Fractalkine in Health and Disease
Abstract
1. The Chemokine CX3CL1, Fractalkine
2. Soluble Fractalkine
3. Fractalkine Receptor and Signal Transduction
4. Integrins
5. Biological Functions of FKN
6. Fractalkine in Cancer
| FKN Function | Cancer Type and Model | Mechanisms | References |
|---|---|---|---|
| Anti-tumor | Murine lung cancer 3LL cells | CD8 and NK cells | [64,65] |
| Anti-tumor | Murine EL4 lymphoma cells | NK cells | [66] |
| Anti-tumor and good prognosis | NSCLC patients and murine lung cancer cells | NK cells, reduced proliferation of cancer cells | [67] |
| Good prognosis | Human colorectal cancer | TIL infiltration | [68] |
| Anti-tumor | Murine NXS2 neuroblastoma | Combination with IL-2 therapy | [69] |
| Anti-tumor | Murine B16F10 melanoma and colon-26 cancer models with DC-FKN transfer | CD4 and CD8 T cells | [70] |
| Good prognosis | Human gastric adenocarcinoma and hepatocellular carcinoma; FKN expression | Correlation with expression | [71] |
| Anti-tumor | Murine hepatocellular carcinoma MM45T.Li cells | CD4 and CD8 T cells | [72] |
| Anti-tumor | Murine C26 colorectal cancer model | CD4 and CD8 T cells | [73] |
| Anti-tumor | Human colorectal cancer cells in mouse xenograft | CTL activities | [74] |
| Good prognosis | Human breast carcinoma | Elevated expression, CD8, DC, and NK infiltration | [76] |
| Good prognosis | Human soft tissue sarcomas | Elevated expression, reduced proliferation of cancer cells | [77] |
| Worse prognosis | NSCLC patients with a history of smoking | Enhanced cancer cell invasion | [78] |
| Good prognosis | NSCLC patients | Elevated mRNA expression in tumors | [79] |
| Good prognosis | Glioma patients | NK recruitment and activity | [80] |
| Good prognosis | Breast cancer | T and NK cell recruitment, synergy with trastuzumab | [81] |
| Anti-tumor | Mouse breast cancer | CD8 T cells | [82] |
| Anti-tumor | Human melanoma | As a therapy associated with TCR-modified T cell transfer | [83] |
| Worse prognosis | Stomach, liver, and urothelial cancer | Correlation studies | [41] |
| Worse prognosis | Testis cancer and prostate cancer | Elevated FKN–CX3CR1 signaling axis | [84,87,88,89,90] |
| Worse prognosis | Breast cancer | Elevated FKN–CX3CR1 signaling axis | [46,85,86] |
| Pro-tumor effects | Murine B16 melanoma | Silencing of surface FKN delays tumor growth | [91] |
| Worse prognosis | Human multiple myeloma patients and cell lines | Elevated expression in bone marrow and in tumor cells | [92,93] |
| Worse prognosis | Human gastric cancer samples and cell lines | Elevation of CX3CR1 and FKN in cancer cells | [94,95] |
| Pro-tumor | Human pancreatic cancer | Resistance to apoptosis, reprogramming of glucose metabolism | [96,97,98] |
| Pro-tumor and worse prognosis | Human ovarian carcinoma | Enhanced proliferation of cancer cells through AKT activation | [99] |
| Pro-tumor | Human leukemia | Invasion of cancer cells | [100] |
| Worse prognosis | Human clear-cell renal carcinoma | Increased CX3CR1 expression | [44] |
7. Fractalkine in Cancer Immunotherapy
7.1. Immune Checkpoint Blockade Immunotherapies
7.2. Fractalkine as a Biomarker of Response in Lung Cancer Immunotherapy
7.3. FKN as a Therapeutic Anti-Cancer Agent
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nomiyama, H.; Imai, T.; Kusuda, J.; Miura, R.; Callen, D.F.; Yoshie, O. Human chemokines fractalkine (SCYD1), MDC (SCYA22) and TARC (SCYA17) are clustered on chromosome 16q13. Cytogenet. Cell Genet. 1998, 81, 10–11. [Google Scholar] [CrossRef]
- White, G.E.; Greaves, D.R. Fractalkine: A survivor’s guide: Chemokines as antiapoptotic mediators. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lloyd, C.; Zhou, H.; Dolich, S.; Deeds, J.; Gonzalo, J.A.; Vath, J.; Gosselin, M.; Ma, J.; Dussault, B.; et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 1997, 387, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, G.K.; Murphy, K.J. Neuron-glia crosstalk in health and disease: Fractalkine and CX3CR1 take centre stage. Open Biol. 2013, 3, 130181. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Imaizumi, T.; Fujimoto, K.; Matsuo, N.; Kimura, K.; Cui, X.; Matsumiya, T.; Tanji, K.; Shibata, T.; Tamo, W.; et al. Synergistic stimulation, by tumor necrosis factor-alpha and interferon-gamma, of fractalkine expression in human astrocytes. Neurosci. Lett. 2001, 303, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Matsumiya, T.; Fujimoto, K.; Okamoto, K.; Cui, X.; Ohtaki, U.; Hidemi; Yoshida; Satoh, K. Interferon-gamma stimulates the expression of CX3CL1/fractalkine in cultured human endothelial cells. Tohoku J. Exp. Med. 2000, 192, 127–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sukkar, M.B.; Issa, R.; Xie, S.; Oltmanns, U.; Newton, R.; Chung, K.F. Fractalkine/CX3CL1 production by human airway smooth muscle cells: Induction by IFN-gamma and TNF-alpha and regulation by TGF-beta and corticosteroids. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L1230–L1240. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Imaizumi, T.; Yoshida, H.; Takanashi, S.; Okumura, K.; Satoh, K. Interferon-gamma stimulates fractalkine expression in human bronchial epithelial cells and regulates mononuclear cell adherence. Am. J. Respir. Cell Mol. Biol. 2001, 25, 233–238. [Google Scholar] [CrossRef]
