Breast Cancer Treatment Strategies Targeting the Tumor Microenvironment: How to Convert “Cold” Tumors to “Hot” Tumors
Abstract
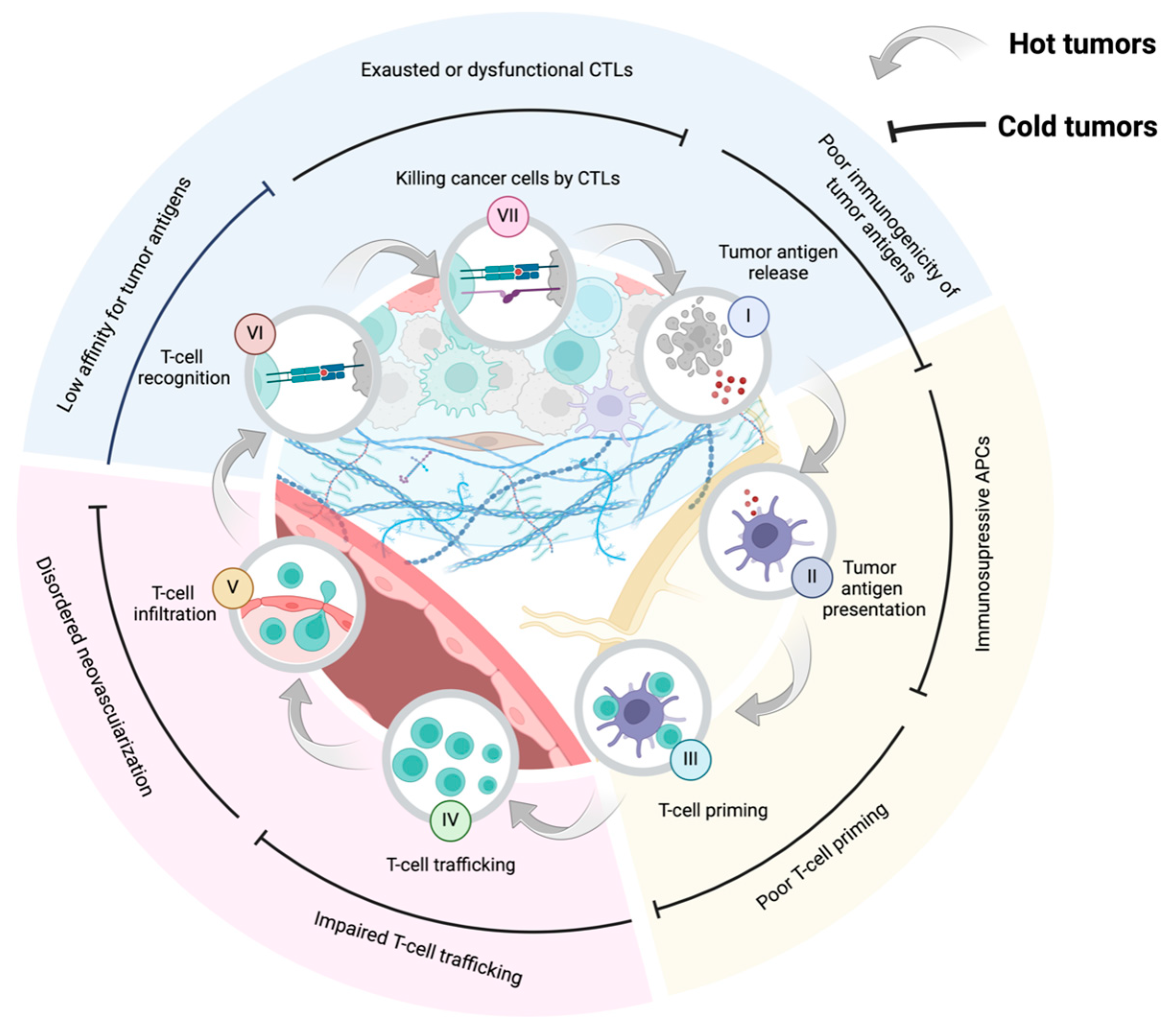
1. Introduction
2. Tumor Microenvironment, the “Soil” on Which Cancer Cells Depend for Survival
3. Strategies of Editing the Tumor Microenvironment for Breast Cancer Treatment: Nano-Based Approaches
3.1. T Cells
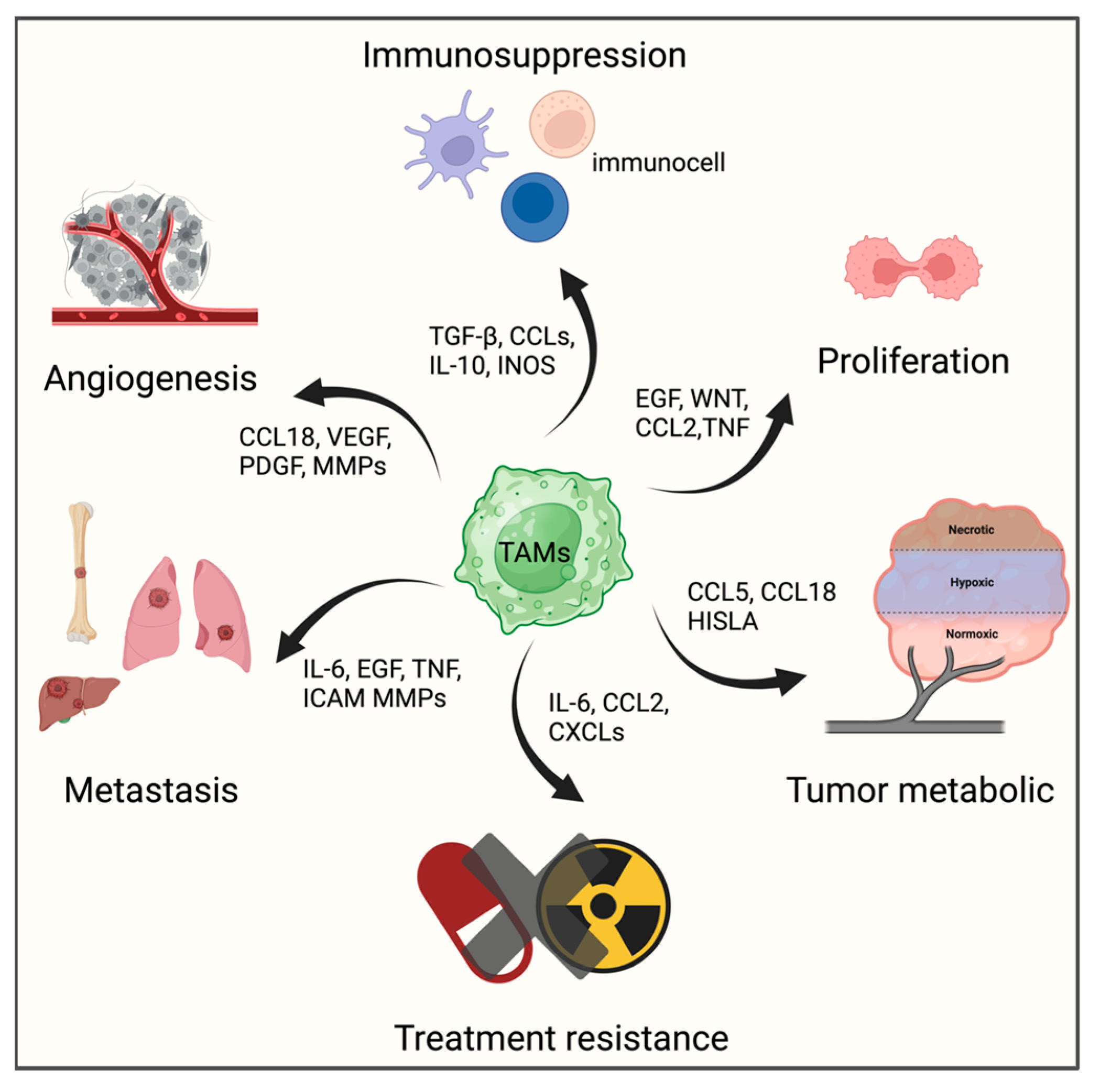
3.2. Tumor-associated Macrophages, TAMs
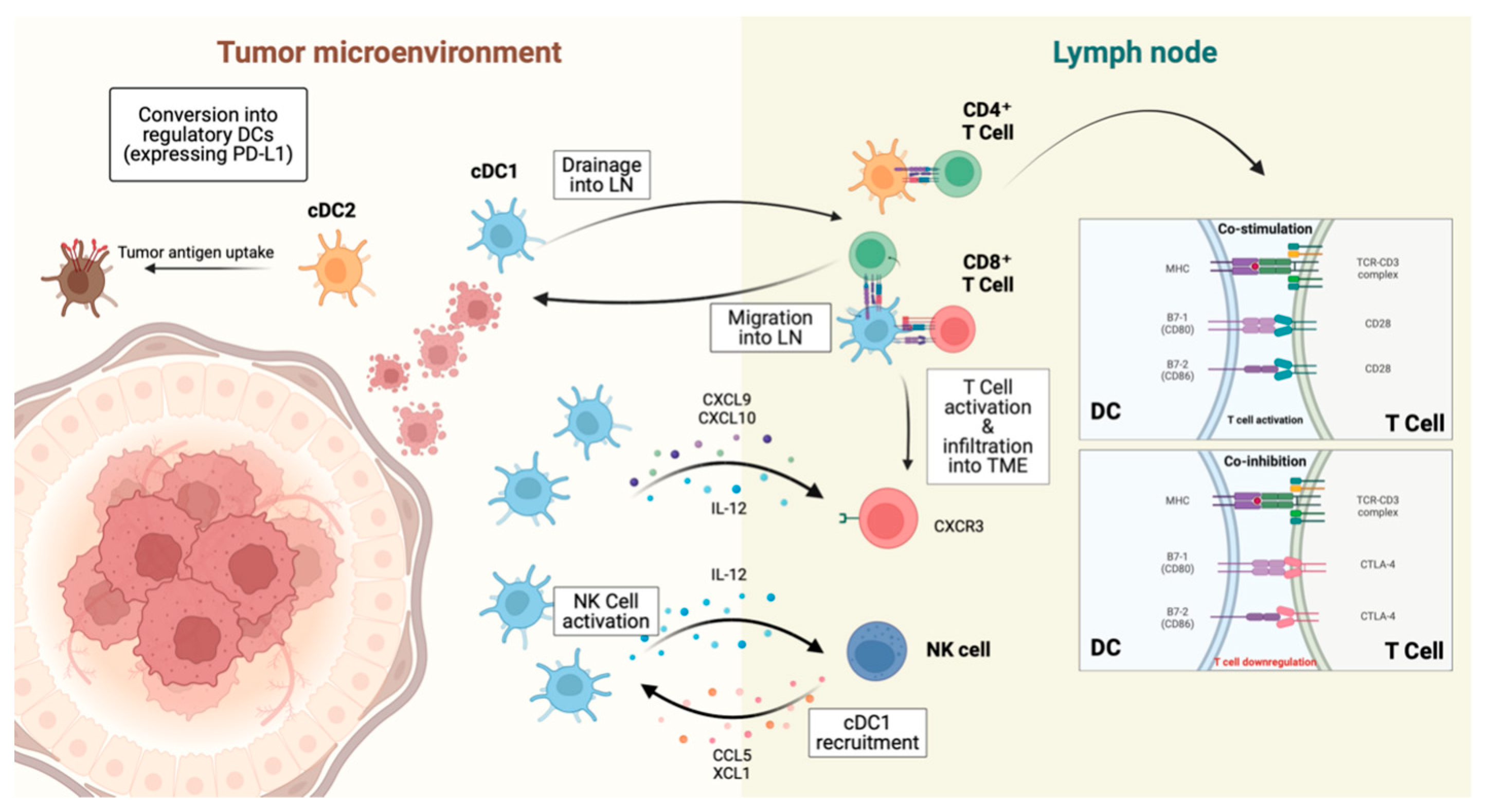
3.3. Dendritic Cells, DCs
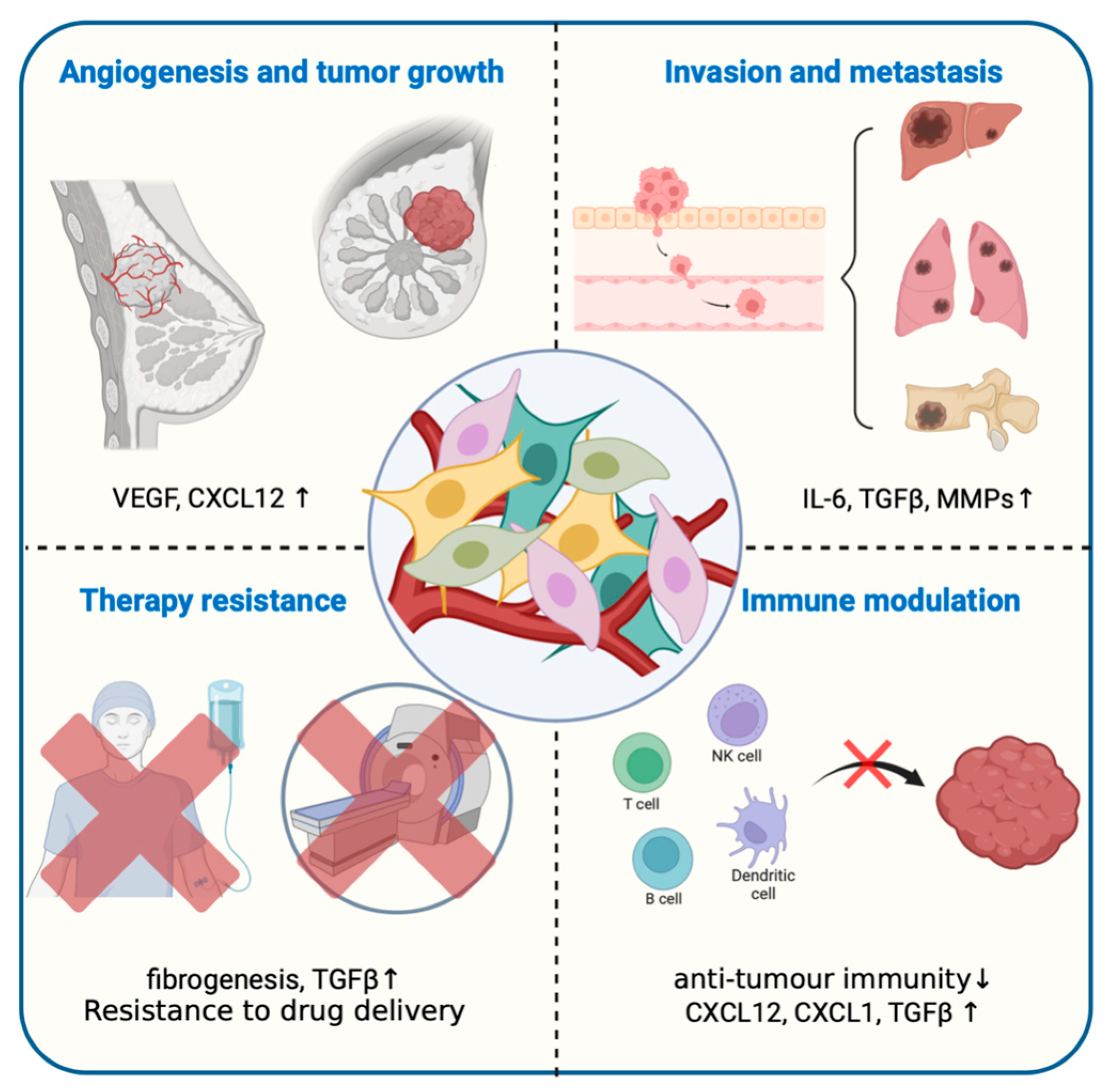
3.4. Cancer-Associated Fibroblasts, CAFs
3.5. Tumor-Associated Neutrophils, TANs
3.6. ErbB/HER Signaling
3.7. Physical and Chemical Properties
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Geyer, F.C.; Pareja, F.; Weigelt, B.; Rakha, E.; Ellis, I.O.; Schnitt, S.J.; Reis-Filho, J.S. The Spectrum of Triple-Negative Breast Disease: High- and Low-Grade Lesions. Am. J. Pathol. 2017, 187, 2139–2151. [Google Scholar] [CrossRef]
- Lee, J.S.; Yost, S.E.; Yuan, Y. Neoadjuvant Treatment for Triple Negative Breast Cancer: Recent Progresses and Challenges. Cancers 2020, 12, 1404. [Google Scholar] [CrossRef]
- Sharma, P.; López-Tarruella, S.; García-Saenz, J.A.; Khan, Q.J.; Gómez, H.L.; Prat, A.; Moreno, F.; Jerez-Gilarranz, Y.; Barnadas, A.; Picornell, A.C.; et al. Pathological Response and Survival in Triple-Negative Breast Cancer Following Neoadjuvant Carboplatin plus Docetaxel. Clin. Cancer Res. 2018, 24, 5820–5829. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Viale, G.; Curigliano, G. Recent advances in triple negative breast cancer: The immunotherapy era. BMC Med. 2019, 17, 90. [Google Scholar] [CrossRef]
- Rui, R.; Zhou, L.; He, S. Cancer immunotherapies: Advances and bottlenecks. Front. Immunol. 2023, 14, 1212476. [Google Scholar] [CrossRef]
- Law, A.M.; Lim, E.; Ormandy, C.J.; Gallego-Ortega, D. The innate and adaptive infiltrating immune systems as targets for breast cancer immunotherapy. Endocr. Relat. Cancer 2017, 24, R123–R144. [Google Scholar] [CrossRef]
- Deepak, K.G.K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.N.; Malla, R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sun, Z.J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, T.; Jiang, C. Recent Advancements in Nanomedicine for ‘Cold’ Tumor Immunotherapy. Nanomicro Lett. 2021, 13, 92. [Google Scholar] [CrossRef]
- Broz, M.L.; Krummel, M.F. The emerging understanding of myeloid cells as partners and targets in tumor rejection. Cancer Immunol. Res. 2015, 3, 313–319. [Google Scholar] [CrossRef]
- DeVito, N.C.; Plebanek, M.P.; Theivanthiran, B.; Hanks, B.A. Role of Tumor-Mediated Dendritic Cell Tolerization in Immune Evasion. Front. Immunol. 2019, 10, 2876. [Google Scholar] [CrossRef]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.I.; Cheng, P.; Cho, H.I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef] [PubMed]
- Gil Del Alcazar, C.R.; Huh, S.J.; Ekram, M.B.; Trinh, A.; Liu, L.L.; Beca, F.; Zi, X.; Kwak, M.; Bergholtz, H.; Su, Y.; et al. Immune Escape in Breast Cancer During In Situ to Invasive Carcinoma Transition. Cancer Discov. 2017, 7, 1098–1115. [Google Scholar] [CrossRef]
