Long-Term Remission with Novel Combined Immune-Targeted Treatment for Histiocytic Sarcoma Accompanied by Follicular Lymphoma: Case Report and Literature Review
Abstract
1. Introduction
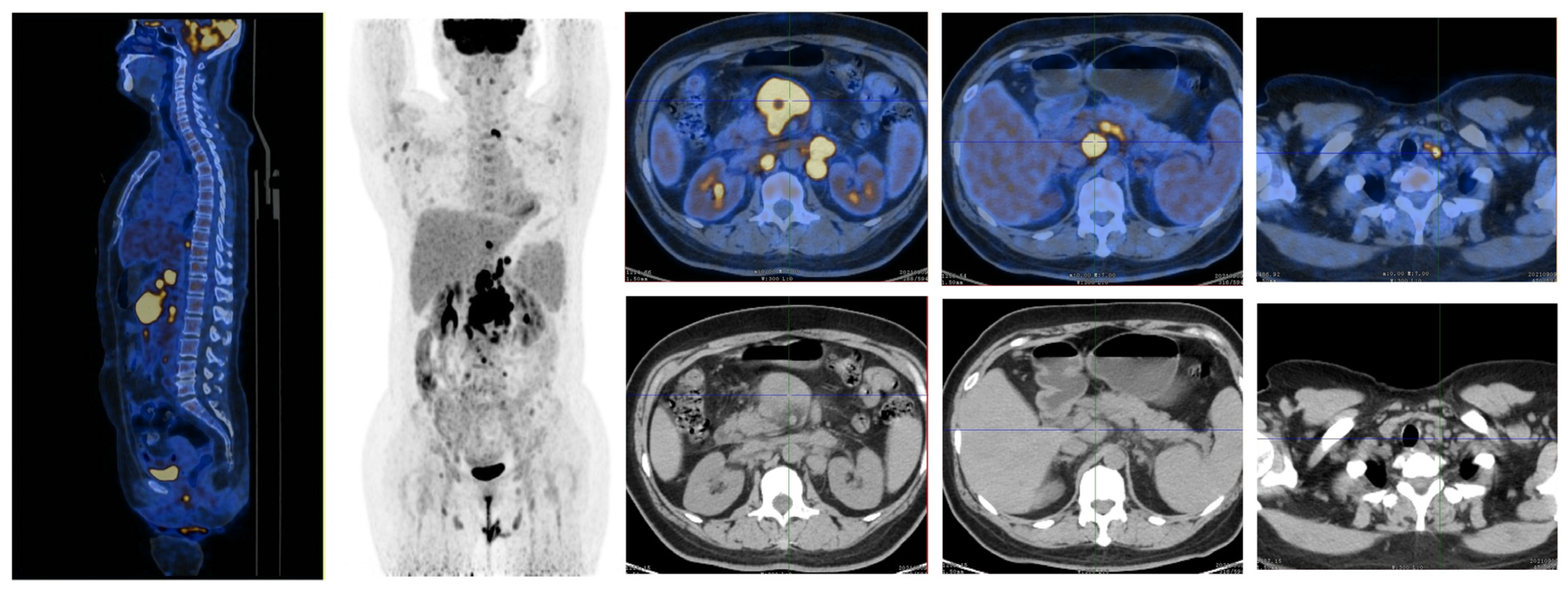
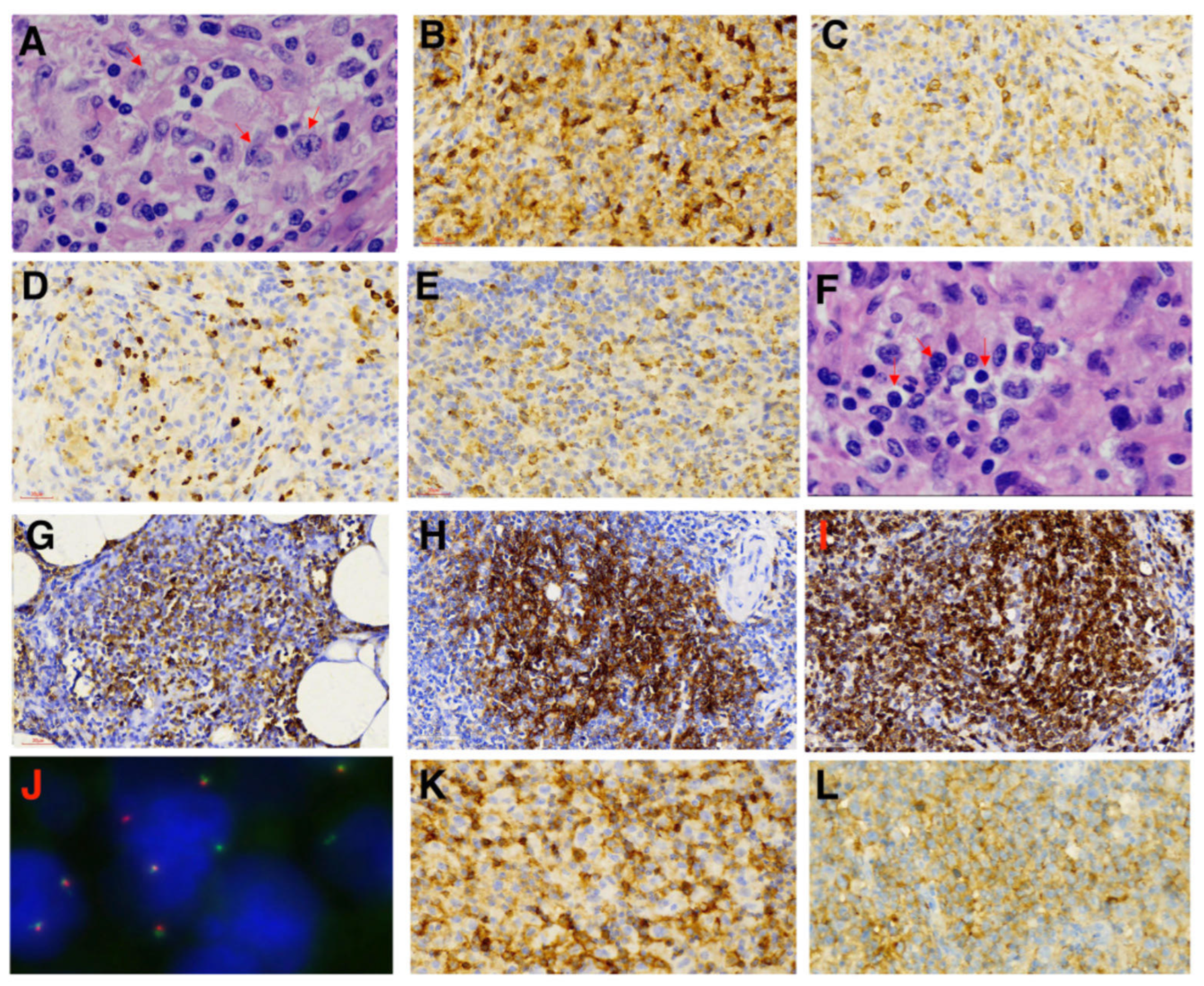
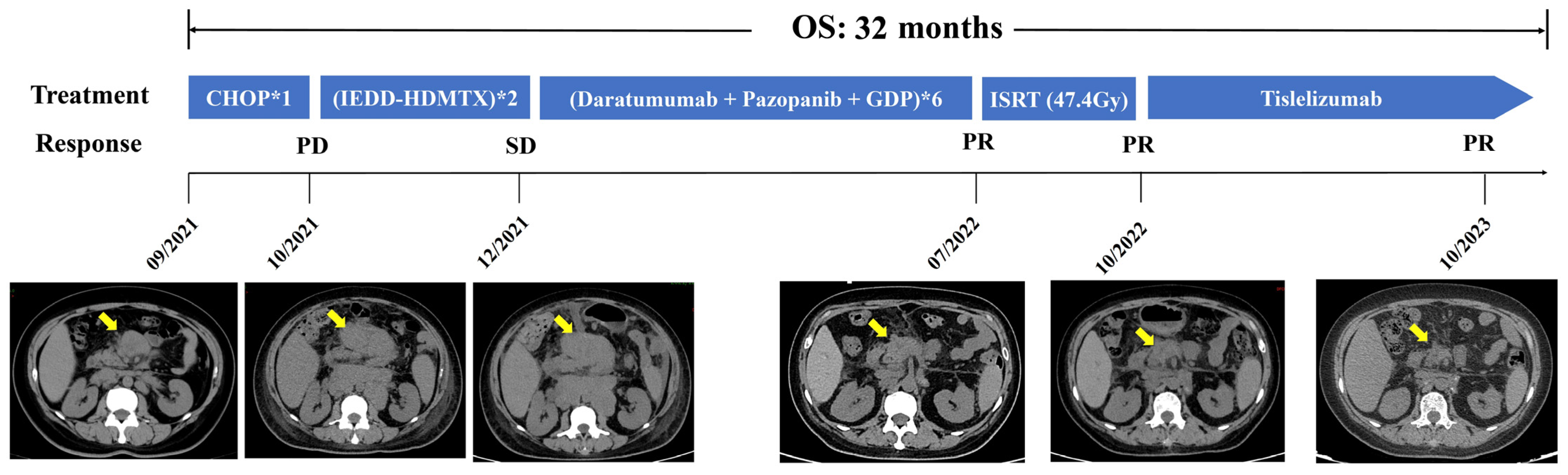
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Feldman, A.L.; Arber, D.A.; Pittaluga, S.; Martinez, A.; Burke, J.S.; Raffeld, M.; Camos, M.; Warnke, R.; Jaffe, E.S. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: Evidence for transdifferentiation of the follicular lymphoma clone. Blood 2008, 111, 5433–5439. [Google Scholar] [CrossRef]
- Wang, E.; Hutchinson, C.B.; Huang, Q.; Sebastian, S.; Rehder, C.; Kanaly, A.; Moore, J.; Datto, M. Histiocytic sarcoma arising in indolent small B-cell lymphoma: Report of two cases with molecular/genetic evidence suggestive of a ‘transdifferentiation’ during the clonal evolution. Leuk. Lymphoma 2010, 51, 802–812. [Google Scholar] [CrossRef]
- Hure, M.C.; Elco, C.P.; Ward, D.; Hutchinson, L.; Meng, X.; Dorfman, D.M.; Yu, H. Histiocytic sarcoma arising from clonally related mantle cell lymphoma. J. Clin. Oncol. 2012, 30, e49–e53. [Google Scholar] [CrossRef]
- Kommalapati, A.; Tella, S.H.; Durkin, M.; Go, R.S.; Goyal, G. Histiocytic sarcoma: A population-based analysis of incidence, demographic disparities, and long-term outcomes. Blood 2018, 131, 265–268. [Google Scholar] [CrossRef]
- Gupta, D.; Gupta, A.; Nalwa, A.; Yadav, T.; Chaudhary, R.; Rao, M. Cytopathologic findings in histiocytic sarcoma of thyroid mimicking as anaplastic carcinoma: A report of a rare case with review of literature. Diagn. Cytopathol. 2021, 49, E218–E221. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, K.; Delgado, R.; Kochiyil, J.; Medina, A.M. Primary Histiocytic Sarcoma in Adult Polycystic Kidney Disease: Case Report and Review of Literature. Int. J. Surg. Pathol. 