- Sugaya, M.; Nakamura, K.; Mitsui, H.; Takekoshi, T.; Saeki, H.; Tamaki, K. Human keratinocytes express fractalkine/CX3CL1. J. Dermatol. Sci. 2003, 31, 179–187. [Google Scholar] [CrossRef]
- Fonovic, U.P.; Jevnikar, Z.; Kos, J. Cathepsin S generates soluble CX3CL1 (fractalkine) in vascular smooth muscle cells. Biol. Chem. 2013, 394, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Gan, A.M.; Butoi, E.; Manea, A.; Pirvulescu, M.M.; Stan, D.; Simion, V.; Calin, M.; Simionescu, M.; Manduteanu, I. Functional analysis of the fractalkine gene promoter in human aortic smooth muscle cells exposed to proinflammatory conditions. FEBS J. 2014, 281, 3869–3881. [Google Scholar] [CrossRef] [PubMed]
- Fong, A.M.; Erickson, H.P.; Zachariah, J.P.; Poon, S.; Schamberg, N.J.; Imai, T.; Patel, D.D. Ultrastructure and function of the fractalkine mucin domain in CX(3)C chemokine domain presentation. J. Biol. Chem. 2000, 275, 3781–3786. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Ten Hagen, K.G. Mucin-type O-glycosylation during development. J. Biol. Chem. 2013, 288, 6921–6929. [Google Scholar] [CrossRef] [PubMed]
- Kehlen, A.; Haegele, M.; Bohme, L.; Cynis, H.; Hoffmann, T.; Demuth, H.U. N-terminal pyroglutamate formation in CX3CL1 is essential for its full biologic activity. Biosci. Rep. 2017, 37, BSR20170712. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhao, Z.; Peng, W.; Wang, P.; Xu, X.; Zhao, C. Glutaminyl cyclases, the potential targets of cancer and neurodegenerative diseases. Eur. J. Pharmacol. 2022, 931, 175178. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, M.A.; Hermand, P.; Saindoy, E.; Guillou, N.; Guellec, J.; Coens, A.; Hattab, C.; Desuzinges-Mandon, E.; Jawhari, A.; Iatmanen-Harbi, S.; et al. CX3CL1 homo-oligomerization drives cell-to-cell adherence. Sci. Rep. 2020, 10, 9069. [Google Scholar] [CrossRef]
- Hermand, P.; Pincet, F.; Carvalho, S.; Ansanay, H.; Trinquet, E.; Daoudi, M.; Combadiere, C.; Deterre, P. Functional adhesiveness of the CX3CL1 chemokine requires its aggregation. Role of the transmembrane domain. J. Biol. Chem. 2008, 283, 30225–30234. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Kulasingam, V.; Alexander, R.T.; Touret, N.; Fong, A.M.; Patel, D.D.; Robinson, L.A. Recycling of the membrane-anchored chemokine, CX3CL1. J. Biol. Chem. 2005, 280, 19858–19866. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Gayen, M.; Singh, N.; Gao, F.; He, W.; Hu, X.; Tsai, L.H.; Yan, R. The intracellular domain of CX3CL1 regulates adult neurogenesis and Alzheimer’s amyloid pathology. J. Exp. Med. 2019, 216, 1891–1903. [Google Scholar] [CrossRef]
- Fujita, M.; Takada, Y.K.; Takada, Y. Integrins alphavbeta3 and alpha4beta1 act as coreceptors for fractalkine, and the integrin-binding defective mutant of fractalkine is an antagonist of CX3CR1. J. Immunol. 2012, 189, 5809–5819. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.K.; Fong, A.M.; Swain, P.A.; Chen, S.; Yu, Y.R.; Salafranca, M.N.; Greenleaf, W.B.; Imai, T.; Patel, D.D. Mutational analysis of the fractalkine chemokine domain. Basic amino acid residues differentially contribute to CX3CR1 binding, signaling, and cell adhesion. J. Biol. Chem. 2001, 276, 21632–21641. [Google Scholar] [CrossRef] [PubMed]
- Garton, K.J.; Gough, P.J.; Blobel, C.P.; Murphy, G.; Greaves, D.R.; Dempsey, P.J.; Raines, E.W. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J. Biol. Chem. 2001, 276, 37993–38001. [Google Scholar] [CrossRef]
- Hundhausen, C.; Misztela, D.; Berkhout, T.A.; Broadway, N.; Saftig, P.; Reiss, K.; Hartmann, D.; Fahrenholz, F.; Postina, R.; Matthews, V.; et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 2003, 102, 1186–1195. [Google Scholar] [CrossRef]
- Bourd-Boittin, K.; Basset, L.; Bonnier, D.; L’Helgoualc’h, A.; Samson, M.; Theret, N. CX3CL1/fractalkine shedding by human hepatic stellate cells: Contribution to chronic inflammation in the liver. J. Cell. Mol. Med. 2009, 13, 1526–1535. [Google Scholar] [CrossRef]
- Dean, R.A.; Overall, C.M. Proteomics discovery of metalloproteinase substrates in the cellular context by iTRAQ labeling reveals a diverse MMP-2 substrate degradome. Mol. Cell. Proteom. MCP 2007, 6, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Hasegawa, H.; Kohno, M.; Ito, M.R.; Terada, M.; Imai, T.; Yoshie, O.; Nose, M.; Fujita, S. Antagonist of fractalkine (CX3CL1) delays the initiation and ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum. 2005, 52, 1522–1533. [Google Scholar] [CrossRef]