- Jerby-Arnon, L.; Shah, P.; Cuoco, M.S.; Rodman, C.; Su, M.J.; Melms, J.C.; Leeson, R.; Kanodia, A.; Mei, S.; Lin, J.R.; et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018, 175, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, P.; Ai, M.; Liu, A.; Budhani, P.; Bartkowiak, T.; Sheng, J.; Ager, C.; Nicholas, C.; Jaiswal, A.R.; Sun, Y.; et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J. Clin. Investig. 2018, 128, 5137–5149. [Google Scholar] [CrossRef] [PubMed]
- Lakins, M.A.; Ghorani, E.; Munir, H.; Martins, C.P.; Shields, J.D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T Cells to protect tumour cells. Nat. Commun. 2018, 9, 948. [Google Scholar] [CrossRef]
- Kato, T.; Noma, K.; Ohara, T.; Kashima, H.; Katsura, Y.; Sato, H.; Komoto, S.; Katsube, R.; Ninomiya, T.; Tazawa, H.; et al. Cancer-Associated Fibroblasts Affect Intratumoral CD8+ and FoxP3+ T Cells Via IL6 in the Tumor Microenvironment. Clin. Cancer Res. 2018, 24, 4820–4833. [Google Scholar] [CrossRef]
- Kumar, V.; Donthireddy, L.; Marvel, D.; Condamine, T.; Wang, F.; Lavilla-Alonso, S.; Hashimoto, A.; Vonteddu, P.; Behera, R.; Goins, M.A.; et al. Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell 2017, 32, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Emon, B.; Bauer, J.; Jain, Y.; Jung, B.; Saif, T. Biophysics of Tumor Microenvironment and Cancer Metastasis—A Mini Review. Comput. Struct. Biotechnol. J. 2018, 16, 279–287. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Samani, A.; Zubovits, J.; Plewes, D. Elastic moduli of normal and pathological human breast tissues: An inversion-technique-based investigation of 169 samples. Phys. Med. Biol. 2007, 52, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, E.; Poincloux, R.; Gauffre, F.; Maridonneau-Parini, I.; Le Cabec, V. Matrix architecture dictates three-dimensional migration modes of human macrophages: Differential involvement of proteases and podosome-like structures. J. Immunol. 2010, 184, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Davis, C.T.; Liu, W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. 2015, 72, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Shi, Q.; Le, X.; Wang, B.; Abbruzzese, J.L.; Xiong, Q.; He, Y.; Xie, K. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene 2001, 20, 3751–3756. [Google Scholar] [CrossRef]
- Walton, Z.E.; Patel, C.H.; Brooks, R.C.; Yu, Y.; Ibrahim-Hashim, A.; Riddle, M.; Porcu, A.; Jiang, T.; Ecker, B.L.; Tameire, F.; et al. Acid Suspends the Circadian Clock in Hypoxia through Inhibition of mTOR. Cell 2018, 174, 72–87. [Google Scholar] [CrossRef]
- Shah, L.; Latif, A.; Williams, K.J.; Tirella, A. Role of stiffness and physico-chemical properties of tumour microenvironment on breast cancer cell stemness. Acta Biomater. 2022, 152, 273–289. [Google Scholar] [CrossRef]
- Erra Díaz, F.; Dantas, E.; Geffner, J. Unravelling the Interplay between Extracellular Acidosis and Immune Cells. Mediators Inflamm. 2018, 2018, 1218297. [Google Scholar] [CrossRef]