2021, 29, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, R.; Kanazawa, Y.; Matsuno, K.; Kakinuma, D.; Tokura, T.; Marumo, A.; Yui, S.; Ando, F.; Masuda, Y.; Hagiwara, N.; et al. An advanced case of gastric histiocytic sarcoma treated with chemotherapy and gastrectomy: A case report and review of literature. Clin. J. Gastroenterol. 2021, 14, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, N.; Lara-Endara, J.; Redrobán, L.; Leiva, M.; Armijos, C.; Russo, L. Primary splenic histiocytic sarcoma associated with hemophagocytic lymphohistiocytosis: A case report and review of literature of next-generation sequencing involving FLT3, NOTCH2, and KMT2A mutations. Cancer Rep. 2022, 5, e1496. [Google Scholar] [CrossRef]
- Péricart, S.; Waysse, C.; Siegfried, A.; Struski, S.; Delabesse, E.; Laurent, C.; Evrard, S. Subsequent development of histiocytic sarcoma and follicular lymphoma: Cytogenetics and next-generation sequencing analyses provide evidence for transdifferentiation of early common lymphoid precursor—A case report and review of literature. Virchows Arch. 2020, 476, 609–614. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Dong, Y.; Li, S.; Qin, H. Primary central nervous system histiocytic sarcoma with somatic NF2 mutation: Case report and review of literature. Clin. Neuropathol. 2022, 41, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pol, S.; Bangs, C.D.; Cherry, A.; Arber, D.A.; Gratzinger, D. Two cases of histiocytic sarcoma with BCL2 translocations and occult or subsequent follicular lymphoma. Hum. Pathol. 2016, 55, 39–43. [Google Scholar] [CrossRef]
- Sabatini, P.J.B.; Tremblay-Lemay, R.; Ahmadi Moghaddam, P.; Delabie, J.M.A.; Sakhdari, A. Marginal zone lymphoma transdifferentiated to histiocytic sarcoma. Br. J. Haematol. 2021, 194, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Bassarova, A.; Trøen, G.; Fosså, A.; Ikonomou, I.M.; Beiske, K.; Nesland, J.M.; Delabie, J. Transformation of B cell lymphoma to histiocytic sarcoma: Somatic mutations of PAX-5 gene with loss of expression cannot explain transdifferentiation. J. Hematop. 