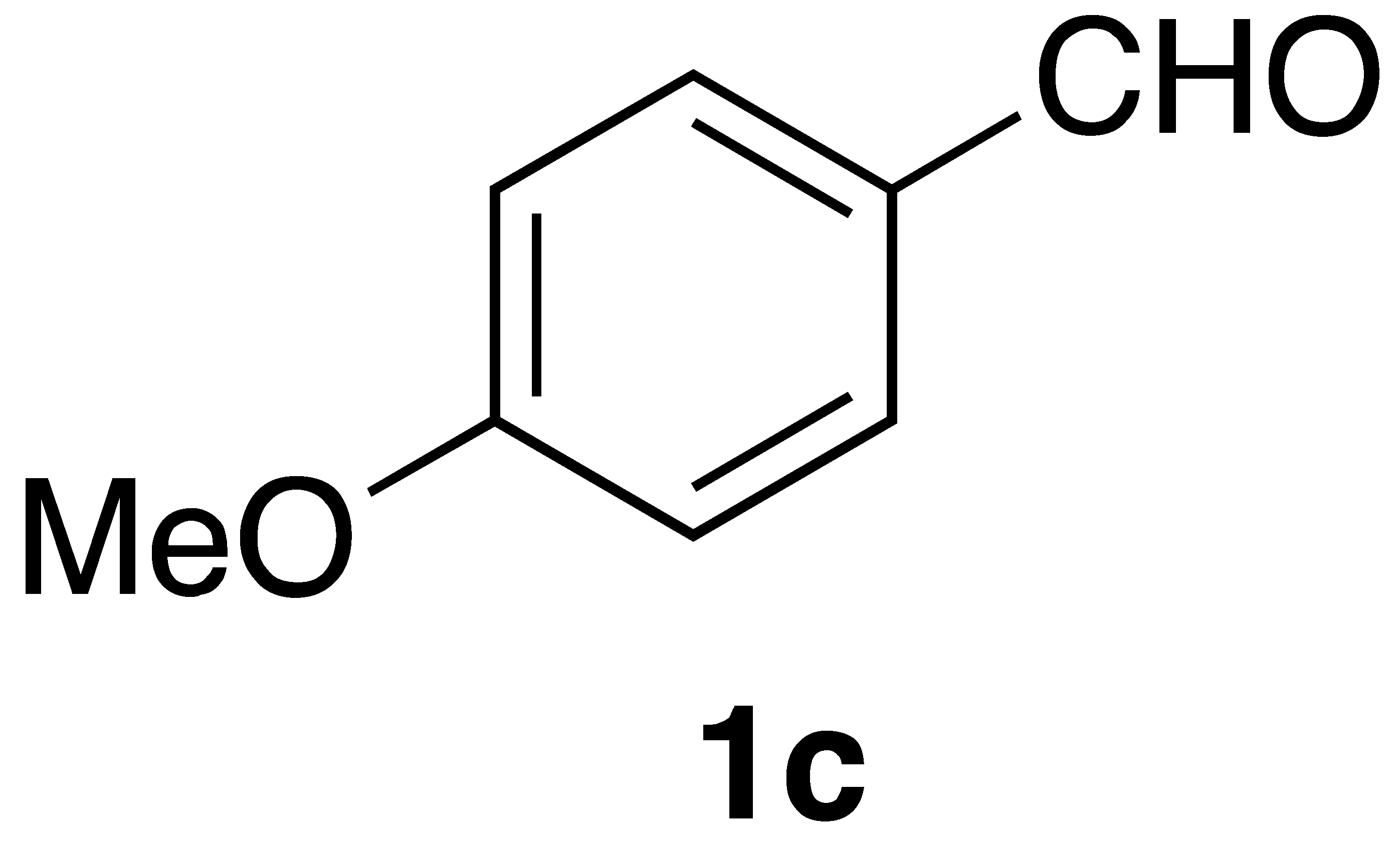 | 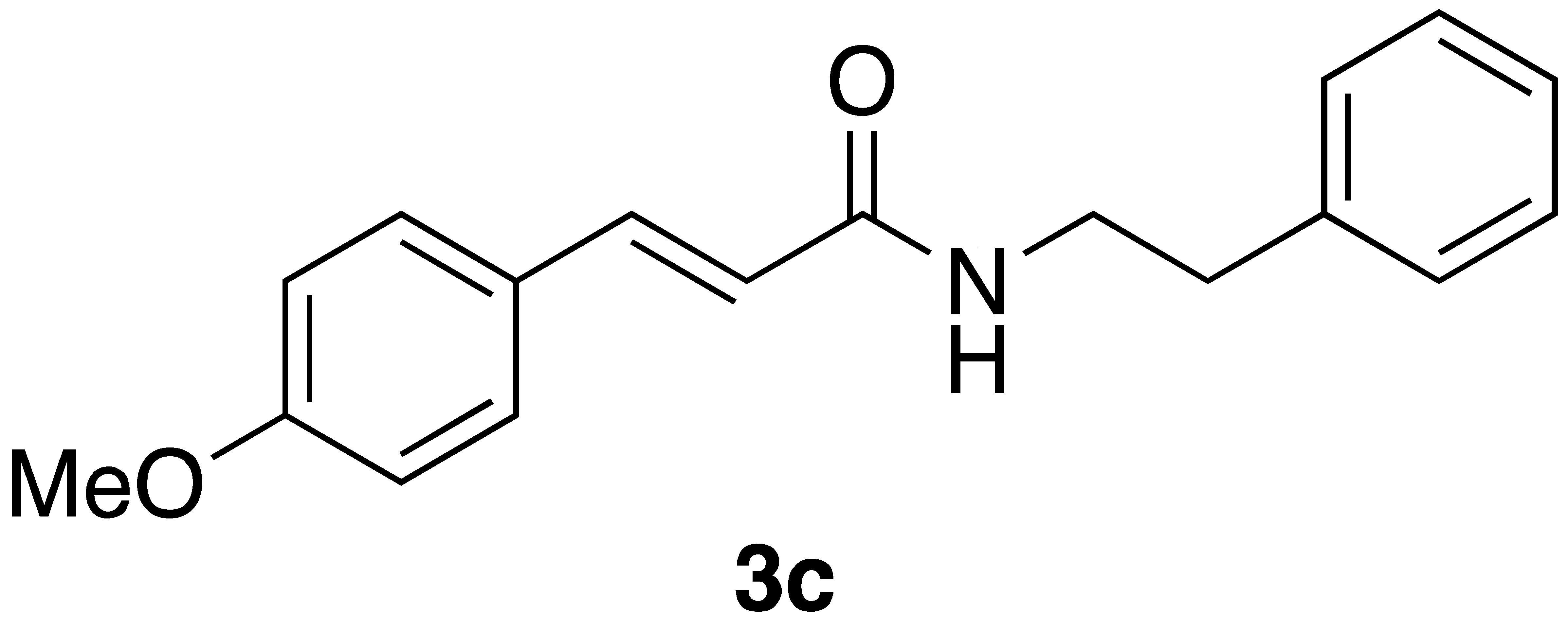 | Flow | 12% |
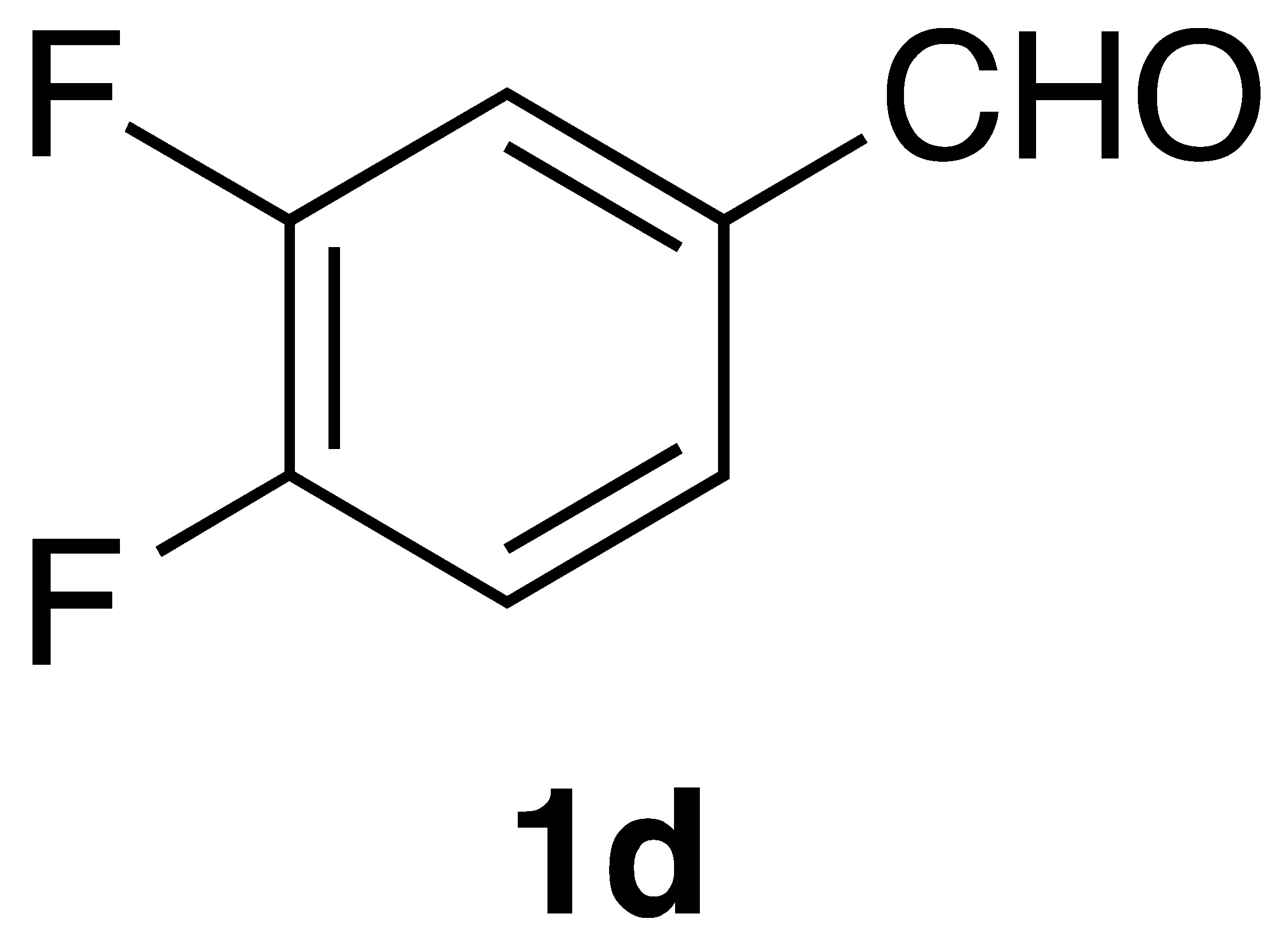 | 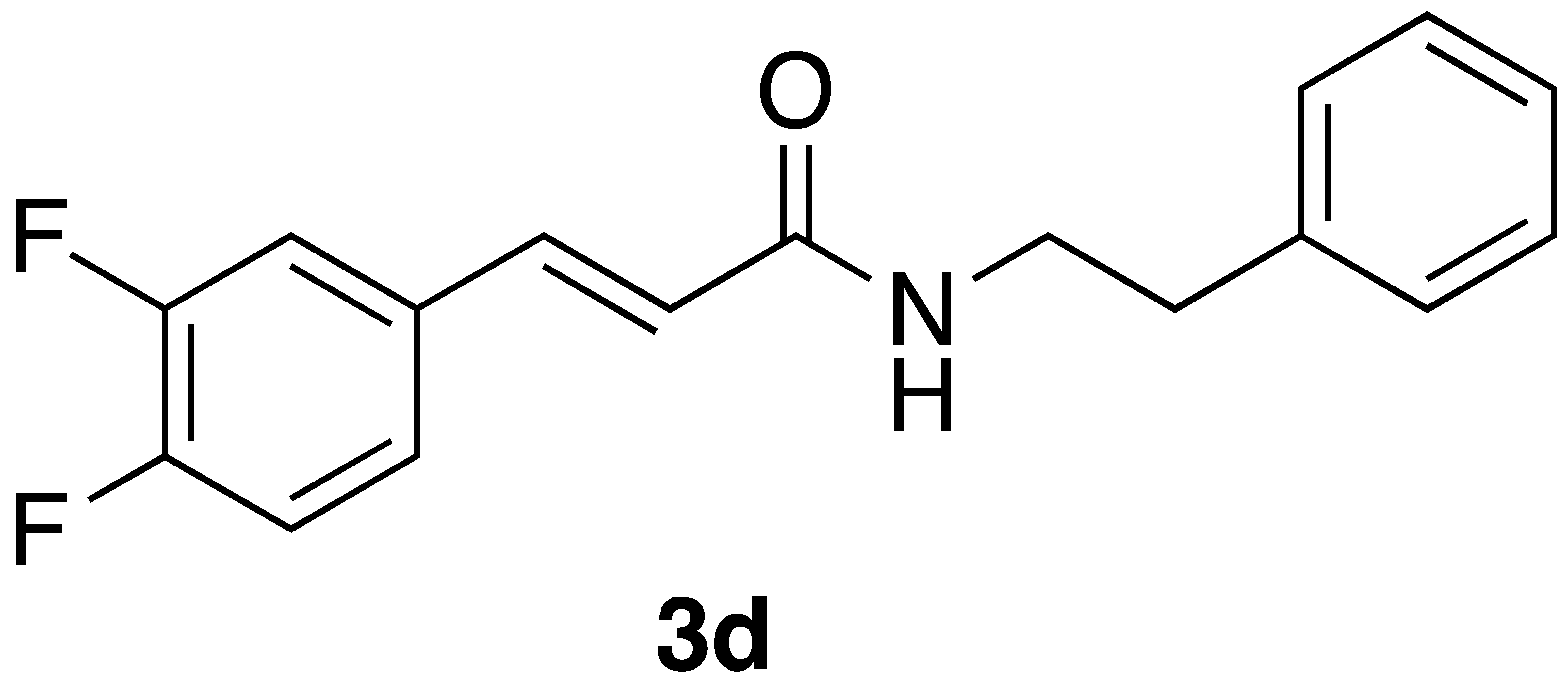 | Flow | 14% |
 | 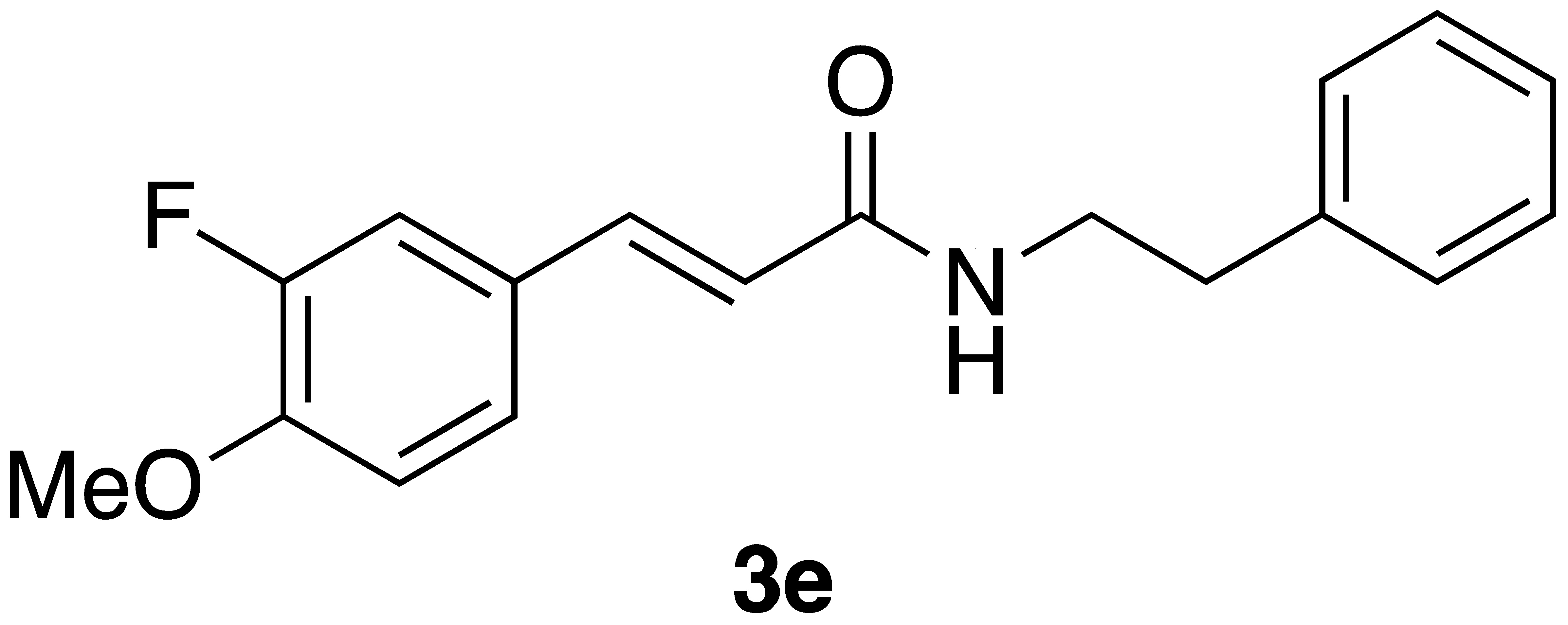 | Flow | 37% |
