Molecular Cloning and Functional Identification of a Pericarp- and Testa-Abundant Gene’s (AhN8DT-2) Promoter from Arachis hypogaea
Abstract
:1. Introduction
2. Results
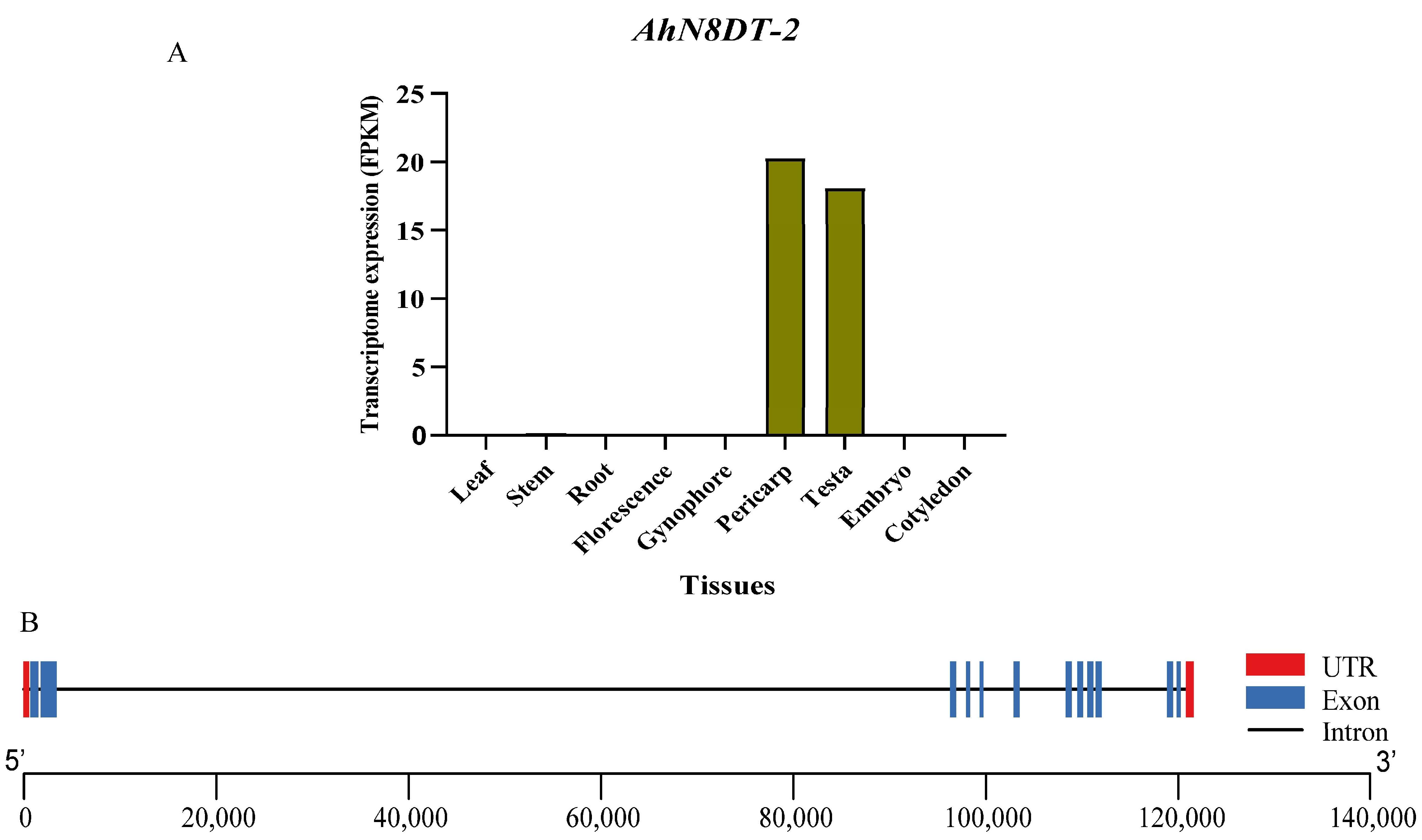
2.1. Identification and Analysis of Pericarp- and Testa-Abundant Gene
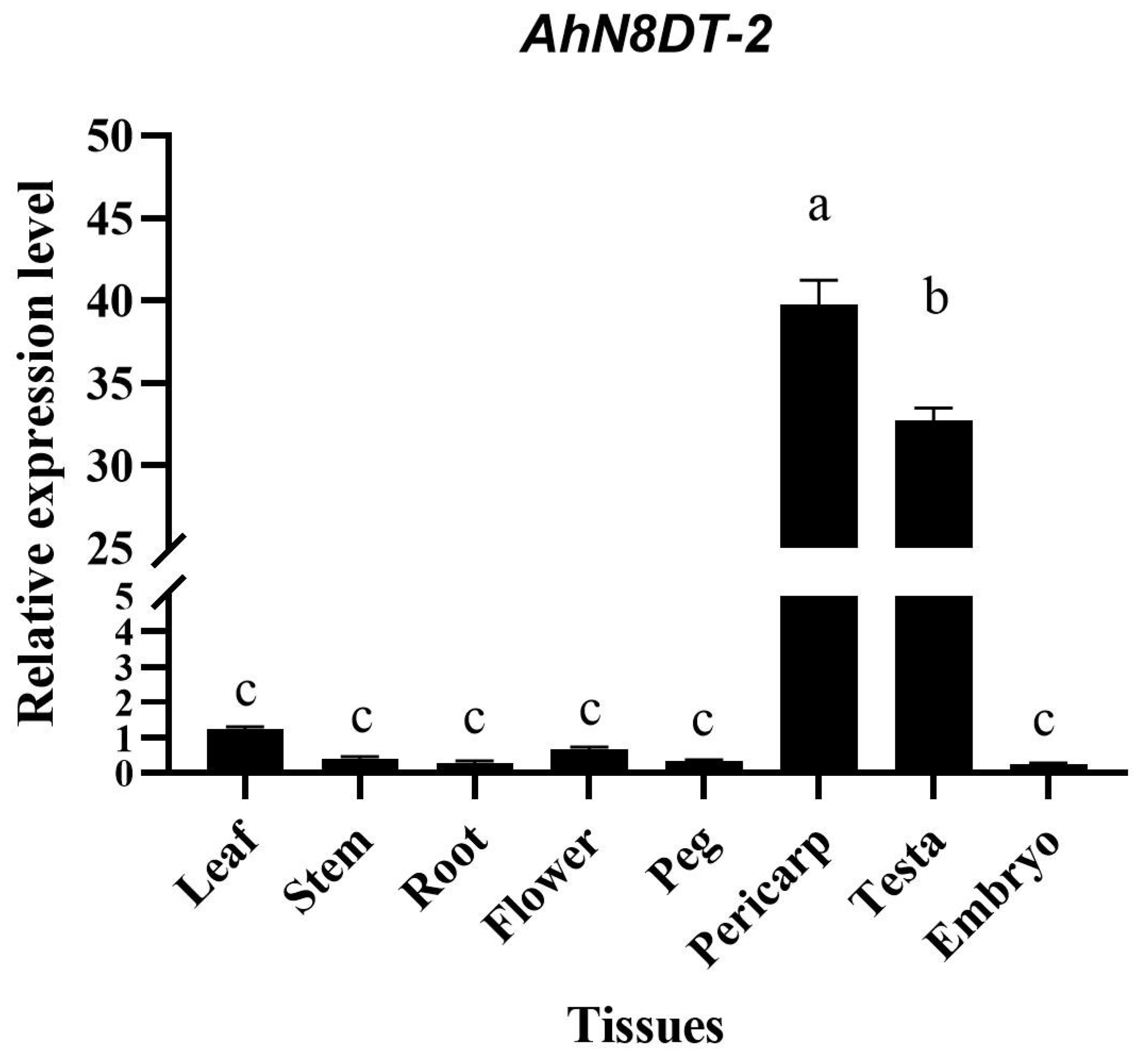
2.2. Expression Validation of AhN8DT-2 Gene in Different Tissues
2.3. In Silico Analysis of AhN8DT-2 Promoter
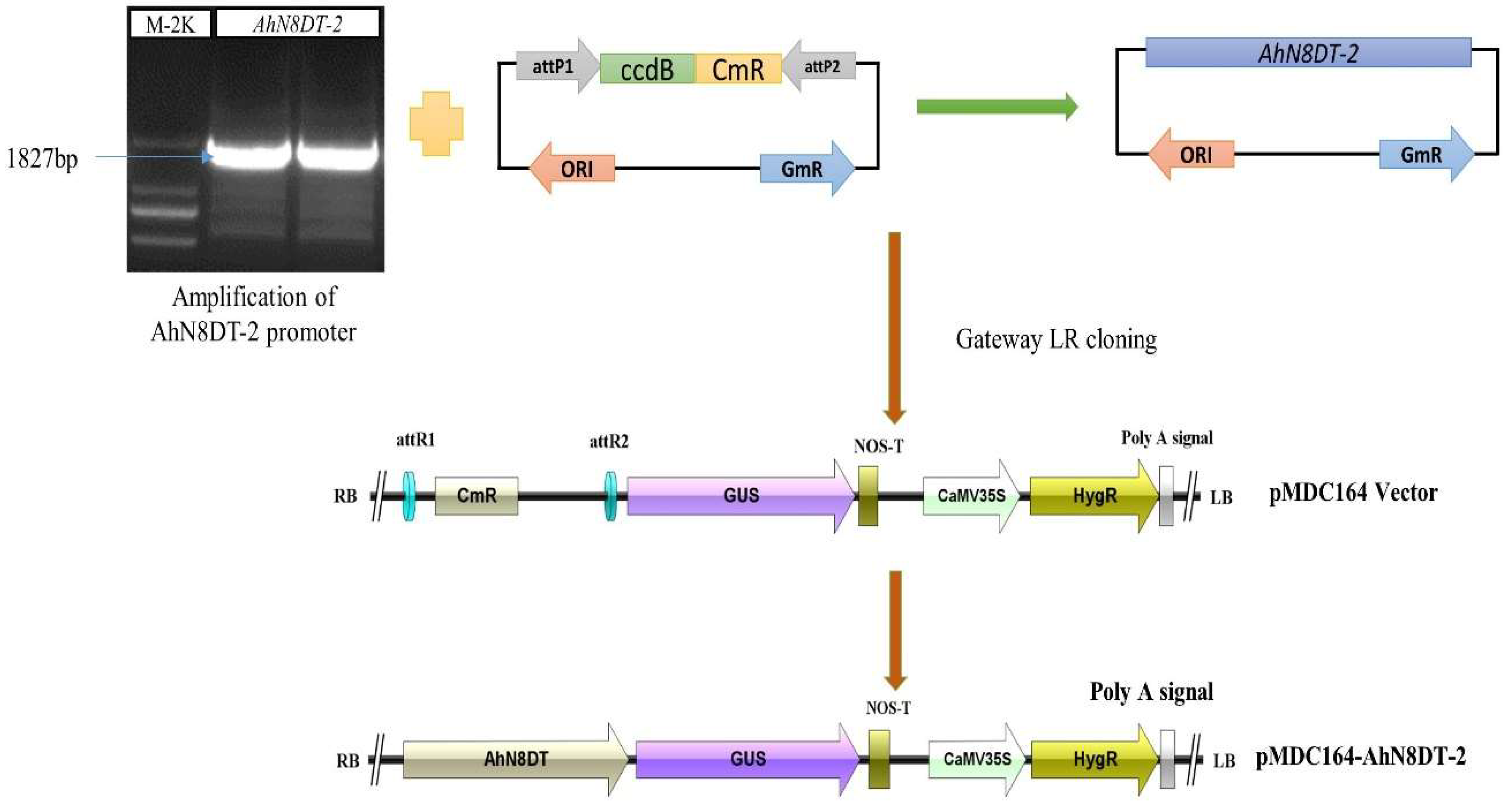
2.4. Cloning of AhN8DT-2 Promoter and Generation of Transgenic Arabidopsis Plants
2.5. Molecular Analysis of Transgenic Arabidopsis and Generation of Pure Lines
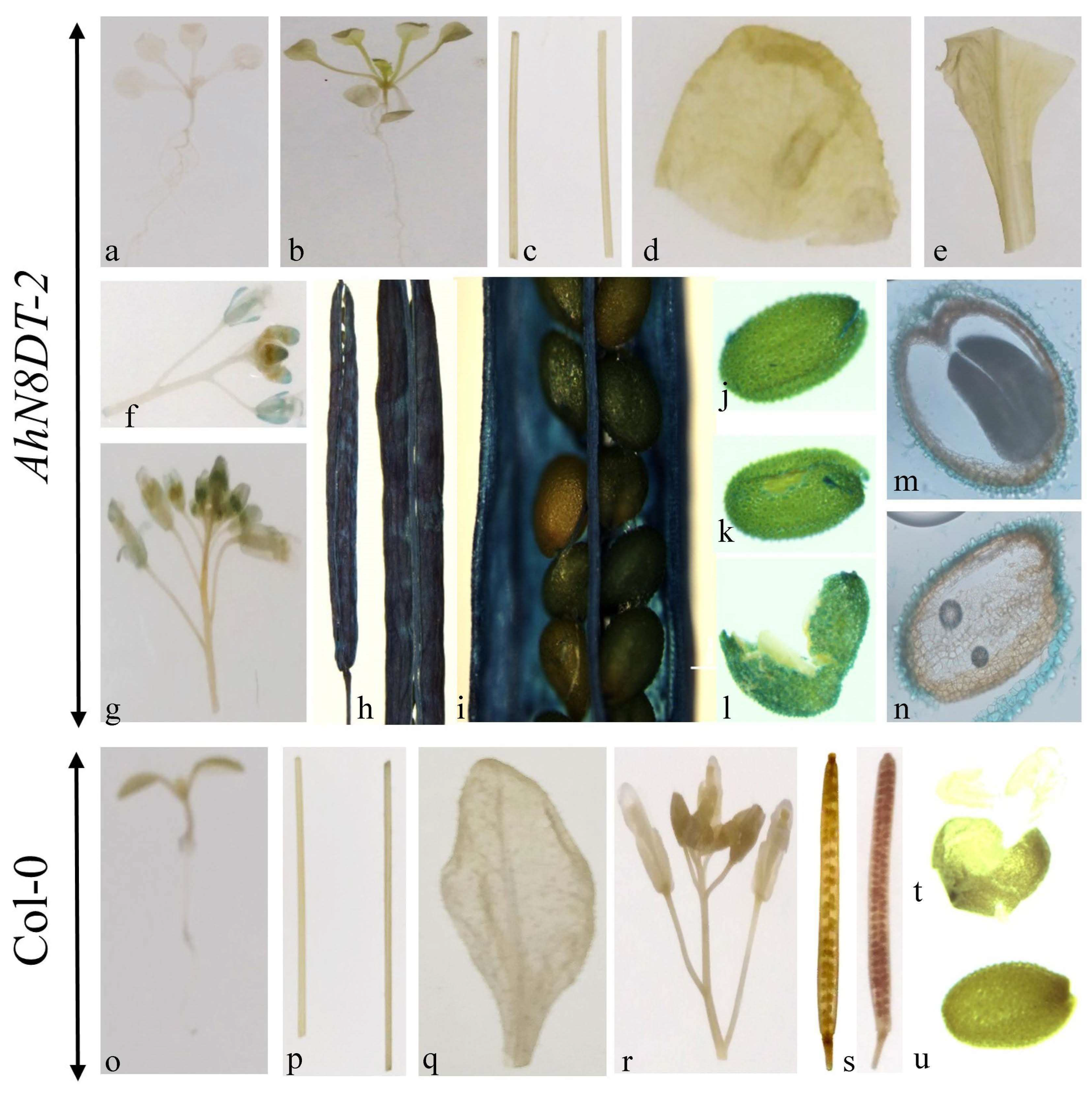
2.6. Tissue-Specific Induction of AhN8DT-2 Promoter
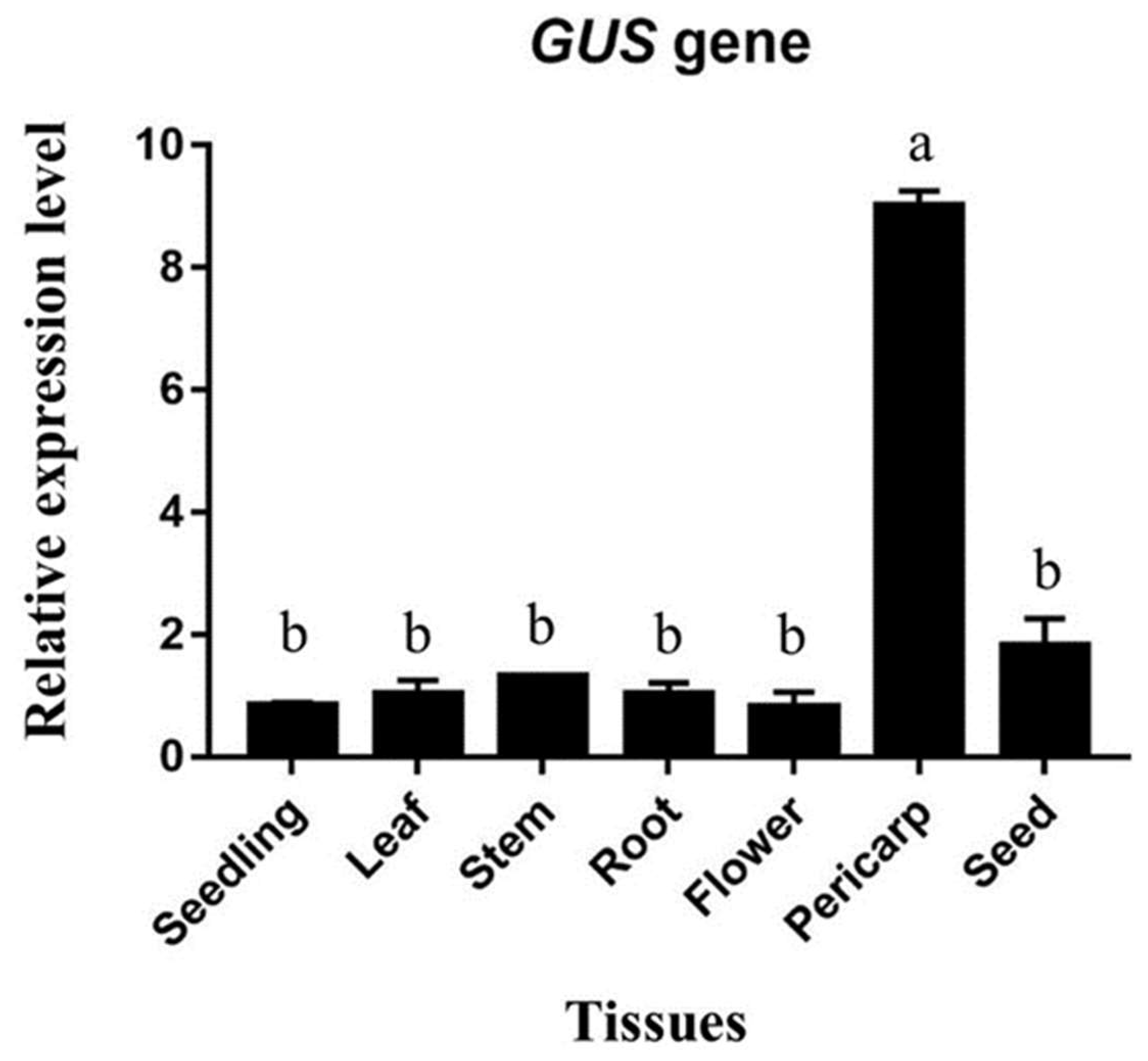
2.7. Quantification of GUS Gene Expression by qRT-PCR
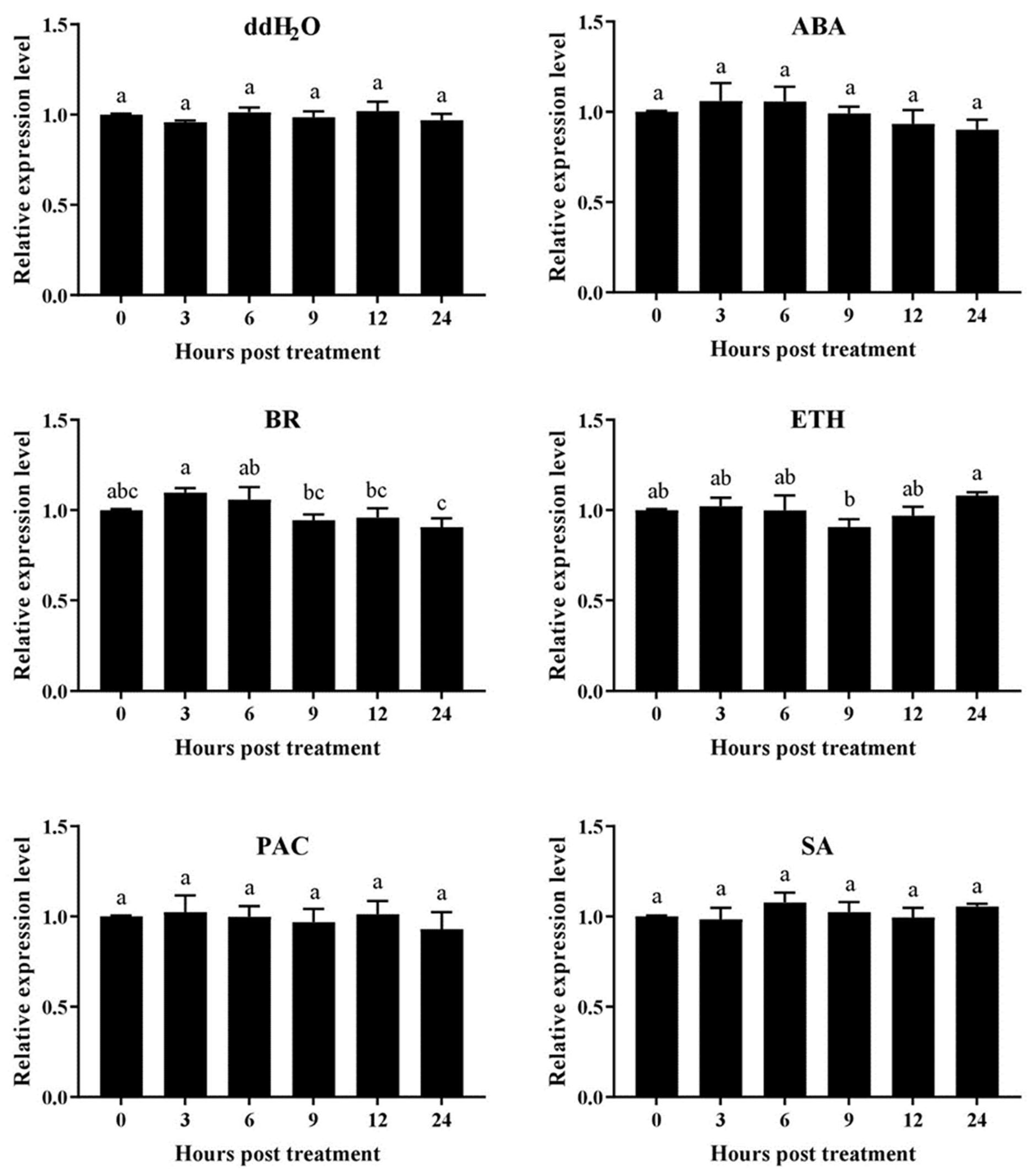
2.8. Expression Responses of AhN8DT-2P to Different Hormonal Treatments
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Identification of Pericarp- and Testa-Abundant Expression Gene
4.3. Bioinformatics Analysis of AhN8DT-2 Gene and Promoter
4.4. Expression Validation of AhN8DT-2 Gene in Various Tissues
4.5. DNA Isolation and Promoter Amplification
4.6. Cloning of AhN8DT-2 Promoter into pMDC164 Vector
4.7. Transformation into Arabidopsis
4.8. Screening of Positive Plants and Generation of Pure Lines