2009, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.; Rufle, A.; Dirnhofer, S.; Lohri, A.; Willi, N.; Cathomas, G.; Tzankov, A.; Juskevicius, D. Follicular lymphoma transformation into histiocytic sarcoma: Indications for a common neoplastic progenitor. Leukemia 2014, 28, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Etancelin, P.; Boussaid, I. Two sides of the same coin: Transdifferentiation from Burkitt lymphoma to histiocytic sarcoma. Blood 2023, 142, 1576. [Google Scholar] [CrossRef] [PubMed]
- Cheon, M.; Yoo, J.; Kim, H.S.; Lee, M. Enhanced Computed Tomography and (18)F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in the Uncommon Histiocytic Sarcoma of Small Intestine Arising after Gastric Large B-Cell Lymphoma. Diagnostics 2023, 13, 3189. [Google Scholar] [CrossRef] [PubMed]
- Farris, M.; Hughes, R.T.; Lamar, Z.; Soike, M.H.; Menke, J.R.; Ohgami, R.S.; Winkfield, K. Histiocytic Sarcoma Associated with Follicular Lymphoma: Evidence for Dramatic Response with Rituximab and Bendamustine Alone and a Review of the Literature. Clin. Lymphoma Myeloma Leuk. 2019, 19, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Al-Kawaaz, M.; Martin, B.A.; Hegazi, M.M.; Tan, B.; Gratzinger, D. Histiocytic Sarcoma with CCND1 Gene Rearrangement Clonally Related and Transdifferentiated from Mantle Cell Lymphoma. Am. J. Clin. Pathol. 2022, 158, 449–455. [Google Scholar] [CrossRef]
- Shao, H.; Xi, L.; Raffeld, M.; Feldman, A.L.; Ketterling, R.P.; Knudson, R.; Rodriguez-Canales, J.; Hanson, J.; Pittaluga, S.; Jaffe, E.S. Clonally related histiocytic/dendritic cell sarcoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: A study of seven cases. Mod. Pathol. 2011, 24, 1421–1432. [Google Scholar] [CrossRef]
- Cheng, F.; Yu, F.; Wang, X.; Huang, K.; Lu, H.; Wang, Z. A Pedigree Analysis and Clonal Correlations of the Coexistence of B-Cell Lymphoma and Histiocytic/Dendritic Cell Tumor. Int. J. Surg. Pathol. 2021, 29, 906–914. [Google Scholar] [CrossRef]
- D’amore, E.S.; Mainardi, C.; Mussolin, L.; Carraro, E.; Alaggio, R.; Lazzari, E.; Fusetti, S.; Ghirotto, C.; Marzollo, A.; Biddeci, G.; et al. Histiocytic sarcoma arising in a child affected by Burkitt lymphoma, with t(8;14)(q24;q32) positivity in both tumors. Pediatr. Hematol. Oncol. 2021, 38, 1–7. [Google Scholar] [CrossRef]