- Hoover, D.M.; Mizoue, L.S.; Handel, T.M.; Lubkowski, J. The crystal structure of the chemokine domain of fractalkine shows a novel quaternary arrangement. J. Biol. Chem. 2000, 275, 23187–23193. [Google Scholar] [CrossRef]
- Finneran, D.; Li, Q.; Subbarayan, M.S.; Joly-Amado, A.; Kamath, S.; Dengler, D.G.; Gordon, M.N.; Jackson, M.R.; Morgan, D.; Bickford, P.C.; et al. Concentration and proteolysis of CX3CL1 may regulate the microglial response to CX3CL1. Glia 2023, 71, 245–258. [Google Scholar] [CrossRef]
- Nakayama, T.; Watanabe, Y.; Oiso, N.; Higuchi, T.; Shigeta, A.; Mizuguchi, N.; Katou, F.; Hashimoto, K.; Kawada, A.; Yoshie, O. Eotaxin-3/CC chemokine ligand 26 is a functional ligand for CX3CR1. J. Immunol. 2010, 185, 6472–6479. [Google Scholar] [CrossRef]
- Combadiere, C.; Salzwedel, K.; Smith, E.D.; Tiffany, H.L.; Berger, E.A.; Murphy, P.M. Identification of CX3CR1. A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV-1. J. Biol. Chem. 1998, 273, 23799–23804. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Hieshima, K.; Haskell, C.; Baba, M.; Nagira, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Nomiyama, H.; Schall, T.J.; et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997, 91, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Fong, A.M.; Robinson, L.A.; Steeber, D.A.; Tedder, T.F.; Yoshie, O.; Imai, T.; Patel, D.D. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J. Exp. Med. 1998, 188, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Niessner, A.; Marculescu, R.; Haschemi, A.; Endler, G.; Zorn, G.; Weyand, C.M.; Maurer, G.; Mannhalter, C.; Wojta, J.; Wagner, O.; et al. Opposite effects of CX3CR1 receptor polymorphisms V249I and T280M on the development of acute coronary syndrome. A possible implication of fractalkine in inflammatory activation. Thromb. Haemost. 2005, 93, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Hoki, T.; Oba, T.; Saito, H.; Attwood, K.; Sabel, M.S.; Chang, A.E.; Odunsi, K.; Ito, F. CX3CR1-CD8+ T cells are critical in antitumor efficacy but functionally suppressed in the tumor microenvironment. JCI Insight 2020, 5, e133920. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Hoki, T.; Oba, T.; Jain, V.; Chen, H.; Attwood, K.; Battaglia, S.; George, S.; Chatta, G.; Puzanov, I.; et al. T-cell CX3CR1 expression as a dynamic blood-based biomarker of response to immune checkpoint inhibitors. Nat. Commun. 2021, 12, 1402. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, A.; Bu, X.; Wang, Y.; Gomez, M.; Torchia, J.A.; Hua, P.; Hung, S.H.; Davies, M.A.; Lizee, G.A.; von Andrian, U.; et al. The CX3CL1-CX3CR1 chemokine axis can contribute to tumor immune evasion and blockade with a novel CX3CR1 monoclonal antibody enhances response to anti-PD-1 immunotherapy. Front. Immunol. 2023, 14, 1237715. [Google Scholar] [CrossRef] [PubMed]
- Sirois-Gagnon, D.; Chamberland, A.; Perron, S.; Brisson, D.; Gaudet, D.; Laprise, C. Association of common polymorphisms in the fractalkine receptor (CX3CR1) with obesity. Obesity 2011, 19, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Faure, S.; Meyer, L.; Costagliola, D.; Vaneensberghe, C.; Genin, E.; Autran, B.; Delfraissy, J.F.; McDermott, D.H.; Murphy, P.M.; Debre, P.; et al. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science 2000, 287, 2274–2277. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Li, T.; Feng, X. G-Protein Coupled Receptors (GPCRs): Signaling Pathways, Characterization, and Functions in Insect Physiology and Toxicology. Int. J. Mol. Sci. 2021, 22, 5260. [Google Scholar] [CrossRef]
- Korbecki, J.; Siminska, D.; Kojder, K.; Grochans, S.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Fractalkine/CX3CL1 in Neoplastic Processes. Int. J. Mol. Sci. 2020, 21, 3723. [Google Scholar] [CrossRef]
- Maciejewski-Lenoir, D.; Chen, S.; Feng, L.; Maki, R.; Bacon, K.B. Characterization of fractalkine in rat brain cells: Migratory and activation signals for CX3CR-1-expressing microglia. J. Immunol. 1999, 163, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Namkoong, S.; Kim, Y.M.; Kim, C.K.; Lee, H.; Ha, K.S.; Chung, H.T.; Kwon, Y.G.; Kim, Y.M. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2836–H2846. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Qi, L.; Chen, X.; Du, J.; Zhang, Z.; Liu, S. Expression of CX3CR1 associates with cellular migration, metastasis, and prognosis in human clear cell renal cell carcinoma. Urol. Oncol. 2014, 32, 162–170. [Google Scholar] [CrossRef]
- Shulby, S.A.; Dolloff, N.G.; Stearns, M.E.; Meucci, O.; Fatatis, A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res. 2004, 64, 4693–4698. [Google Scholar] [CrossRef]
- Liang, Y.; Yi, L.; Liu, P.; Jiang, L.; Wang, H.; Hu, A.; Sun, C.; Dong, J. CX3CL1 involves in breast cancer metastasizing to the spine via the Src/FAK signaling pathway. J. Cancer 2018, 9, 3603–3612. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liang, Y.; Chan, Q.; Jiang, L.; Dong, J. CX3CL1 promotes lung cancer cell migration and invasion via the Src/focal adhesion kinase signaling pathway. Oncol. Rep. 2019, 41, 1911–1917. [Google Scholar] [CrossRef]