- Pantelidou, C.; Sonzogni, O.; De Oliveria Taveira, M.; Mehta, A.K.; Kothari, A.; Wang, D.; Visal, T.; Li, M.K.; Pinto, J.; Castrillon, J.A.; et al. PARP Inhibitor Efficacy Depends on CD8+ T-cell Recruitment via Intratumoral STING Pathway Activation in BRCA-Deficient Models of Triple-Negative Breast Cancer. Cancer Discov. 2019, 9, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Dang, Y.; Coveler, A.L.; Childs, J.S.; Higgins, D.M.; Liu, Y.; Zhou, J.; Mackay, S.; Salazar, L.G. A Phase I/II Trial of HER2 Vaccine-Primed Autologous T-Cell Infusions in Patients with Treatment Refractory HER2-Overexpressing Breast Cancer. Clin. Cancer Res. 2023, 29, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Chaib, M.; Sipe, L.M.; Yarbro, J.R.; Bohm, M.S.; Counts, B.R.; Tanveer, U.; Pingili, A.K.; Daria, D.; Marion, T.N.; Carson, J.A.; et al. PKC agonism restricts innate immune suppression promotes antigen cross-presentation and synergizes with agonistic CD40 antibody therapy to activate CD8+ T cells in breast cancer. Cancer Lett. 2022, 531, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Lee, H.H.; Jiang, Z.; Chan, L.C.; Hortobagyi, G.N.; Yu, D.; Hung, M.C. Ribonuclease 1 Enhances Antitumor Immunity against Breast Cancer by Boosting T cell Activation. Int. J. Biol. Sci. 2023, 19, 2957–2973. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.T.R.; Emens, L.A. Emerging combination immunotherapy strategies for breast cancer: Dual immune checkpoint modulation antibody-drug conjugates and bispecific antibodies. Breast Cancer Res. Treat. 2022, 191, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Rius Ruiz, I.; Vicario, R.; Morancho, B.; Morales, C.B.; Arenas, E.J.; Herter, S.; Freimoser-Grundschober, A.; Somandin, J.; Sam, J.; Ast, O.; et al. p95HER2-T cell bispecific antibody for breast cancer treatment. Sci. Transl. Med. 2018, 10, eaat1445. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, K.; Wang, K.; Zhu, H. Treatment-related adverse events of antibody-drug conjugates in clinical trials: A systematic review and meta-analysis. Cancer 2023, 129, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Copeland-Halperin, R.S.; Liu, J.E.; Yu, A.F. Cardiotoxicity of HER2-targeted therapies. Curr. Opin. Cardiol. 2019, 34, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, M.; Rojo, F.; Ocaña, A.; Anido, J.; Guzman, M.; Cortes, J.; Di Cosimo, S.; Matias-Guiu, X.; Ramon y Cajal, S.; Arribas, J.; et al. Expression of p95HER2 a truncated form of the HER2 receptor and response to anti-HER2 therapies in breast cancer. J. Natl. Cancer Inst. 2007, 99, 628–638. [Google Scholar] [CrossRef]
- Liu, H.; Bai, L.; Huang, L.; Ning, N.; Li, L.; Li, Y.; Dong, X.; Du, Q.; Xia, M.; Chen, Y.; et al. Bispecific antibody targeting TROP2xCD3 suppresses tumor growth of triple negative breast cancer. J. Immunother. Cancer 2021, 9, e003468. [Google Scholar] [CrossRef]
- Mittal, D.; Vijayan, D.; Neijssen, J.; Kreijtz, J.; Habraken, M.M.J.M.; Van Eenennaam, H.; Van Elsas, A.; Smyth, M.J. Blockade of ErbB2 and PD-L1 using a bispecific antibody to improve targeted anti-ErbB2 therapy. Oncoimmunology 2019, 8, e1648171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.A.; Wu, T.H.; Wang, Y.M.; Cheng, T.L.; Chen, I.J.; Lu, Y.C.; Chuang, K.H.; Wang, C.K.; Chen, C.Y.; Lin, R.A.; et al. Humanized bispecific antibody (mPEG × HER2) rapidly confers PEGylated nanoparticles tumor specificity for multimodality imaging in breast cancer. J. Nanobiotechnol. 2020, 18, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shi, J.; Shi, X.; Xu, X.; Gao, L.; Li, S.; Liu, M.; Gao, M.; Jin, S.; Zhou, J.; et al. Development and evaluation of a human CD47/HER2 bispecific antibody for Trastuzumab-resistant breast cancer immunotherapy. Drug Resist. Updates 2024, 74, 101068. [Google Scholar] [CrossRef] [PubMed]