- Zhang, D.; McGuirk, J.; Ganguly, S.; Persons, D.L. Histiocytic/dendritic cell sarcoma arising from follicular lymphoma involving the bone: A case report and review of literature. Int. J. Hematol. 2009, 89, 529–532. [Google Scholar] [CrossRef]
- Schünemann, C.; Göhring, G.; Behrens, Y.L.; Kreipe, H.-H.; Ganser, A.; Thol, F. Histiocytic sarcoma progressing from follicular lymphoma and mimicking acquired hemophagocytic lymphohistiocytosis. Ann. Hematol. 2020, 99, 2441–2443. [Google Scholar] [CrossRef]
- Shi, C.M.; Lytle, A.M.; Milman, T.; Penne, R.; Bagg, A. Composite Histiocytic Sarcoma and Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma of the Ocular Adnexa. Ophthalmic Plast. Reconstr. Surg. 2024, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Egan, C.; Lack, J.; Skarshaug, S.; Pham, T.A.; Abdullaev, Z.; Xi, L.; Pack, S.; Pittaluga, S.; Jaffe, E.S.; Raffeld, M. The mutational landscape of histiocytic sarcoma associated with lymphoid malignancy. Mod. Pathol. 2020, 34, 336–347. [Google Scholar] [CrossRef]
- Hung, Y.P.; Qian, X. Histiocytic Sarcoma. Arch. Pathol. Lab. Med. 2020, 144, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Tocut, M.; Vaknine, H.; Potachenko, P.; Elias, S.; Zandman-Goddard, G. Histiocytic Sarcoma. Isr. Med. Assoc. J. 2020, 22, 645–647. [Google Scholar] [PubMed]
- Campedel, L.; Kharroubi, D.; Vozy, A.; Spano, J.P.; Emile, J.F.; Haroche, J. Malignant Histiocytosis With PD-L1 Expression-Dramatic Response to Nivolumab. Mayo Clin. Proc. 2022, 97, 1401–1403. [Google Scholar] [CrossRef]
- Imataki, O.; Uemura, M.; Fujita, H.; Kadowaki, N. Application of PD-L1 blockade in refractory histiocytic sarcoma: A case report. Mol. Clin. Oncol. 2022, 17, 1–6. [Google Scholar] [CrossRef]