- Huang, L.; Ma, B.; Ma, J.; Wang, F. Fractalkine/CX3CR1 axis modulated the development of pancreatic ductal adenocarcinoma via JAK/STAT signaling pathway. Biochem. Biophys. Res. Commun. 2017, 493, 1510–1517. [Google Scholar] [CrossRef]
- Fujita, M.; Takada, Y.K.; Takada, Y. The chemokine fractalkine can activate integrins without CX3CR1 through direct binding to a ligand-binding site distinct from the classical RGD-binding site. PLoS ONE 2014, 9, e96372. [Google Scholar] [CrossRef]
- Takada, Y.K.; Fujita, M.; Takada, Y. Pro-Inflammatory Chemokines CCL5, CXCL12, and CX3CL1 Bind to and Activate Platelet Integrin alphaIIbbeta3 in an Allosteric Manner. Cells 2022, 11, 3059. [Google Scholar] [CrossRef]
- Kim, K.W.; Vallon-Eberhard, A.; Zigmond, E.; Farache, J.; Shezen, E.; Shakhar, G.; Ludwig, A.; Lira, S.A.; Jung, S. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood 2011, 118, e156–e167. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.D.; Chadwick, N.; Warren, B.F.; Jewell, D.P.; Gordon, S.; Powrie, F.; Greaves, D.R. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am. J. Pathol. 2001, 158, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.E.; Xia, Y.; Chen, S.; Wang, Y.; Ye, R.D.; Harrison, J.K.; Bacon, K.B.; Zerwes, H.G.; Feng, L. NF-kappaB-dependent fractalkine induction in rat aortic endothelial cells stimulated by IL-1beta, TNF-alpha, and LPS. J. Leukoc. Biol. 2000, 67, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Berkhout, T.; Moores, K.; Groot, P.; Chapman, G. Fractalkine is expressed by smooth muscle cells in response to IFN-gamma and TNF-alpha and is modulated by metalloproteinase activity. J. Immunol. 2002, 168, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Sakaguchi, T.; Gu, X.; Colgan, S.P.; Reinecker, H.C. Fractalkine-mediated signals regulate cell-survival and immune-modulatory responses in intestinal epithelial cells. Gastroenterology 2002, 122, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Fukuda, S.; Mori, T.; Matsuda, K.; Yamaguchi, T.; Tanikawa, C.; Ogawa, M.; Nakamura, Y.; Arakawa, H. Identification of fractalkine, a CX3C-type chemokine, as a direct target of p53. Cancer Res. 2000, 60, 3722–3726. [Google Scholar]
- Gordon, T.; Jones, R.J.; Smith, M.E.; Watson, J.E. The influence of intracellular components on the chemosensitivity of skeletal muscle. J. Physiol. 1975, 247, 42P–43P. [Google Scholar] [PubMed]
- Goda, S.; Imai, T.; Yoshie, O.; Yoneda, O.; Inoue, H.; Nagano, Y.; Okazaki, T.; Imai, H.; Bloom, E.T.; Domae, N.; et al. CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J. Immunol. 2000, 164, 4313–4320. [Google Scholar] [CrossRef] [PubMed]
- Gemma, C.; Bachstetter, A.D. The role of microglia in adult hippocampal neurogenesis. Front. Cell. Neurosci. 2013, 7, 229. [Google Scholar] [CrossRef]
- Wang, S.K.; Xue, Y.; Rana, P.; Hong, C.M.; Cepko, C.L. Soluble CX3CL1 gene therapy improves cone survival and function in mouse models of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2019, 116, 10140–10149. [Google Scholar] [CrossRef]
- Adamski, V.; Hattermann, K.; Kubelt, C.; Cohrs, G.; Lucius, R.; Synowitz, M.; Sebens, S.; Held-Feindt, J. Entry and exit of chemotherapeutically-promoted cellular dormancy in glioblastoma cells is differentially affected by the chemokines CXCL12, CXCL16, and CX3CL1. Oncogene 2020, 39, 4421–4435. [Google Scholar] [CrossRef] [PubMed]
- Santoso, C.S.; Li, Z.; Lal, S.; Yuan, S.; Gan, K.A.; Agosto, L.M.; Liu, X.; Pro, S.C.; Sewell, J.A.; Henderson, A.; et al. Comprehensive mapping of the human cytokine gene regulatory network. Nucleic Acids Res. 2020, 48, 12055–12073. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.A.; Ruan, X.; Kumar, P. Activation of microglia: A neuroinflammatory role for CAP37. Glia 2003, 41, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, M.; Wang, B.; Yuan, Z.; Guo, Z.; Chen, T.; Yu, Y.; Qin, Z.; Cao, X. Fractalkine transgene induces T-cell-dependent antitumor immunity through chemoattraction and activation of dendritic cells. Int. J. Cancer 2003, 103, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, T.; Wang, B.; Zhang, M.; An, H.; Guo, Z.; Yu, Y.; Qin, Z.; Cao, X. Chemoattraction, adhesion and activation of natural killer cells are involved in the antitumor immune response induced by fractalkine/CX3CL1. Immunol. Lett. 2003, 89, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, E.; Combadiere, B.; Bonduelle, O.; Iga, M.; Gao, J.L.; Maho, M.; Boissonnas, A.; Murphy, P.M.; Debre, P.; Combadiere, C. Fractalkine mediates natural killer-dependent antitumor responses in vivo. Cancer Res. 2003, 63, 7468–7474. [Google Scholar]
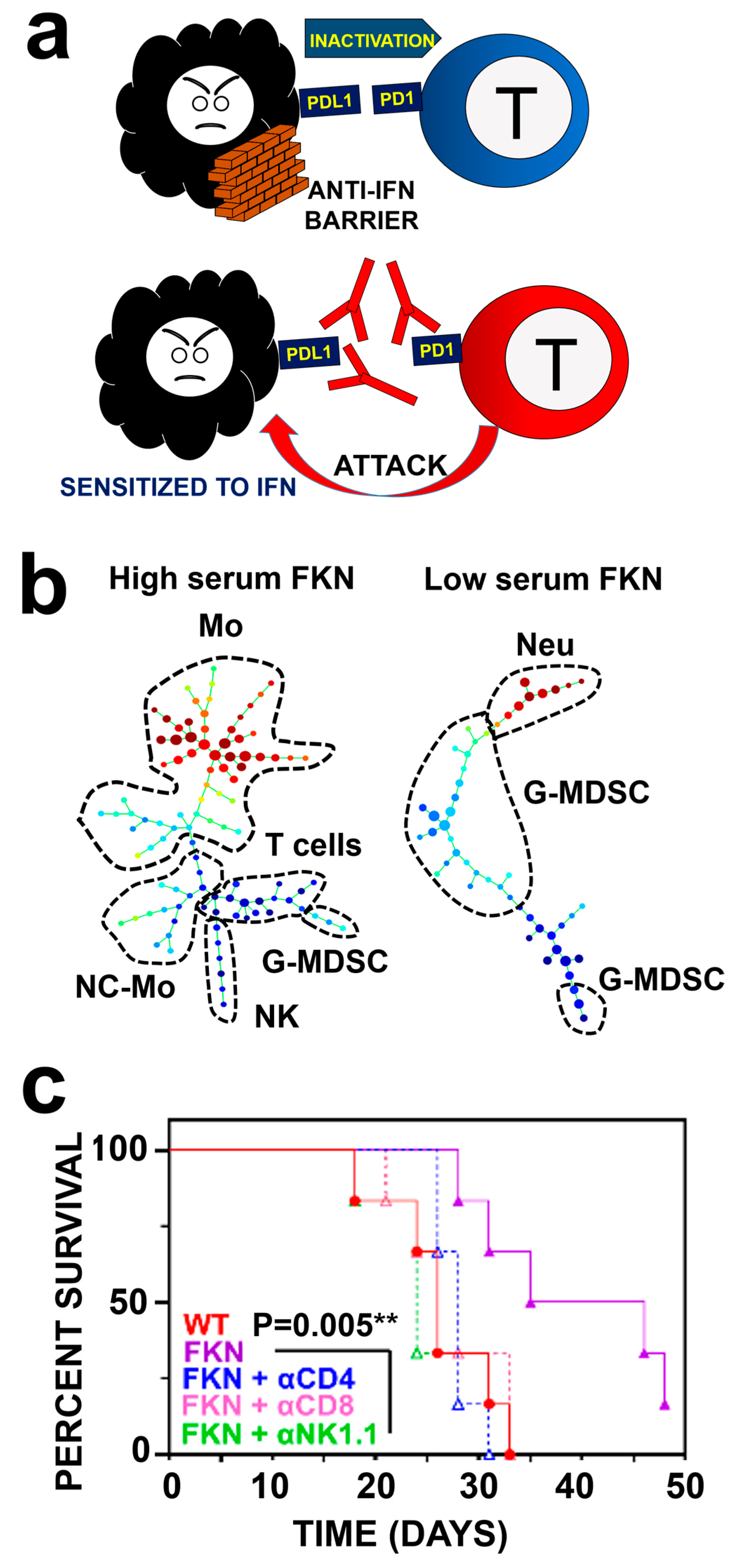