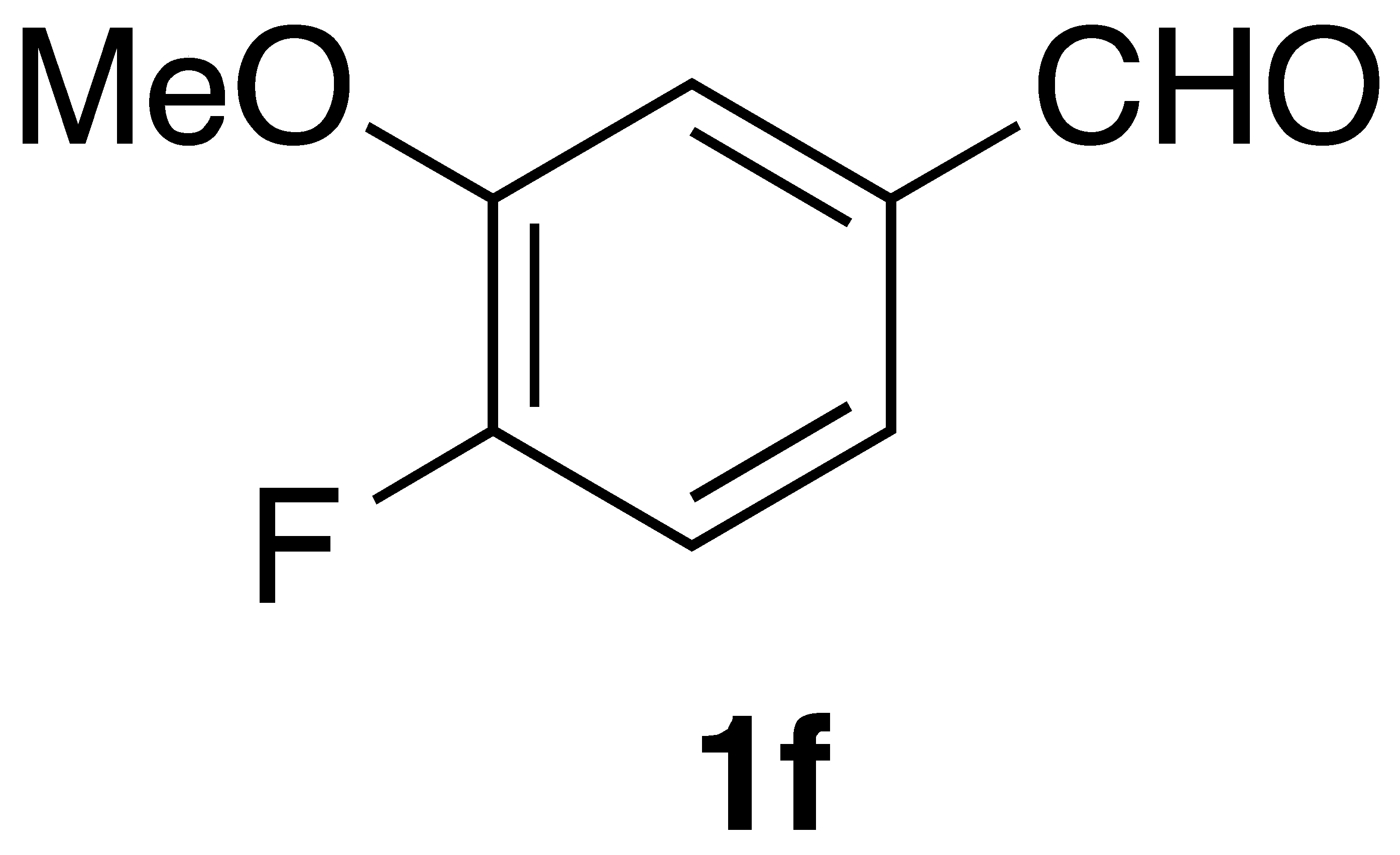 | 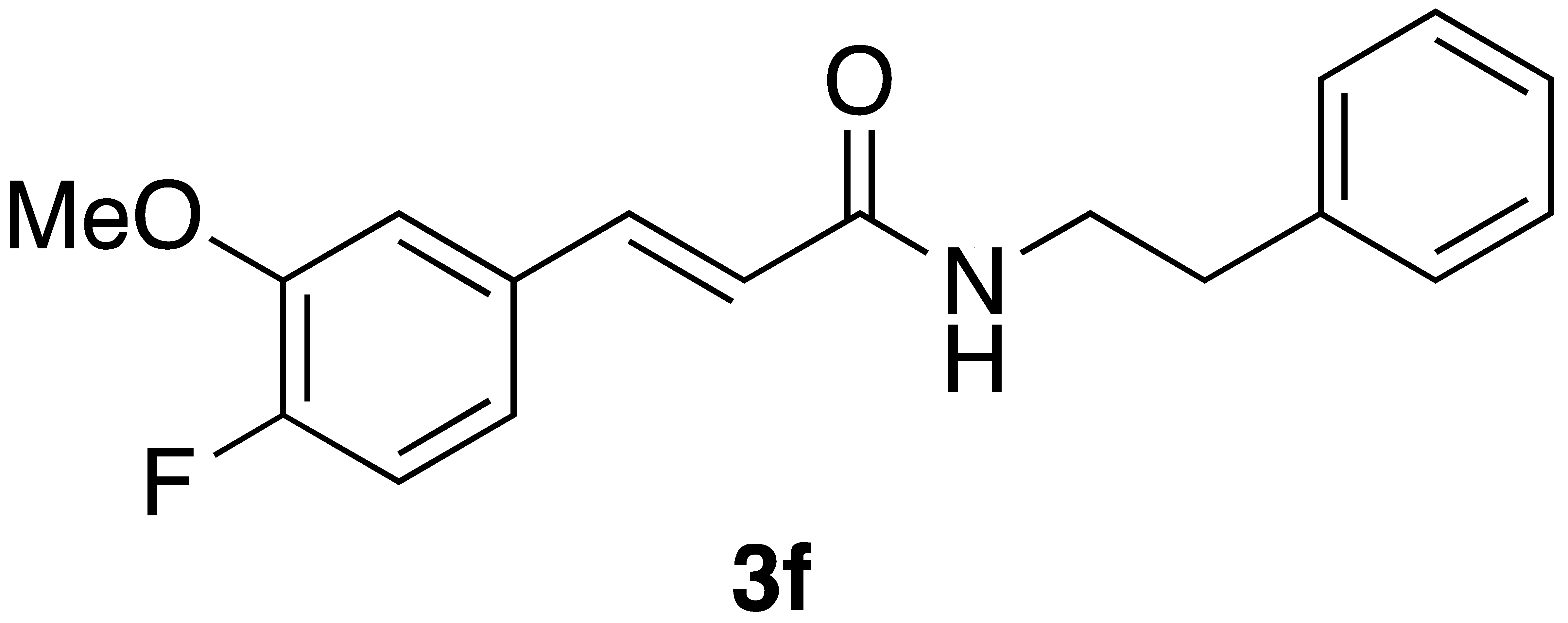 | Flow On-water | 33% 49% |
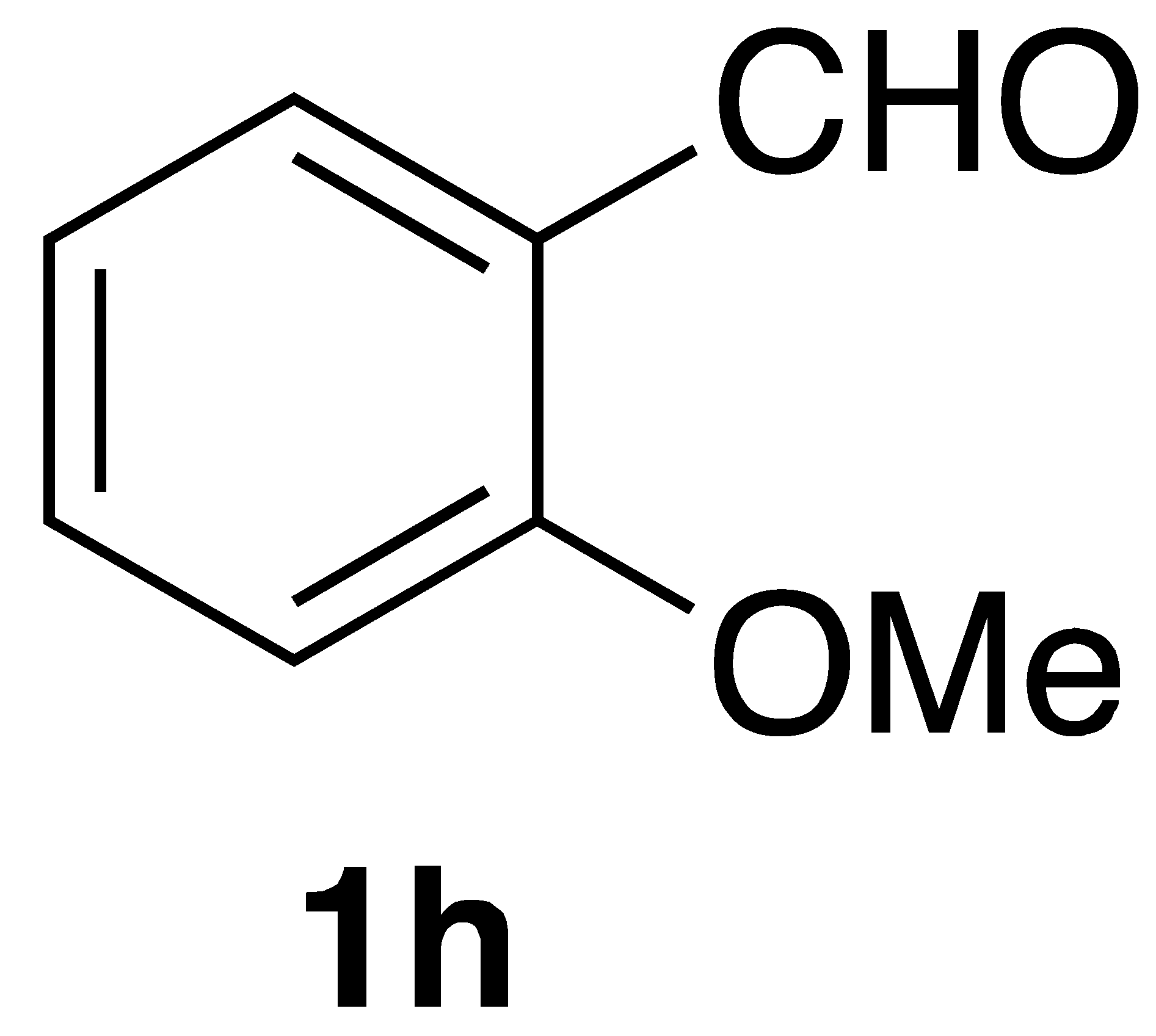
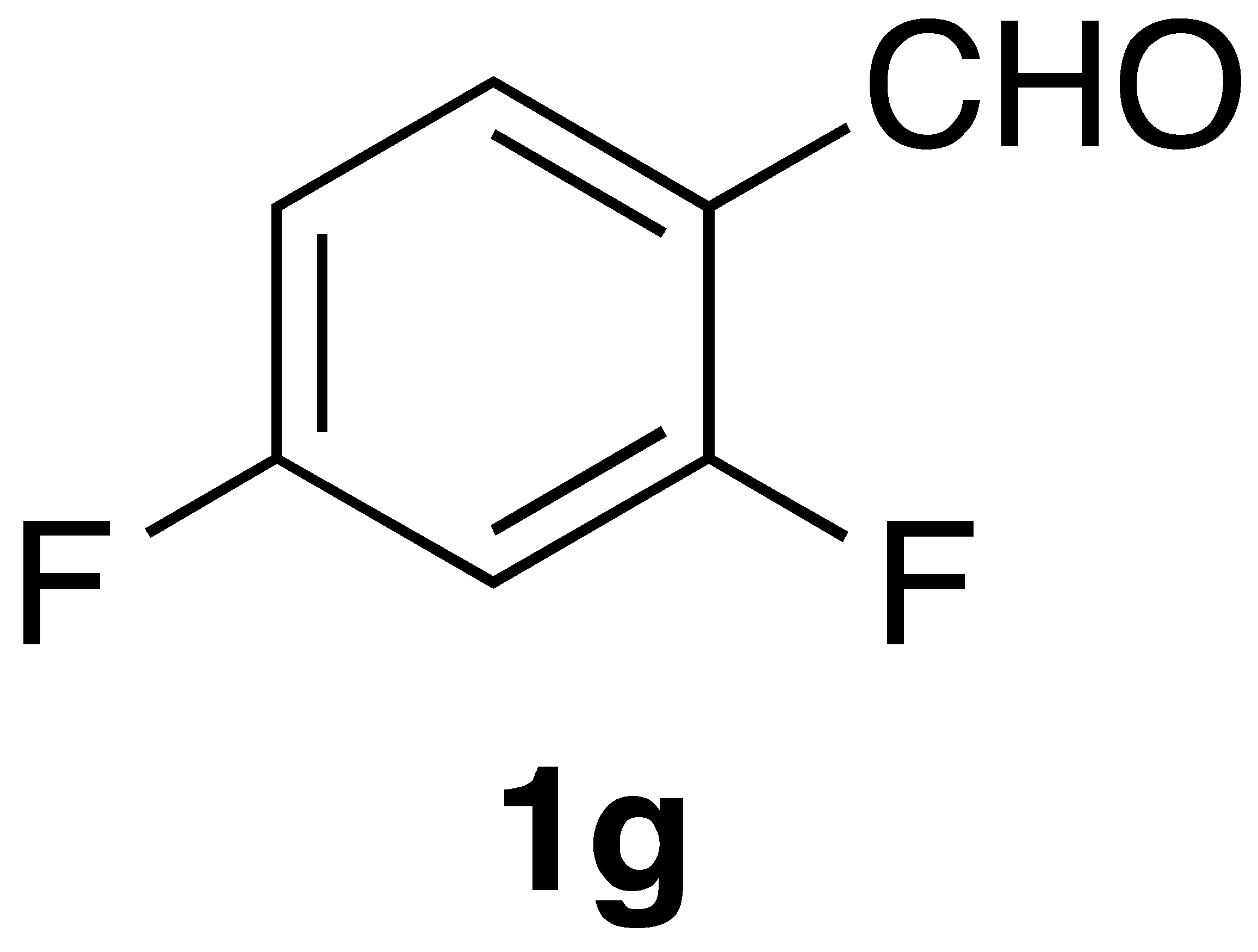 |  | On-water | 52% |
 |  | On-water | 41% |
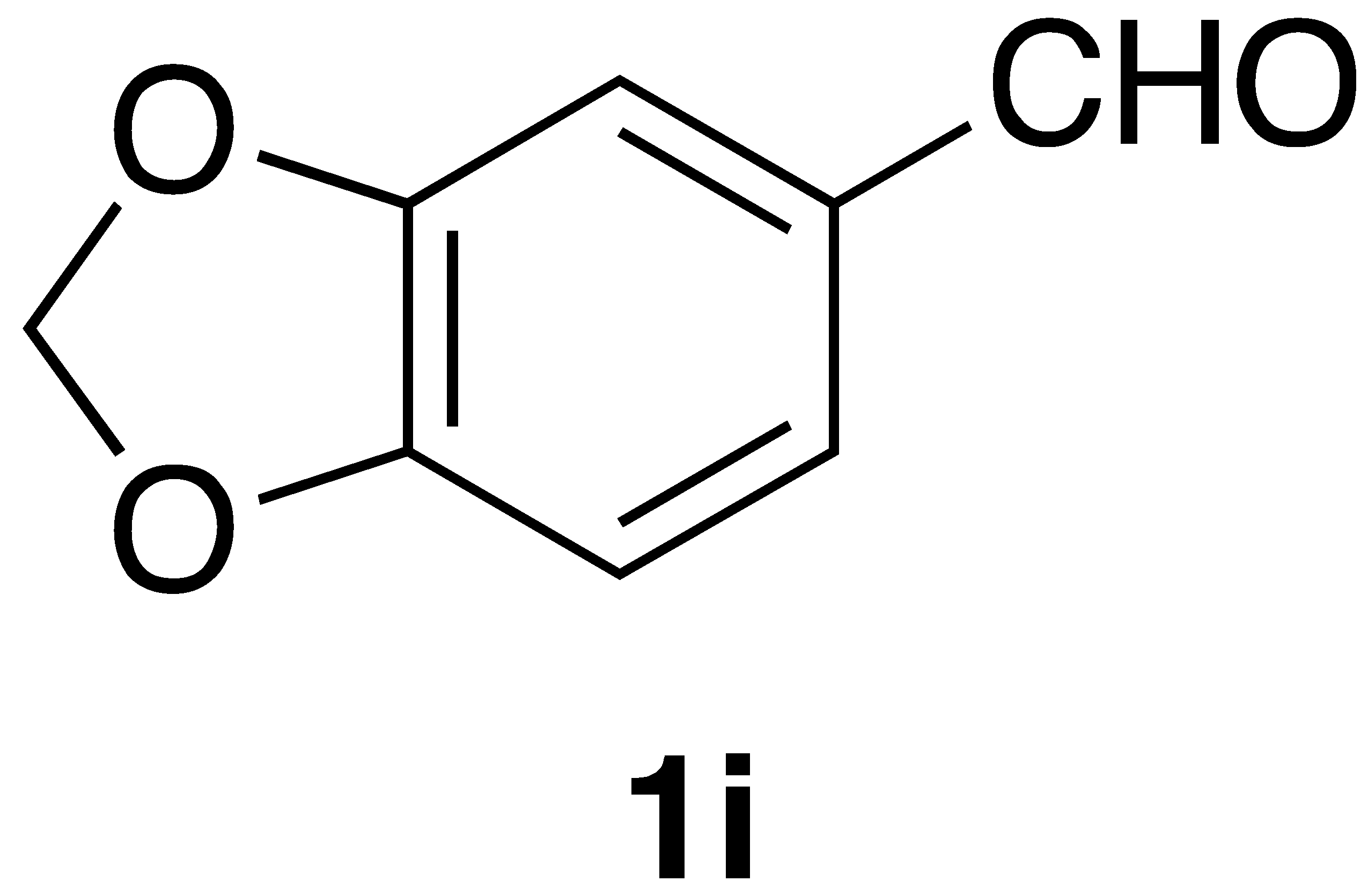 |  | On-water | 66% |