- May, J.M.; Waddle, M.R.; Miller, D.H.; Stross, W.C.; Kaleem, T.A.; May, B.C.; Miller, R.C.; Jiang, L.; Strong, G.W.; Trifiletti, D.M.; et al. Primary histiocytic sarcoma of the central nervous system: A case report with platelet derived growth factor receptor mutation and PD-L1/PD-L2 expression and literature review. Radiat. Oncol. 2018, 13, 167. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, Y.; Jiang, Y.; Zheng, W.; Tan, Y.; Yang, Z.; Wang, Z.; Xu, F.; Cheng, Z.; Yuan, L.; et al. Case report: Targeting the PD-1 receptor and genetic mutations validated in primary histiocytic sarcoma with hemophagocytic lymphohistiocytosis. Front. Immunol. 2023, 14, 1127599. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, X.; Wang, Z.; Lu, H.; Lin, X.; Lu, Q. Histiocytic sarcoma combined with acute monocytic leukemia: A case report. Diagn. Pathol. 2015, 10, 1–6. [Google Scholar] [CrossRef]
- Michonneau, D.; Kaltenbach, S.; Derrieux, C.; Trinquand, A.; Brouzes, C.; Gibault, L.; North, M.O.; Delarue, R.; Varet, B.; Emile, J.F.; et al. BRAF(V600E) mutation in a histiocytic sarcoma arising from hairy cell leukemia. J. Clin. Oncol. 2014, 32, e117–e121. [Google Scholar] [CrossRef]
- Shanmugam, V.; Griffin, G.K.; Jacobsen, E.D.; Fletcher, C.D.M.; Sholl, L.M.; Hornick, J.L. Identification of diverse activating mutations of the RAS-MAPK pathway in histiocytic sarcoma. Mod. Pathol. 2019, 32, 830–843. [Google Scholar] [CrossRef]
- Egan, C.; Nicolae, A.; Lack, J.; Chung, H.-J.; Skarshaug, S.; Pham, T.A.; Navarro, W.; Abdullaev, Z.; Aguilera, N.S.; Xi, L.; et al. Genomic profiling of primary histiocytic sarcoma reveals two molecular subgroups. Haematologica 2020, 105, 951–960. [Google Scholar] [CrossRef]
- Hung, Y.P.; Lovitch, S.B.; Qian, X. Histiocytic sarcoma: New insights into FNA cytomorphology and molecular characteristics. Cancer Cytopathol. 2017, 125, 604–614. [Google Scholar] [CrossRef]
- Narita, K.; Noro, R.; Seike, M.; Matsumoto, M.; Fujita, K.; Matsumura, J.; Takahashi, M.; Kawamoto, M.; Gemma, A. Successful treatment of histiocytic sarcoma and concurrent HIV infection using a combination of CHOP and antiretroviral therapy. Intern. Med. 2013, 52, 2805–2809. [Google Scholar] [CrossRef][Green Version]
- Philip, D.S.J.; Sherief, A.; Narayanan, G.; Nair, S.G.; Av, J. Histiocytic Sarcoma: Clinical Features and Outcomes of Patients Treated at a Tertiary Cancer Care Center. Cureus 2022, 14, e25814. [Google Scholar] [CrossRef]