- Bocanegra, A.; Fernandez-Hinojal, G.; Ajona, D.; Blanco, E.; Zuazo, M.; Garnica, M.; Chocarro, L.; Alfaro-Arnedo, E.; Pineiro-Hermida, S.; Morente, P.; et al. Plasma fractalkine contributes to systemic myeloid diversity and PD-L1/PD-1 blockade in lung cancer. EMBO Rep. 2023, 24, e55884. [Google Scholar] [CrossRef]
- Ohta, M.; Tanaka, F.; Yamaguchi, H.; Sadanaga, N.; Inoue, H.; Mori, M. The high expression of Fractalkine results in a better prognosis for colorectal cancer patients. Int. J. Oncol. 2005, 26, 41–47. [Google Scholar] [CrossRef]
- Zeng, Y.; Jiang, J.; Huebener, N.; Wenkel, J.; Gaedicke, G.; Xiang, R.; Lode, H.N. Fractalkine gene therapy for neuroblastoma is more effective in combination with targeted IL-2. Cancer Lett. 2005, 228, 187–193. [Google Scholar] [CrossRef]
- Nukiwa, M.; Andarini, S.; Zaini, J.; Xin, H.; Kanehira, M.; Suzuki, T.; Fukuhara, T.; Mizuguchi, H.; Hayakawa, T.; Saijo, Y.; et al. Dendritic cells modified to express fractalkine/CX3CL1 in the treatment of preexisting tumors. Eur. J. Immunol. 2006, 36, 1019–1027. [Google Scholar] [CrossRef]
- Matsubara, T.; Ono, T.; Yamanoi, A.; Tachibana, M.; Nagasue, N. Fractalkine-CX3CR1 axis regulates tumor cell cycle and deteriorates prognosis after radical resection for hepatocellular carcinoma. J. Surg. Oncol. 2007, 95, 241–249. [Google Scholar] [CrossRef]
- Tang, L.; Hu, H.D.; Hu, P.; Lan, Y.H.; Peng, M.L.; Chen, M.; Ren, H. Gene therapy with CX3CL1/Fractalkine induces antitumor immunity to regress effectively mouse hepatocellular carcinoma. Gene Ther. 2007, 14, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Cambien, B.; Karimdjee, B.F.; Barthel, R.; Staccini, P.; Luci, C.; Breittmayer, V.; Anjuere, F.; Schmid-Alliana, A.; Schmid-Antomarchi, H. Tissue-specific differential antitumour effect of molecular forms of fractalkine in a mouse model of metastatic colon cancer. Gut 2007, 56, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.; Erreni, M.; van Brakel, M.; Debets, R.; Allavena, P. Enhanced recruitment of genetically modified CX3CR1-positive human T cells into Fractalkine/CX3CL1 expressing tumors: Importance of the chemokine gradient. J. Immunother. Cancer 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Huebener, N.; Fest, S.; Weixler, S.; Schroeder, U.; Gaedicke, G.; Xiang, R.; Schramm, A.; Eggert, A.; Reisfeld, R.A.; et al. Fractalkine (CX3CL1)- and interleukin-2-enriched neuroblastoma microenvironment induces eradication of metastases mediated by T cells and natural killer cells. Cancer Res. 2007, 67, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Lee, J.S.; Yoon, J.H. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J. Surg. Oncol. 2012, 106, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kehlen, A.; Greither, T.; Wach, S.; Nolte, E.; Kappler, M.; Bache, M.; Holzhausen, H.J.; Lautenschlager, C.; Gobel, S.; Wurl, P.; et al. High coexpression of CCL2 and CX3CL1 is gender-specifically associated with good prognosis in soft tissue sarcoma patients. Int. J. Cancer 2014, 135, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Chang, H.; Sun, S.J.; Liao, C.Y.; Wang, L.Y.; Ko, J.L.; Chang, J.T. Differential impact of CX3CL1 on lung cancer prognosis in smokers and non-smokers. Mol. Carcinog. 2018, 57, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Zhu, X.; Li, Q.; Liang, X.; Xie, J.; Hu, S.; Peng, W.; Li, C. Increased CX3CL1 mRNA expression level is a positive prognostic factor in patients with lung adenocarcinoma. Oncol. Lett. 2019, 17, 4877–4890. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhao, Q.; Huang, L.; Zheng, Y.; Li, L.; He, Q.; Zhang, C.; Li, F.; Maimela, N.R.; Sun, Z.; et al. The R132H mutation in IDH1 promotes the recruitment of NK cells through CX3CL1/CX3CR1 chemotaxis and is correlated with a better prognosis in gliomas. Immunol. Cell Biol. 2019, 97, 457–469. [Google Scholar] [CrossRef]
- Dreyer, T.F.; Kuhn, S.; Stange, C.; Heithorst, N.; Schilling, D.; Jelsma, J.; Sievert, W.; Seitz, S.; Stangl, S.; Hapfelmeier, A.; et al. The Chemokine CX3CL1 Improves Trastuzumab Efficacy in HER2 Low-Expressing Cancer In Vitro and In Vivo. Cancer Immunol. Res. 2021, 9, 779–789. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Yang, N.; Shi, Q.; Su, H.; Lin, T.; He, Z.; Wang, W.; Guo, H.; Shen, P. Crosstalk between IL-15Ralpha(+) tumor-associated macrophages and breast cancer cells reduces CD8(+) T cell recruitment. Cancer Commun. 2022, 42, 536–557. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, T.S.; Peters, C.W.; Quiros, C.; Kidd, C.K.; Kawakami, M.; Klomhaus, A.M.; Baselga-Carretero, I.; Kaplan-Lefko, P.; Macabali, M.H.; Perez Garcilazo, I.; et al. Infusion Product TNFalpha, Th2, and STAT3 Activities Are Associated with Clinical Responses to Transgenic T-cell Receptor Cell Therapy. Cancer Immunol. Res. 2023, 11, 1589–1597. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef]