- de Vries Schultink, A.H.M.; Doornbos, R.P.; Bakker, A.B.H.; Bol, K.; Throsby, M.; Geuijen, C.; Maussang, D.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R. Translational PK-PD modeling analysis of MCLA-128 a HER2/HER3 bispecific monoclonal antibody to predict clinical efficacious exposure and dose. Investig. New Drugs 2018, 36, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Rau, A.; Lieb, W.S.; Seifert, O.; Honer, J.; Birnstock, D.; Richter, F.; Aschmoneit, N.; Olayioye, M.A.; Kontermann, R.E. Inhibition of Tumor Cell Growth and Cancer Stem Cell Expansion by a Bispecific Antibody Targeting EGFR and HER3. Mol. Cancer Ther. 2020, 19, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Greiling, T.M.; Dehner, C.; Chen, X.; Hughes, K.; Iñiguez, A.J.; Boccitto, M.; Ruiz, D.Z.; Renfroe, S.C.; Vieira, S.M.; Ruff, W.E.; et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci. Transl. Med. 2018, 10, eaan2306. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Wang, Y.; Li, R.; Rossi, D.L.; Liu, D.; Rossi, E.A.; Cardillo, T.M.; Goldenberg, D.M. Combination Therapy with Bispecific Antibodies and PD-1 Blockade Enhances the Antitumor Potency of T Cells. Cancer Res. 2017, 77, 5384–5394. [Google Scholar] [CrossRef] [PubMed]
- Kamada, H.; Taki, S.; Nagano, K.; Inoue, M.; Ando, D.; Mukai, Y.; Higashisaka, K.; Yoshioka, Y.; Tsutsumi, Y.; Tsunoda, S. Generation and characterization of a bispecific diabody targeting both EPH receptor A10 and CD3. Biochem. Biophys. Res. Commun. 2015, 456, 908–912. [Google Scholar] [CrossRef]
- Burges, A.; Wimberger, P.; Kümper, C.; Gorbounova, V.; Sommer, H.; Schmalfeldt, B.; Pfisterer, J.; Lichinitser, M.; Makhson, A.; Moiseyenko, V.; et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: A phase I/II study. Clin. Cancer Res. 2007, 13, 3899–3905. [Google Scholar] [CrossRef]
- Hong, R.; Zhou, Y.; Tian, X.; Wang, L.; Wu, X. Selective inhibition of IDO1 D-1-methyl-tryptophan (D-1MT) effectively increased EpCAM/CD3-bispecific BiTE antibody MT110 efficacy against IDO1hi breast cancer via enhancing immune cells activity. Int. Immunopharmacol. 2018, 54, 118–124. [Google Scholar] [CrossRef]
- Fisher, T.S.; Hooper, A.T.; Lucas, J.; Clark, T.H.; Rohner, A.K.; Peano, B.; Elliott, M.W.; Tsaparikos, K.; Wang, H.; Golas, J.; et al. A CD3-bispecific molecule targeting P-cadherin demonstrates T cell-mediated regression of established solid tumors in mice. Cancer Immunol. Immunother. 2018, 67, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Shi, X.; Han, M.; Smbatyan, G.; Lenz, H.J.; Zhang, Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J. Am. Chem. Soc. 2018, 140, 16413–16417. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, B.; Howard, C.B.; Mahler, S.M.; Thurecht, K.J.; Wu, Y.; Huang, L.; Xu, Z.P. Multifunctional lipid-coated calcium phosphate nanoplatforms for complete inhibition of large triple negative breast cancer via targeted combined therapy. Biomaterials 2019, 216, 119232. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Lei, C.; Yu, Y.; Liu, S.; Li, T.; Lin, F.; Fan, X.; Shen, Y.; Ding, M.; Tang, Y.; et al. EGFR/Notch Antagonists Enhance the Response to Inhibitors of the PI3K-Akt Pathway by Decreasing Tumor-Initiating Cell Frequency. Clin. Cancer Res. 2019, 25, 2835–2847. [Google Scholar] [CrossRef] [PubMed]