- Tsujimura, H.; Miyaki, T.; Yamada, S.; Sugawara, T.; Ise, M.; Iwata, S.; Yonemoto, T.; Ikebe, D.; Itami, M.; Kumagai, K. Successful treatment of histiocytic sarcoma with induction chemotherapy consisting of dose-escalated CHOP plus etoposide and upfront consolidation auto-transplantation. Int. J. Hematol. 2014, 100, 507–510. [Google Scholar] [CrossRef]
- Zeidan, A.; Bolaños-Meade, J.; Kasamon, Y.; Aoki, J.; Borowitz, M.; Swinnen, L.; Symons, H.; Luznik, L.; Fuchs, E.; Jones, R.; et al. Human leukocyte antigen-haploidentical hematopoietic stem cell transplant for a patient with histiocytic sarcoma. Leuk. Lymphoma 2012, 54, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Schlick, K.; Aigelsreiter, A.; Pichler, M.; Reitter, S.; Neumeister, P.; Hoefler, G.; Beham-Schmid, C.; Linkesch, W. Histiocytic sarcoma—Targeted therapy: Novel therapeutic options? A series of 4 cases. Onkologie 2012, 35, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.M.; Solit, D.B.; Tap, W.D. Trametinib in Histiocytic Sarcoma with an Activating MAP2K1 (MEK1) Mutation. N. Engl. J. Med. 2018, 378, 1945–1947. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Patel, J.L.; Tao, R.; Cannon, R.B.; Monroe, M.; Goyal, G. Near Complete Response to Trametinib Treatment in Histiocytic Sarcoma Harboring a Somatic KRAS Mutation. J. Natl. Compr. Cancer Netw. 2022, 20, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Mokhtari, K.; Emile, J.-F.; Galanaud, D.; Belaid, H.; de Bernard, S.; Benameur, N.; Barlog, V.-C.; Psimaras, D.; Donadieu, J.; et al. Dramatic response of a BRAF V600E-mutated primary CNS histiocytic sarcoma to vemurafenib. Neurology 2014, 83, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, J.L.; Freitag, C.E.; Hemminger, J.A.; Jones, J.A. BRAF V600E expression in histiocytic sarcoma associated with splenic marginal zone lymphoma: A case report. J. Med. Case Rep. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Furui, Y.; Kurata, T.; Komori, K.; Uchida, E.; Miyairi, Y.; Chiba, A.; Ogiso, Y.; Sakashita, K. A case of recurrent refractory cervical primary histiocytic sarcoma treated with pembrolizumab. Int. Cancer Conf. J. 2022, 11, 280–285. [Google Scholar] [CrossRef]