- Tardaguila, M.; Mira, E.; Garcia-Cabezas, M.A.; Feijoo, A.M.; Quintela-Fandino, M.; Azcoitia, I.; Lira, S.A.; Manes, S. CX3CL1 promotes breast cancer via transactivation of the EGF pathway. Cancer Res. 2013, 73, 4461–4473. [Google Scholar] [CrossRef]
- Reed, J.R.; Stone, M.D.; Beadnell, T.C.; Ryu, Y.; Griffin, T.J.; Schwertfeger, K.L. Fibroblast growth factor receptor 1 activation in mammary tumor cells promotes macrophage recruitment in a CX3CL1-dependent manner. PLoS ONE 2012, 7, e45877. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liang, Y.; Jiang, L.; Wang, H.; Wang, S.; Dong, J. CX3CL1/fractalkine enhances prostate cancer spinal metastasis by activating the Src/FAK pathway. Int. J. Oncol. 2018, 53, 1544–1556. [Google Scholar] [CrossRef]
- Xiao, L.J.; Chen, Y.Y.; Lin, P.; Zou, H.F.; Lin, F.; Zhao, L.N.; Li, D.; Guo, L.; Tang, J.B.; Zheng, X.L.; et al. Hypoxia increases CX3CR1 expression via HIF-1 and NF-kappaB in androgen-independent prostate cancer cells. Int. J. Oncol. 2012, 41, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, Y.; Cui, R.; Li, D.; Xiao, L.; Lin, P.; Du, Y.; Sun, H.; Yu, X.; Zheng, X. Upregulation of fractalkine contributes to the proliferative response of prostate cancer cells to hypoxia via promoting the G1/S phase transition. Mol. Med. Rep. 2015, 12, 7907–7914. [Google Scholar] [CrossRef]
- Tang, J.; Xiao, L.; Cui, R.; Li, D.; Zheng, X.; Zhu, L.; Sun, H.; Pan, Y.; Du, Y.; Yu, X. CX3CL1 increases invasiveness and metastasis by promoting epithelial-to-mesenchymal transition through the TACE/TGF-alpha/EGFR pathway in hypoxic androgen-independent prostate cancer cells. Oncol. Rep. 2016, 35, 1153–1162. [Google Scholar] [CrossRef]
- Ren, T.; Chen, Q.; Tian, Z.; Wei, H. Down-regulation of surface fractalkine by RNA interference in B16 melanoma reduced tumor growth in mice. Biochem. Biophys. Res. Commun. 2007, 364, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Marchica, V.; Toscani, D.; Corcione, A.; Bolzoni, M.; Storti, P.; Vescovini, R.; Ferretti, E.; Dalla Palma, B.; Vicario, E.; Accardi, F.; et al. Bone Marrow CX3CL1/Fractalkine is a New Player of the Pro-Angiogenic Microenvironment in Multiple Myeloma Patients. Cancers 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Wada, A.; Ito, A.; Iitsuka, H.; Tsuneyama, K.; Miyazono, T.; Murakami, J.; Shibahara, N.; Sakurai, H.; Saiki, I.; Nakayama, T.; et al. Role of chemokine CX3CL1 in progression of multiple myeloma via CX3CR1 in bone microenvironments. Oncol. Rep. 2015, 33, 2935–2939. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.M.; Cao, S.; Yu, W.D.; Liu, Y.L.; Wang, J.T. Overexpression of CX3CR1 is associated with cellular metastasis, proliferation and survival in gastric cancer. Oncol. Rep. 2015, 33, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.Y.; Zhou, T.; Chen, W.; Yin, X.D.; Yao, J.H.; Zhang, Y.F. Preliminary study correlating CX3CL1/CX3CR1 expression with gastric carcinoma and gastric carcinoma perineural invasion. World J. Gastroenterol. 2014, 20, 4428–4432. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cai, J.; Du, S.; Guo, Z.; Xin, B.; Wang, J.; Wei, W.; Shen, X. Fractalkine/CX3CR1 induces apoptosis resistance and proliferation through the activation of the AKT/NF-kappaB cascade in pancreatic cancer cells. Cell Biochem. Funct. 2017, 35, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhao, T.; Sun, J.; Wang, X.; Liu, J.; Gao, S.; Yu, M.; Hao, J. The CX3CL1/CX3CR1 reprograms glucose metabolism through HIF-1 pathway in pancreatic adenocarcinoma. J. Cell. Biochem. 2013, 114, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Stout, M.C.; Narayan, S.; Pillet, E.S.; Salvino, J.M.; Campbell, P.M. Inhibition of CX(3)CR1 reduces cell motility and viability in pancreatic adenocarcinoma epithelial cells. Biochem. Biophys. Res. Commun. 2018, 495, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, F.; Nasreddine, S.; Donnadieu, A.C.; Emilie, D.; Combadiere, C.; Prevot, S.; Machelon, V.; Balabanian, K. Identification of the chemokine CX3CL1 as a new regulator of malignant cell proliferation in epithelial ovarian cancer. PLoS ONE 2011, 6, e21546. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, E.; Bertolotto, M.; Deaglio, S.; Tripodo, C.; Ribatti, D.; Audrito, V.; Blengio, F.; Matis, S.; Zupo, S.; Rossi, D.; et al. A novel role of the CX3CR1/CX3CL1 system in the cross-talk between chronic lymphocytic leukemia cells and tumor microenvironment. Leukemia 2011, 25, 1268–1277. [Google Scholar] [CrossRef]
- Topalian, S.L.; Weiner, G.J.; Pardoll, D.M. Cancer immunotherapy comes of age. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 4828–4836. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernandez-Rubio, L.; Morente, P.; Fernandez-Hinojal, G.; Echaide, M.; Garnica, M.; et al. Understanding LAG-3 Signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar] [CrossRef] [PubMed]
- Arasanz, H.; Gato-Canas, M.; Zuazo, M.; Ibanez-Vea, M.; Breckpot, K.; Kochan, G.; Escors, D. PD1 signal transduction pathways in T cells. Oncotarget 2017, 8, 51936–51945. [Google Scholar] [CrossRef] [PubMed]