4.9. Histochemical GUS Expression
4.10. Real-Time Expression of UidA Gene
4.11. Cryostat Sectioning Analysis of Transgenic Arabidopsis Seeds
4.12. Evaluation of AhN8DT-2P under Different Phytohormones
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Hinds, M.J. Fatty acid composition of Caribbean-grown peanuts (Arachis hypogaea L.) at three maturity stages. Food Chem. 1995, 53, 7–14. [Google Scholar] [CrossRef]
- Gupta, K.; Gupta, S.; Faigenboim-Doron, A.; Patil, A.S.; Levy, Y.; Carrus, S.C.; Hovav, R. Deep transcriptomic study reveals the role of cell wall biosynthesis and organization networks in the developing shell of peanut pod. BMC Plant Biol. 2021, 21, 509. [Google Scholar] [CrossRef] [PubMed]
- Mupunga, I.; Mngqawa, P.; Katerere, D.R. Peanuts, aflatoxins and undernutrition in children in Sub-Saharan Africa. Nutrients 2017, 9, 1287. [Google Scholar] [CrossRef] [PubMed]
- Cobos, C.J.; Tengey, T.K.; Balasubramanian, V.K.; Williams, L.D.; Sudini, H.K.; Varshney, R.K.; Falalou, H.; Burow, M.D.; Mendu, V. Employing Peanut Seed Coat Cell Wall Mediated Resistance against Aspergillus flavus Infection and Aflatoxin Contamination. Plant Sci. 2018. preprint. [Google Scholar]
- Sharif, Y.; Mamadou, G.; Yang, Q.; Cai, T.; Zhuang, Y.; Chen, K.; Deng, Y.; Khan, S.A.; Ali, N.; Zhang, C. Genome-Wide Investigation of Apyrase (APY) Genes in Peanut (Arachis hypogaea L.) and Functional Characterization of a Pod-Abundant Expression Promoter AhAPY2-1p. Int. J. Mol. Sci. 2023, 24, 4622. [Google Scholar] [CrossRef] [PubMed]