- Frampton, J.E. Pazopanib: A Review in Advanced Renal Cell Carcinoma. Target. Oncol. 2017, 12, 543–554. [Google Scholar] [CrossRef]
- Ward, J.E.; Stadler, W.M. Pazopanib in renal cell carcinoma. Clin. Cancer Res. 2010, 16, 5923–5927. [Google Scholar] [CrossRef]
- Kommalapati, A.; Tella, S.H.; Go, R.S.; Goyal, G. Predictors of survival, treatment patterns, and outcomes in histiocytic sarcoma. Leuk. Lymphoma 2018, 60, 553–555. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Xiao, F.; Fang, J.; Liu, Z.; Shen, Y.; Zhu, D.; Zhang, Y.; Hou, J.; Huang, H. Long-Term Remission with Novel Combined Immune-Targeted Treatment for Histiocytic Sarcoma Accompanied by Follicular Lymphoma: Case Report and Literature Review. Int. J. Mol. Sci. 2024, 25, 7293. https://doi.org/10.3390/ijms25137293
Zhang M, Xiao F, Fang J, Liu Z, Shen Y, Zhu D, Zhang Y, Hou J, Huang H. Long-Term Remission with Novel Combined Immune-Targeted Treatment for Histiocytic Sarcoma Accompanied by Follicular Lymphoma: Case Report and Literature Review. International Journal of Molecular Sciences. 2024; 25(13):7293. https://doi.org/10.3390/ijms25137293
Chicago/Turabian StyleZhang, Minyue, Fei Xiao, Jianchen Fang, Zebing Liu, Yanying Shen, Di Zhu, Yiwei Zhang, Jian Hou, and Honghui Huang. 2024. "Long-Term Remission with Novel Combined Immune-Targeted Treatment for Histiocytic Sarcoma Accompanied by Follicular Lymphoma: Case Report and Literature Review" International Journal of Molecular Sciences 25, no. 13: 7293. https://doi.org/10.3390/ijms25137293
APA StyleZhang, M., Xiao, F., Fang, J., Liu, Z., Shen, Y., Zhu, D., Zhang, Y., Hou, J., & Huang, H. (2024). Long-Term Remission with Novel Combined Immune-Targeted Treatment for Histiocytic Sarcoma Accompanied by Follicular Lymphoma: Case Report and Literature Review. International Journal of Molecular Sciences, 25(13), 7293. https://doi.org/10.3390/ijms25137293