- Escors, D.; Gato-Canas, M.; Zuazo, M.; Arasanz, H.; Garcia-Granda, M.J.; Vera, R.; Kochan, G. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct. Target. Ther. 2018, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Peggs, K.S.; Quezada, S.A.; Korman, A.J.; Allison, J.P. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr. Opin. Immunol. 2006, 18, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Gato-Canas, M.; Zuazo, M.; Arasanz, H.; Ibanez-Vea, M.; Lorenzo, L.; Fernandez-Hinojal, G.; Vera, R.; Smerdou, C.; Martisova, E.; Arozarena, I.; et al. PDL1 Signals through Conserved Sequence Motifs to Overcome Interferon-Mediated Cytotoxicity. Cell Rep. 2017, 20, 1818–1829. [Google Scholar] [CrossRef]
- Zuazo, M.; Arasanz, H.; Fernandez-Hinojal, G.; Garcia-Granda, M.J.; Gato, M.; Bocanegra, A.; Martinez, M.; Hernandez, B.; Teijeira, L.; Morilla, I.; et al. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol. Med. 2019, 11, e10293. [Google Scholar] [CrossRef]
- Chocarro de Erauso, L.; Blanco, E.; Fernandez-Rubio, L.; Garnica, M.; Zuazo, M.; Garcia, M.J.; Bocanegra, A.; Echaide, M.; Johnston, C.; Edwards, C.J.; et al. PD-1/LAG-3 Co-signaling Profiling Uncovers CBL Ubiquitin Ligases as Key Immunotherapy Targets. EMBO Mol. Med. 2024. [Google Scholar] [CrossRef]
- Zuazo, M.; Arasanz, H.; Bocanegra, A.; Chocarro, L.; Vera, R.; Escors, D.; Kagamu, H.; Kochan, G. Systemic CD4 immunity: A powerful clinical biomarker for PD-L1/PD-1 immunotherapy. EMBO Mol. Med. 2020, 12, e12706. [Google Scholar] [CrossRef] [PubMed]
- Kagamu, H.; Kitano, S.; Yamaguchi, O.; Yoshimura, K.; Horimoto, K.; Kitazawa, M.; Fukui, K.; Shiono, A.; Mouri, A.; Nishihara, F.; et al. CD4(+) T-cell Immunity in the Peripheral Blood Correlates with Response to Anti-PD-1 Therapy. Cancer Immunol. Res. 2020, 8, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.H.; Carmi, Y.; Reticker-Flynn, N.E.; Kwek, S.S.; Madhireddy, D.; Martins, M.M.; Gherardini, P.F.; Prestwood, T.R.; Chabon, J.; Bendall, S.C.; et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 2017, 168, 487–502.e415. [Google Scholar] [CrossRef] [PubMed]
- Horton, B.L.; Morgan, D.M.; Momin, N.; Zagorulya, M.; Torres-Mejia, E.; Bhandarkar, V.; Wittrup, K.D.; Love, J.C.; Spranger, S. Lack of CD8(+) T cell effector differentiation during priming mediates checkpoint blockade resistance in non-small cell lung cancer. Sci. Immunol. 2021, 6, eabi8800. [Google Scholar] [CrossRef] [PubMed]
- Mathios, D.; Kim, J.E.; Mangraviti, A.; Phallen, J.; Park, C.K.; Jackson, C.M.; Garzon-Muvdi, T.; Kim, E.; Theodros, D.; Polanczyk, M.; et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci. Transl. Med. 2016, 8, 370ra180. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Naigeon, M.; Auclin, E.; Duchemann, B.; Cassard, L.; Jouniaux, J.M.; Boselli, L.; Grivel, J.; Desnoyer, A.; Mezquita, L.; et al. Circulating T-cell Immunosenescence in Patients with Advanced Non-small Cell Lung Cancer Treated with Single-agent PD-1/PD-L1 Inhibitors or Platinum-based Chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Silva-Pilipich, N.; Bocanegra, A.; Chocarro, L.; Procopio, A.; Ausin, K.; Fernandez-Irigoyen, J.; Fernandez, L.; Razquin, N.; Igea, A.; et al. Oleuropein-driven reprogramming of the myeloid cell compartment to sensitise tumours to PD-1/PD-L1 blockade strategies. Br. J. Cancer 2024, 130, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, A.; Fernandez-Hinojal, G.; Zuazo-Ibarra, M.; Arasanz, H.; Garcia-Granda, M.J.; Hernandez, C.; Ibanez, M.; Hernandez-Marin, B.; Martinez-Aguillo, M.; Lecumberri, M.J.; et al. PD-L1 Expression in Systemic Immune Cell Populations as a Potential Predictive Biomarker of Responses to PD-L1/PD-1 Blockade Therapy in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1631. [Google Scholar] [CrossRef]
- Krieg, C.; Nowicka, M.; Guglietta, S.; Schindler, S.; Hartmann, F.J.; Weber, L.M.; Dummer, R.; Robinson, M.D.; Levesque, M.P.; Becher, B. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 2018, 24, 144–153. [Google Scholar] [CrossRef]
- Arasanz, H.; Bocanegra, A.I.; Morilla, I.; Fernandez-Irigoyen, J.; Martinez-Aguillo, M.; Teijeira, L.; Garnica, M.; Blanco, E.; Chocarro, L.; Ausin, K.; et al. Circulating Low Density Neutrophils Are Associated with Resistance to First Line Anti-PD1/PDL1 Immunotherapy in Non-Small Cell Lung Cancer. Cancers 2022, 14, 3846. [Google Scholar] [CrossRef] [PubMed]
- Schultze, J.L.; Mass, E.; Schlitzer, A. Emerging Principles in Myelopoiesis at Homeostasis and during Infection and Inflammation. Immunity 2019, 50, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Gubin, M.M.; Esaulova, E.; Ward, J.P.; Malkova, O.N.; Runci, D.; Wong, P.; Noguchi, T.; Arthur, C.D.; Meng, W.; Alspach, E.; et al. High-Dimensional Analysis Delineates Myeloid and Lymphoid Compartment Remodeling during Successful Immune-Checkpoint Cancer Therapy. Cell 2018, 175, 1014–1030.e1019. [Google Scholar] [CrossRef] [PubMed]