- Del Bano, J.; Florès-Florès, R.; Josselin, E.; Goubard, A.; Ganier, L.; Castellano, R.; Chames, P.; Baty, D.; Kerfelec, B. A Bispecific Antibody-Based Approach for Targeting Mesothelin in Triple Negative Breast Cancer. Front. Immunol. 2019, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, C.; Li, J.; Liu, J.; Lin, L.; Li, L.; Cao, D.; Li, Q.; Wang, Z. Single domain based bispecific antibody Muc1-Bi-1 and its humanized form Muc1-Bi-2 induce potent cancer cell killing in muc1 positive tumor cells. PLoS ONE 2018, 13, e0191024. [Google Scholar] [CrossRef] [PubMed]
- Berezhnoy, A.; Sumrow, B.J.; Stahl, K.; Shah, K.; Liu, D.; Li, J.; Hao, S.S.; De Costa, A.; Kaul, S.; Bendell, J.; et al. Development and Preliminary Clinical Activity of PD-1-Guided CTLA-4 Blocking Bispecific DART Molecule. Cell Rep. Med. 2020, 1, 100163. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.; Manitz, J.; Patel, M.R.; D’Angelo, S.P.; Apolo, A.B.; Rajan, A.; Kasturi, V.; Speit, I.; Bajars, M.; Warth, J.; et al. Efficacy and immune-related adverse event associations in avelumab-treated patients. J. Immunother. Cancer 2020, 8, e001427. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Heery, C.R.; Schlom, J.; Madan, R.A.; Cao, L.; Kang, Z.; Lamping, E.; Marté, J.L.; Donahue, R.N.; Grenga, I.; et al. Phase I Trial of M7824 (MSB0011359C) a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ in Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1287–1295. [Google Scholar] [CrossRef]
- Strauss, J.; Gatti-Mays, M.E.; Cho, B.C.; Hill, A.; Salas, S.; McClay, E.; Redman, J.M.; Sater, H.A.; Donahue, R.N.; Jochems, C.; et al. Bintrafusp alfa a bifunctional fusion protein targeting TGF-β and PD-L1 in patients with human papillomavirus-associated malignancies. J. Immunother. Cancer 2020, 8, e001395. [Google Scholar] [CrossRef]
- Xie, Y.Q.; Arik, H.; Wei, L.; Zheng, Y.; Suh, H.; Irvine, D.J.; Tang, L. Redox-responsive interleukin-2 nanogel specifically and safely promotes the proliferation and memory precursor differentiation of tumor-reactive T-cells. Biomater. Sci. 2019, 7, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Moon, B.; Liao, J.; Guo, J.; Zou, Z.; Han, Y.; Cao, S.; Wang, Y.; Fu, Y.X.; Peng, H. A tumor-specific pro-IL-12 activates preexisting cytotoxic T cells to control established tumors. Sci. Immunol. 2022, 7, eabi6899. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zheng, Y.; Melo, M.B.; Mabardi, L.; Castaño, A.P.; Xie, Y.Q.; Li, N.; Kudchodkar, S.B.; Wong, H.C.; Jeng, E.K.; et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 2018, 36, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, G.; Moon, T.J.; Loutrianakis, G.; Sims, H.M.; Umapathy, M.P.; Lorkowski, M.E.; Bielecki, P.A.; Wiese, M.L.; Atukorale, P.U.; Karathanasis, E. Comparison of the uptake of untargeted and targeted immunostimulatory nanoparticles by immune cells in the microenvironment of metastatic breast cancer. J. Mater. Chem. B 2022, 10, 224–235. [Google Scholar] [CrossRef]
- Qiu, X.; Zhao, T.; Luo, R.; Qiu, R.; Li, Z. Tumor-Associated Macrophages: Key Players in Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 772615. [Google Scholar] [CrossRef]
- Lu, H.; Clauser, K.R.; Tam, W.L.; Fröse, J.; Ye, X.; Eaton, E.N.; Reinhardt, F.; Donnenberg, V.S.; Bhargava, R.; Carr, S.A.; et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 2014, 16, 1105–1117. [Google Scholar] [CrossRef]