- Sharif, Y.; Chen, H.; Deng, Y.; Ali, N.; Khan, S.; Zhang, C.; Xie, W.; Chen, K.; Cai, T.; Yang, Q. Cloning and Functional Characterization of a Pericarp Abundant Expression Promoter (AhGLP17-1P) From Peanut (Arachis hypogaea L.). Front. Genet. 2022, 12, 821281. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Tsurumaru, Y.; Yamamoto, H.; Yazaki, K. Molecular characterization of a membrane-bound prenyltransferase specific for isoflavone from Sophora flavescens. J. Biol. Chem. 2011, 286, 24125–24134. [Google Scholar] [CrossRef] [PubMed]
- Munakata, R.; Kitajima, S.; Nuttens, A.; Tatsumi, K.; Takemura, T.; Ichino, T.; Galati, G.; Vautrin, S.; Berges, H.; Grosjean, J.; et al. Convergent evolution of the UbiA prenyltransferase family underlies the independent acquisition of furanocoumarins in plants. New Phytol. 2020, 225, 2166–2182. [Google Scholar] [CrossRef]
- Sasaki, K.; Mito, K.; Ohara, K.; Yamamoto, H.; Yazaki, K. Cloning and characterization of naringenin 8-prenyltransferase, a flavonoid-specific prenyltransferase of Sophora flavescens. Plant Physiol. 2008, 146, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Chen, H.; Yang, M.; Wang, J.; Pandey, M.K.; Zhang, C.; Chang, W.