- Arasanz, H.; Bocanegra, A.; Morilla, I.; Fernandez-Irigoyen, J.; Martinez-Aguillo, M.; Teijeira, L.; Garnica, M.; Blanco, E.; Chocarro, L.; Ausin, K.; et al. Circulating low density neutrophils are associated with resistance to first-line anti-PD1/PDL1 immunotherapy in non-small cell lung cancer. medRxiv 2022. medRxiv:2022.04.27.22273598. [Google Scholar] [CrossRef]
- Cappelletto, E.; Fasiolo, L.T.; Salizzato, V.; Piccin, L.; Fabozzi, A.; Contato, A.; Bianco, P.D.; Pasello, G.; Chiarion-Sileni, V.; Gion, M.; et al. Cytokine and soluble programmed death-ligand 1 levels in serum and plasma of cancer patients treated with immunotherapy: Preanalytical and analytical considerations. Int. J. Biol. Markers 2024, 39, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Kikuchi, T.; Andarini, S.; Ohkouchi, S.; Suzuki, T.; Nukiwa, T.; Huqun; Hagiwara, K.; Honjo, T.; Saijo, Y. Antitumor immune response by CX3CL1 fractalkine gene transfer depends on both NK and T cells. Eur. J. Immunol. 2005, 35, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Hyakudomi, M.; Matsubara, T.; Hyakudomi, R.; Yamamoto, T.; Kinugasa, S.; Yamanoi, A.; Maruyama, R.; Tanaka, T. Increased expression of fractalkine is correlated with a better prognosis and an increased number of both CD8+ T cells and natural killer cells in gastric adenocarcinoma. Ann. Surg. Oncol. 2008, 15, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Kee, J.Y.; Arita, Y.; Shinohara, K.; Ohashi, Y.; Sakurai, H.; Saiki, I.; Koizumi, K. Antitumor immune activity by chemokine CX3CL1 in an orthotopic implantation of lung cancer model in vivo. Mol. Clin. Oncol. 2013, 1, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ajona, D.; Ortiz-Espinosa, S.; Lozano, T.; Exposito, F.; Calvo, A.; Valencia, K.; Redrado, M.; Remírez, A.; Lecanda, F.; Alignani, D.; et al. Short-term starvation reduces IGF-1 levels to sensitize lung tumors to PD-1 immune checkpoint blockade. Nat. Cancer 2020, 1, 75–85. [Google Scholar] [CrossRef]
- Marchesi, F.; Piemonti, L.; Fedele, G.; Destro, A.; Roncalli, M.; Albarello, L.; Doglioni, C.; Anselmo, A.; Doni, A.; Bianchi, P.; et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008, 68, 9060–9069. [Google Scholar] [CrossRef]
- Erreni, M.; Solinas, G.; Brescia, P.; Osti, D.; Zunino, F.; Colombo, P.; Destro, A.; Roncalli, M.; Mantovani, A.; Draghi, R.; et al. Human glioblastoma tumours and neural cancer stem cells express the chemokine CX3CL1 and its receptor CX3CR1. Eur. J. Cancer 2010, 46, 3383–3392. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Rooper, L.; Xie, J.; Kajdacsy-Balla, A.A.; Barbolina, M.V. Fractalkine receptor CX(3)CR1 is expressed in epithelial ovarian carcinoma cells and required for motility and adhesion to peritoneal mesothelial cells. Mol. Cancer Res. 2012, 10, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Jamieson-Gladney, W.L.; Zhang, Y.; Fong, A.M.; Meucci, O.; Fatatis, A. The chemokine receptor CX(3)CR1 is directly involved in the arrest of breast cancer cells to the skeleton. Breast Cancer Res. BCR 2011, 13, R91. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, J.G.; Demir, I.E.; Friess, H.; Ceyhan, G.O. Fractalkine/CX3CR1: Why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin. Ther. Targets 2010, 14, 207–219. [Google Scholar] [CrossRef]
- Winter, A.N.; Subbarayan, M.S.; Grimmig, B.; Weesner, J.A.; Moss, L.; Peters, M.; Weeber, E.; Nash, K.; Bickford, P.C. Two forms of CX3CL1 display differential activity and rescue cognitive deficits in CX3CL1 knockout mice. J. Neuroinflamm. 2020, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.; Adams, W.A.; Calescibetta, A.; Tu, N.; Dalton, R.; So, T.; Wei, M.; Ward, G.; Kostenko, E.; Christiansen, S.; et al. CX3CR1 deficiency-induced TIL tumor restriction as a novel addition for CAR-T design in solid malignancies. iScience 2023, 26, 106443. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, C.; Chocarro, L.; Echaide, M.; Ausin, K.; Escors, D.; Kochan, G. Fractalkine in Health and Disease. Int. J. Mol. Sci. 2024, 25, 8007. https://doi.org/10.3390/ijms25158007
Rodriguez C, Chocarro L, Echaide M, Ausin K, Escors D, Kochan G. Fractalkine in Health and Disease. International Journal of Molecular Sciences. 2024; 25(15):8007. https://doi.org/10.3390/ijms25158007
Chicago/Turabian StyleRodriguez, Claudia, Luisa Chocarro, Miriam Echaide, Karina Ausin, David Escors, and Grazyna Kochan. 2024. "Fractalkine in Health and Disease" International Journal of Molecular Sciences 25, no. 15: 8007. https://doi.org/10.3390/ijms25158007
APA StyleRodriguez, C., Chocarro, L., Echaide, M., Ausin, K., Escors, D., & Kochan, G. (2024). Fractalkine in Health and Disease. International Journal of Molecular Sciences, 25(15), 8007. https://doi.org/10.3390/ijms25158007