- Yang, J.; Liao, D.; Chen, C.; Liu, Y.; Chuang, T.H.; Xiang, R.; Markowitz, D.; Reisfeld, R.A.; Luo, Y. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells 2013, 31, 248–258. [Google Scholar] [CrossRef]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Li, Z.; Zhu, B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 2021, 11, 1016–1030. [Google Scholar] [CrossRef]
- Komohara, Y.; Jinushi, M.; Takeya, M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014, 105, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef]
- Nie, Y.; Huang, H.; Guo, M.; Chen, J.; Wu, W.; Li, W.; Xu, X.; Lin, X.; Fu, W.; Yao, Y.; et al. Breast Phyllodes Tumors Recruit and Repolarize Tumor-Associated Macrophages via Secreting CCL5 to Promote Malignant Progression Which Can Be Inhibited by CCR5 Inhibition Therapy. Clin. Cancer Res. 2019, 25, 3873–3886. [Google Scholar] [CrossRef]
- Oh, S.A.; Li, M.O. TGF-β: Guardian of T cell function. J. Immunol. 2013, 191, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.T.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Kumar, S.; Nandi, D.; Kulkarni, A. CSF1R- and SHP2-Inhibitor-Loaded Nanoparticles Enhance Cytotoxic Activity and Phagocytosis in Tumor-Associated Macrophages. Adv. Mater. 2019, 31, 1904364. [Google Scholar] [CrossRef]
- Molgora, M.; Colonna, M. Turning enemies into allies-reprogramming tumor-associated macrophages for cancer therapy. Med 2021, 2, 666–681. [Google Scholar] [CrossRef]
- Wanderley, C.W.; Colón, D.F.; Luiz, J.P.M.; Oliveira, F.F.; Viacava, P.R.; Leite, C.A.; Pereira, J.A.; Silva, C.M.; Silva, C.R.; Silva, R.L.; et al. Paclitaxel Reduces Tumor Growth by Reprogramming Tumor-Associated Macrophages to an M1 Profile in a TLR4-Dependent Manner. Cancer Res. 2018, 78, 5891–5900. [Google Scholar] [CrossRef]
- Xie, R.; Ruan, S.; Liu, J.; Qin, L.; Yang, C.; Tong, F.; Lei, T.; Shevtsov, M.; Gao, H.; Qin, Y. Furin-instructed aggregated gold nanoparticles for re-educating tumor associated macrophages and overcoming breast cancer chemoresistance. Biomaterials 2021, 275, 120891. [Google Scholar] [CrossRef]
- Esser, A.K.; Ross, M.H.; Fontana, F.; Su, X.; Gabay, A.; Fox, G.C.; Xu, Y.; Xiang, J.; Schmieder, A.H.; Yang, X.; et al. Nanotherapy delivery of c-myc inhibitor targets Pro-tumor Macrophages and preserves Antitumor Macrophages in Breast Cancer. Theranostics 2020, 10, 7510–7526. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, Q.; Jia, M.; Zhao, J.; Sun, X.; Gong, T.; Zhang, Z. Tumors and Their Microenvironment Dual-Targeting Chemotherapy with Local Immune Adjuvant Therapy for Effective Antitumor Immunity against Breast Cancer. Adv. Sci. 2019, 6, 1801868. [Google Scholar] [CrossRef]
- Niu, M.; Valdes, S.; Naguib, Y.W.; Hursting, S.D.; Cui, Z. Tumor-Associated Macrophage-Mediated Targeted Therapy of Triple-Negative Breast Cancer. Mol. Pharm. 2016, 13, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lip, H.; He, C.; Cai, P.; Wang, Z.; Henderson, J.T.; Rauth, A.M.; Wu, X.Y. Multitargeted Nanoparticles Deliver Synergistic Drugs across the Blood-Brain Barrier to Brain Metastases of Triple Negative Breast Cancer Cells and Tumor-Associated Macrophages. Adv. Healthc. Mater. 2019, 8, 1900543. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Singh, Y.; Sharma, K.; Shrivastav, A.; Sharma, A.; Singh, A.; Meher, J.G.; Singh, P.; Raval, K.; Bora, H.K.; et al. Doxorubicin Hydrochloride Loaded Zymosan-Polyethylenimine Biopolymeric Nanoparticles for Dual ‘Chemoimmunotherapeutic’ Intervention in Breast Cancer. Pharm. Res. 2017, 34, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, L.; Kim, J.A. Modulating mammary tumor growth metastasis and immunosuppression by siRNA-induced MIF reduction in tumor microenvironment. Cancer Gene Ther. 2015, 22, 463–474. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y.; Chen, K.G.; Luo, Y.L.; Wang, J. Cationic Polymeric Nanoparticle Delivering CCR2 siRNA to Inflammatory Monocytes for Tumor Microenvironment Modification and Cancer Therapy. Mol. Pharm. 2018, 15, 3642–3653. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multifunctionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, W.; Cai, X.; Wang, X.; Dang, W.; Tang, H.; Cao, H.; Wang, L.; Chen, T. Hydrazinocurcumin Encapsuled nanoparticles ‘re-educate’ tumor-associated macrophages and exhibit anti-tumor effects on breast cancer following STAT3 suppression. PLoS ONE 2013, 8, e65896. [Google Scholar] [CrossRef]
- Chong, L.; Jiang, Y.W.; Wang, D.; Chang, P.; Xu, K.; Li, J. Targeting and repolarizing M2-like tumor-associated macrophage-mediated MR imaging and tumor immunotherapy by biomimetic nanoparticles. J. Nanobiotechnol. 2023, 21, 401. [Google Scholar] [CrossRef]
- Cai, X.J.; Wang, Z.; Cao, J.W.; Ni, J.J.; Xu, Y.Y.; Yao, J.; Xu, H.; Liu, F.; Yang, G.Y. Anti-angiogenic and anti-tumor effects of metronomic use of novel liposomal zoledronic acid depletes tumor-associated macrophages in triple negative breast cancer. Oncotarget 2017, 8, 84248–84257. [Google Scholar] [CrossRef]
- Hu, Y.; Manasrah, B.K.; McGregor, S.M.; Lera, R.F.; Norman, R.X.; Tucker, J.B.; Scribano, C.M.; Yan, R.E.; Humayun, M.; Wisinski, K.B.; et al. Paclitaxel Induces Micronucleation and Activates Pro-Inflammatory cGAS-STING Signaling in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2021, 20, 2553–2567. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.J.; Park, J.; Joe, Y.; Lee, S.E.; Saeidi, S.; Zhong, X.; Kim, S.H.; Park, S.A.; Na, H.K.; et al. Reprograming of Tumor-Associated Macrophages in Breast Tumor-Bearing Mice under Chemotherapy by Targeting Heme Oxygenase-1. Antioxidants 2021, 10, 470. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Swartz, M.A.; Hubbell, J.A. Targeting dendritic cells with biomaterials: Developing the next generation of vaccines. Trends Immunol. 2006, 27, 573–579. [Google Scholar] [CrossRef]
- Szpor, J.; Streb, J.; Glajcar, A.; Frączek, P.; Winiarska, A.; Tyrak, K.E.; Basta, P.; Okoń, K.; Jach, R.; Hodorowicz-Zaniewska, D. Dendritic Cells Are Associated with Prognosis and Survival in Breast Cancer. Diagnostics 2021, 11, 702. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Men, K.; Huang, R.; Zhou, B.; Zhang, R.; Zou, R.; Yang, L. Modified Fe3O4 Magnetic Nanoparticle Delivery of CpG Inhibits Tumor Growth and Spontaneous Pulmonary Metastases to Enhance Immunotherapy. Nanoscale Res. Lett. 2018, 13, 240. [Google Scholar] [CrossRef]