-C.; Zhang, L.; Zhang, X.; Tang, R. The genome of cultivated peanut provides insight into legume karyotypes, polyploid evolution and crop domestication. Nat. Genet. 2019, 51, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.L.; Chandrasekharan, M.B.; Hall, T.C.; Crowe, A.J. Sequence and spacing of TATA box elements are critical for accurate initiation from the β-phaseolin promoter. J. Biol. Chem. 2004, 279, 8102–8110. [Google Scholar] [CrossRef]
- Shirsat, A.; Wilford, N.; Croy, R.; Boulter, D. Sequences responsible for the tissue specific promoter activity of a pea legumin gene in tobacco. Mol. Gen. Genet. 1989, 215, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Ezcurra, I.; Ellerström, M.; Wycliffe, P.; Stålberg, K.; Rask, L. Interaction between composite elements in the napA promoter: Both the B-box ABA-responsive complex and the RY/G complex are necessary for seed-specific expression. Plant Mol. Biol. 1999, 40, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Ayoubi, T.A.; Van De Yen, W.J. Regulation of gene expression by alternative promoters. FASEB J. 1996, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Porto, M.S.; Pinheiro, M.P.N.; Batista, V.G.L.; dos Santos, R.C.; de Albuquerque Melo Filho, P.; de Lima, L. Plant promoters: An approach of structure and function. Mol Biotechnol. 2014, 56, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Auriac, M.