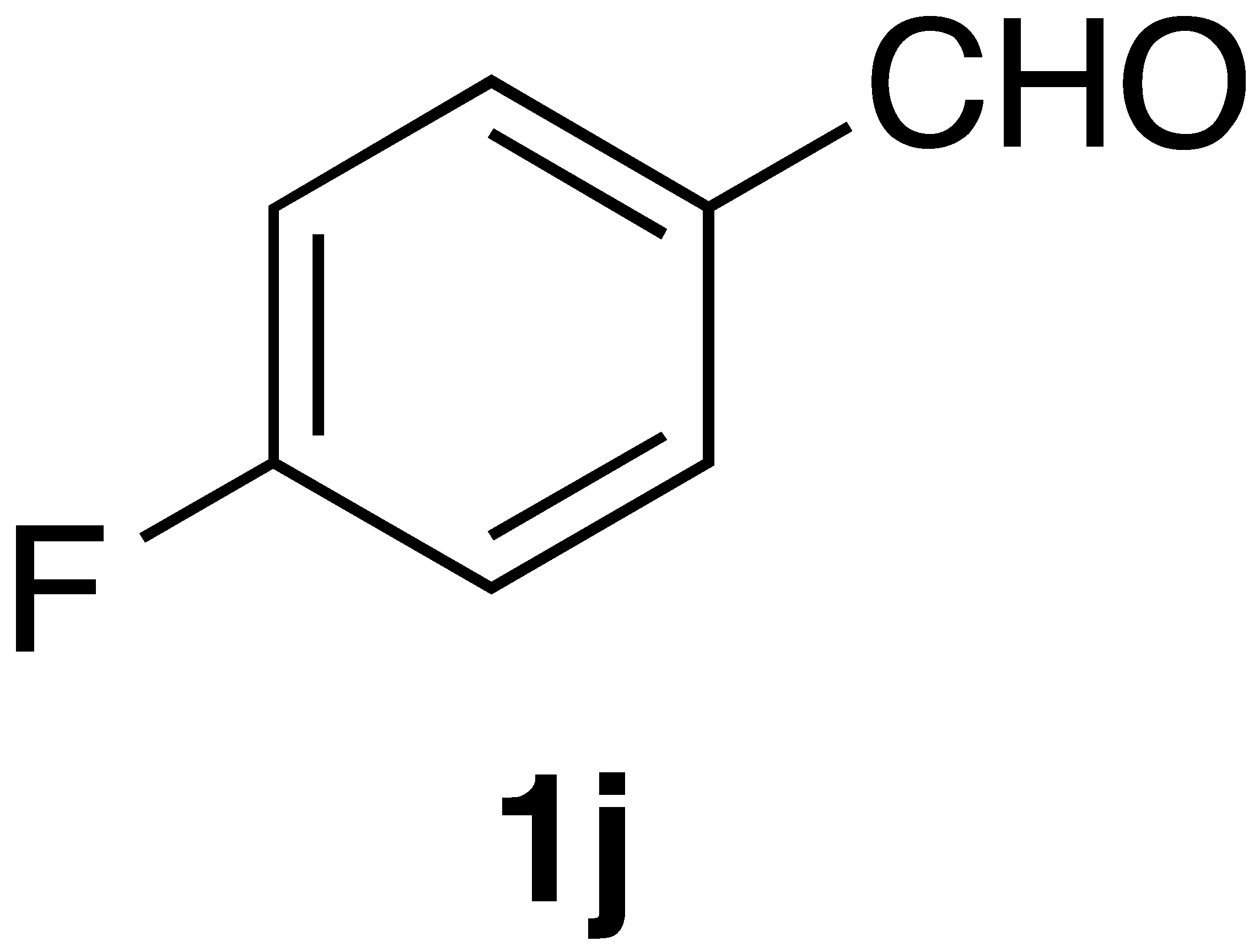 | 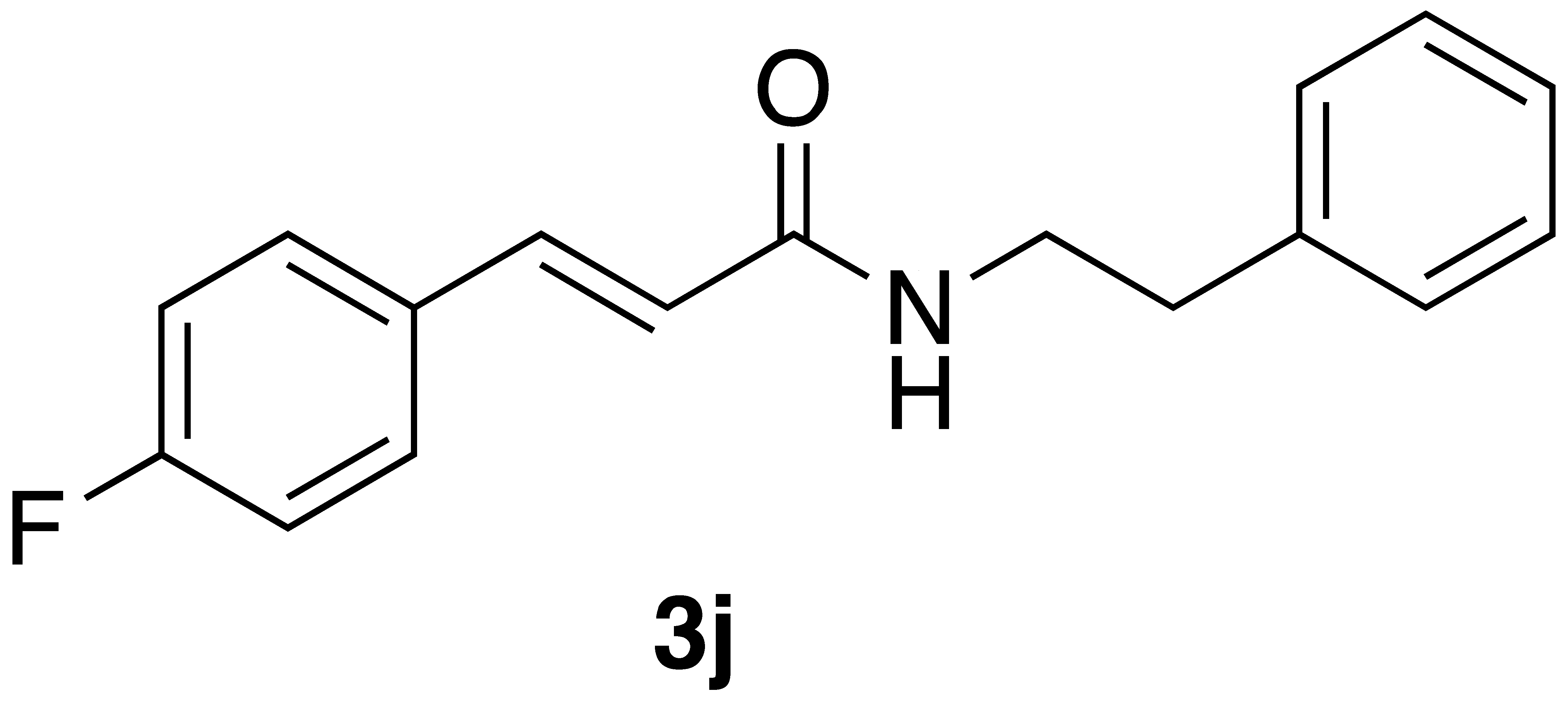 | On-water | 55% |
| Compound | HeLa IC50 (µM) | BE(2)-C IC50 (µM) |
|---|---|---|
| CAPE | 32 ± 12 | 5 ± 2 |
| CAPA | 112 ± 17 | 21 ± 2 |
| 3a | >600 | >600 |
| 3b | >600 | nd 1 |
| 3c | >600 | nd |
| 3d | >600 | >600 |
| 3e | >600 | nd |