-C.; Timmers, A.C. Nodulation studies in the model legume Medicago truncatula: Advantages of using the constitutive EF1α promoter and limitations in detecting fluorescent reporter proteins in nodule tissues. Mol. Plant-Microbe Interact. 2007, 20, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Kishor, P.K.; Bhatnagar-Mathur, P.; Singam, P.; Sharma, K.K.; Vadez, V.; Reddy, P.S. Isolation and functional characterization of three abiotic stress-inducible (Apx, Dhn and Hsc70) promoters from pearl millet (Pennisetum glaucum L.). Mol. Biol. Rep. 2019, 46, 6039–6052. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, C.; Yan, C.; Zhao, X.; Wang, J.; Sun, Q.; Shan, S. Isolation and characterization of a novel seed-specific promoter from peanut (Arachis hypogaea L.). Mol. Biol. Rep. 2019, 46, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Li, Y.; Tang, Y.; Wei, R.; Huijun, Z.; Wang, Y.; Zhang, C. Vacuolar processing enzyme (VvβVPE) from Vitis vinifera, processes seed proteins during ovule development, and accelerates seed germination in VvβVPE heterologously over-expressed Arabidopsis. Plant Sci. 2018, 274, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, S.; Liu, S.; Jiang, L.; Chen, L.; Ren, Y.; Han, X.; Liu, F.; Ji, S.; Liu, X. The vacuolar processing enzyme OsVPE1 is required for efficient glutelin processing in rice. Plant J. 2009, 58, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Nishimura, M.; Hara-Nishimura, I. The sequence and expression of the γ-VPE gene, one member of a family of three genes for vacuolar processing enzymes in Arabidopsis thaliana. Plant Cell Physiol. 1995, 36, 1555–1562. [Google Scholar] [PubMed]
- Tang, Y.; Wang, R.; Gong, P.; Li, S.; Wang, Y.; Zhang, C. Gene cloning, expression and enzyme activity of Vitis vinifera vacuolar processing enzymes (VvVPEs). PLoS ONE 2016, 11, e0160945. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J. The structure of plant gene promoters. In Genetic Engineering; Springer: Berlin/Heidelberg, Germany, 1997; pp. 15–47. [Google Scholar]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Mu, B.; Li, S.; Li, Y.; Zhou, X.; Zhang, C.; Fan, Y.; Chen, R. Engineering of ‘Purple Embryo Maize’ with a multigene expression system derived from a bidirectional promoter and self-cleaving 2A peptides. Plant Biotechnol. J. 2018, 16, 1107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H. Development of “purple endosperm rice” by engineering anthocyanin biosynthesis in the endosperm with a high-efficiency transgene stacking system. Mol. Plant 2017, 10, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Truksa, M.; Datla, N.; Vrinten, P.; Bauer, J.; Zank, T.; Cirpus, P.; Heinz, E.; Qiu, X. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 2005, 23, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Xu, P.; Liu, W.; Liu, Z.; Shan, L. Cloning and characterization of 5′ flanking regulatory sequences of AhLEC1B gene from Arachis hypogaea L. PLoS ONE 2015, 10, e0139213. [Google Scholar] [CrossRef] [PubMed]
- Kummari, D.; Palakolanu, S.R.; Kishor, P.; Bhatnagar-Mathur, P.; Singam, P.; Vadez, V.; Sharma, K.K. An update and perspectives on the use of promoters in plant genetic engineering. J. Biosci. 2020, 45, 119. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A.J. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Higo, H. PLACE: A database of plant cis-acting regulatory DNA elements. Nucleic Acids Res. 1998, 26, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, C.; Cai, T.C.; Deng, Y.; Zhou, S.; Zheng, Y.; Ma, S.; Tang, R.; Varshney, R.K.; Zhuang, W. Identification of low Ca2+ stress-induced embryo apoptosis response genes in Arachis hypogaea by SSH-associated library lift (SSHaLL). Plant Biotechnol. J. 2016, 14, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Yu, J.; Wang, L.; Zhou, S. A modified CTAB protocol for plant DNA extraction. Chin. Bull. Bot. 2013, 48, 72. [Google Scholar]
- Sheikholeslam, S.N.; Weeks, D.P. Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens. Plant Mol. Biol. 1987, 8, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, Y.; Zhuang, Y.; Xie, W.; Zhang, C.; Chen, K.; Deng, Y.; Chen, Y.; Fu, H.; Wang, L.; Chen, X.; et al. Molecular Cloning and Functional Identification of a Pericarp- and Testa-Abundant Gene’s (AhN8DT-2) Promoter from Arachis hypogaea. Int. J. Mol. Sci. 2024, 25, 7671. https://doi.org/10.3390/ijms25147671
Sharif Y, Zhuang Y, Xie W, Zhang C, Chen K, Deng Y, Chen Y, Fu H, Wang L, Chen X, et al. Molecular Cloning and Functional Identification of a Pericarp- and Testa-Abundant Gene’s (AhN8DT-2) Promoter from Arachis hypogaea. International Journal of Molecular Sciences. 2024; 25(14):7671. https://doi.org/10.3390/ijms25147671
Chicago/Turabian StyleSharif, Yasir, Yuhui Zhuang, Wenpin Xie, Chong Zhang, Kun Chen, Ye Deng, Yuting Chen, Huiwen Fu, Lihui Wang, Xiangyu Chen, and et al. 2024. "Molecular Cloning and Functional Identification of a Pericarp- and Testa-Abundant Gene’s (AhN8DT-2) Promoter from Arachis hypogaea" International Journal of Molecular Sciences 25, no. 14: 7671. https://doi.org/10.3390/ijms25147671
APA StyleSharif, Y., Zhuang, Y., Xie, W., Zhang, C., Chen, K., Deng, Y., Chen, Y., Fu, H., Wang, L., Chen, X., Zhuang, W., & Chen, H. (2024). Molecular Cloning and Functional Identification of a Pericarp- and Testa-Abundant Gene’s (AhN8DT-2) Promoter from Arachis hypogaea. International Journal of Molecular Sciences, 25(14), 7671. https://doi.org/10.3390/ijms25147671