| 3f | 63 ± 29 | 91 ± 52 |
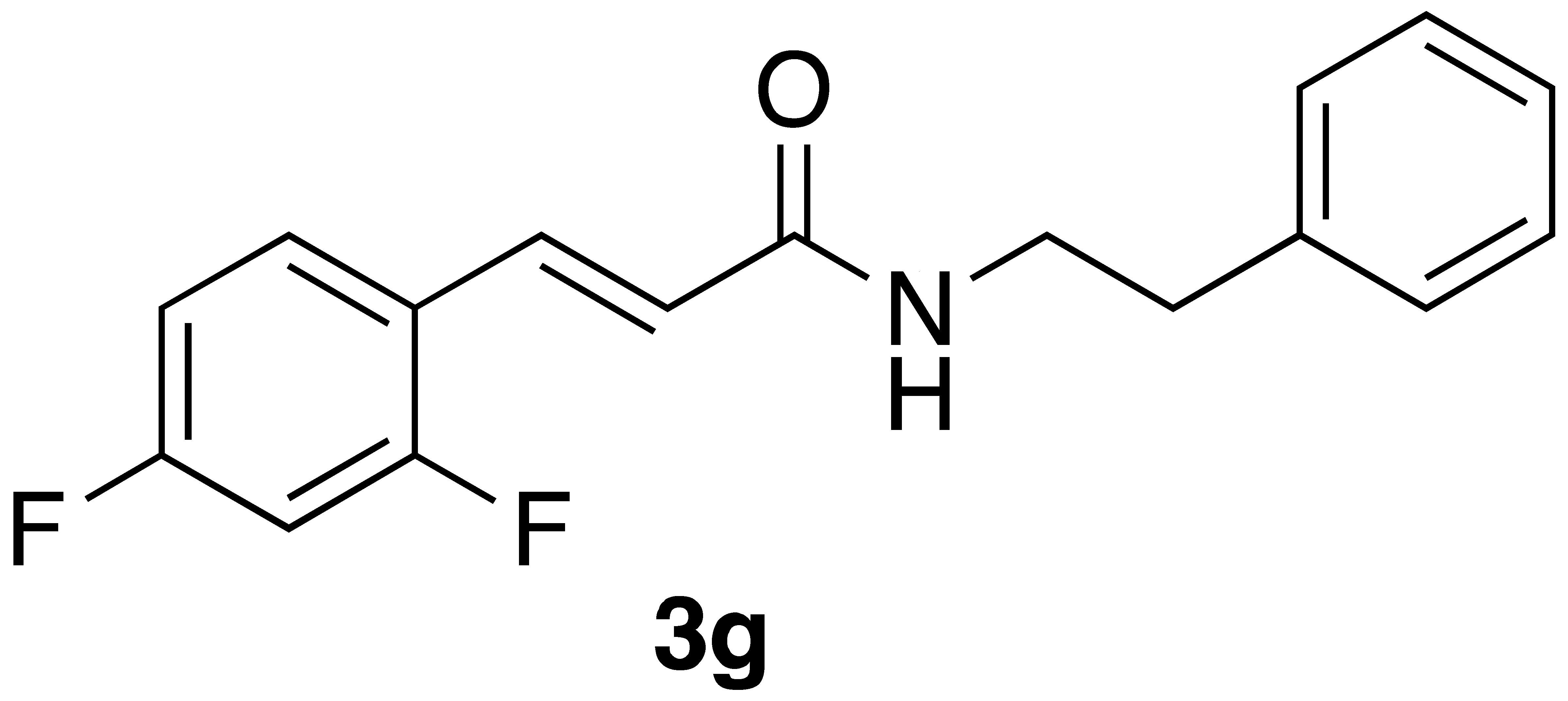
| 3g | 140 ± 58 | 92 ± 48 |
| 3h | 600 ± 66 | 163.8 ± 0.6 |
| 3i | >600 | >600 |
| 3j | >600 | >600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saucedo, A.; Subbarao, M.; Jemal, M.; Mesa-Diaz, N.L.; Smith, J.L.; Vernaza, A.; Du, L.; Kerwin, S.M. Flow and On-Water Synthesis and Cancer Cell Cytotoxicity of Caffeic Acid Phenethyl Amide (CAPA) Derivatives. Int. J. Mol. Sci. 2024, 25, 8051. https://doi.org/10.3390/ijms25158051
Saucedo A, Subbarao M, Jemal M, Mesa-Diaz NL, Smith JL, Vernaza A, Du L, Kerwin SM. Flow and On-Water Synthesis and Cancer Cell Cytotoxicity of Caffeic Acid Phenethyl Amide (CAPA) Derivatives. International Journal of Molecular Sciences. 2024; 25(15):8051. https://doi.org/10.3390/ijms25158051
Chicago/Turabian StyleSaucedo, Anthony, Muppidi Subbarao, Mauricio Jemal, Nakya L. Mesa-Diaz, Jadyn L. Smith, Alexandra Vernaza, Liqin Du, and Sean M. Kerwin. 2024. "Flow and On-Water Synthesis and Cancer Cell Cytotoxicity of Caffeic Acid Phenethyl Amide (CAPA) Derivatives" International Journal of Molecular Sciences 25, no. 15: 8051. https://doi.org/10.3390/ijms25158051
APA StyleSaucedo, A., Subbarao, M., Jemal, M., Mesa-Diaz, N. L., Smith, J. L., Vernaza, A., Du, L., & Kerwin, S. M. (2024). Flow and On-Water Synthesis and Cancer Cell Cytotoxicity of Caffeic Acid Phenethyl Amide (CAPA) Derivatives. International Journal of Molecular Sciences, 25(15), 8051. https://doi.org/10.3390/ijms25158051