- He, Y.; Wang, M.; Li, X.; Yu, T.; Gao, X. Targeted MIP-3β plasmid nanoparticles induce dendritic cell maturation and inhibit M2 macrophage polarisation to suppress cancer growth. Biomaterials 2020, 249, 120046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pang, G.; Chen, C.; Qin, J.; Yu, H.; Liu, Y.; Zhang, X.; Song, Z.; Zhao, J.; Wang, F.; et al. Effective cancer immunotherapy by Ganoderma lucidum polysaccharide-gold nanocomposites through dendritic cell activation and memory T cell response. Carbohydr. Polym. 2019, 205, 192–202. [Google Scholar] [CrossRef]
- Jadidi-Niaragh, F.; Atyabi, F.; Rastegari, A.; Kheshtchin, N.; Arab, S.; Hassannia, H.; Ajami, M.; Mirsanei, Z.; Habibi, S.; Masoumi, F.; et al. CD73 specific siRNA loaded chitosan lactate nanoparticles potentiate the antitumor effect of a dendritic cell vaccine in 4T1 breast cancer bearing mice. J. Control. Release 2017, 246, 46–59. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, L.; Zhang, S.; Wan, G.; Chen, B.; Zhou, P.; Zhang, N.; Wang, Y. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef]
- Wu, T.; Qiao, Q.; Qin, X.; Zhang, D.; Zhang, Z. Immunostimulatory cytokine and doxorubicin co-loaded nanovesicles for cancer immunochemotherapy. Nanomedicine 2019, 18, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.W.; Chen, J.L.; Zhu, J.Y.; Rong, L.; Li, B.; Lei, Q.; Fan, J.X.; Zou, M.Z.; Li, C.; Cheng, S.X.; et al. Highly Integrated Nano-Platform for Breaking the Barrier between Chemotherapy and Immunotherapy. Nano Lett. 2016, 16, 4341–4347. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Song, J.; Wang, B.; Hua, H.; Zhu, H.; Guo, X.; Xiong, S.; Zhao, Y. Dendritic cell vaccine for the effective immunotherapy of breast cancer. Biomed. Pharmacother. 2020, 126, 110046. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Kublo, L.; Cumberland, R.; Gooding, W.; Baar, J. Optimized systemic dosing with CpG DNA enhances dendritic cell-mediated rejection of a poorly immunogenic mammary tumor in BALB/c mice. Clin. Transl. Sci. 2009, 2, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Yoon, S.R.; Kim, T.D.; Choi, I.; Jung, H. Toll-Like Receptors in Natural Killer Cells and Their Application for Immunotherapy. J. Immunol. Res. 2020, 2020, 2045860. [Google Scholar] [CrossRef] [PubMed]
- Ballas, Z.K. Modulation of NK cell activity by CpG oligodeoxynucleotides. Immunol. Res. 2007, 39, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hodge, D.R.; Hurt, E.M.; Farrar, W.L. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer 2005, 41, 2502–2512. [Google Scholar] [CrossRef] [PubMed]
- Ngamcherdtrakul, W.; Reda, M.; Nelson, M.A.; Wang, R.; Zaidan, H.Y.; Bejan, D.S.; Hoang, N.H.; Lane, R.S.; Luoh, S.W.; Leachman, S.A.; et al. In Situ Tumor Vaccination with Nanoparticle Co-Delivering CpG and STAT3 siRNA to Effectively Induce Whole-Body Antitumor Immune Response. Adv. Mater. 2021, 33, 2100628. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef]
- Pei, L.; Liu, Y.; Liu, L.; Gao, S.; Gao, X.; Feng, Y.; Sun, Z.; Zhang, Y.; Wang, C. Roles of cancer-associated fibroblasts (CAFs) in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol. Cancer 2023, 22, 29. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Zuo, T.; Ma, S.; Xokrat, N.; Hu, Z.; Wang, Z.; Xu, R.; Wei, Y.; Shen, Q. Heparanase-driven sequential released nanoparticles for ferroptosis and tumor microenvironment modulations synergism in breast cancer therapy. Biomaterials 2021, 266, 120429. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, T.; Yang, J.; Hu, Z.; Wang, Z.; Xu, R.; Ma, S.; Wei, Y.; Shen, Q. Hierarchically Releasing Bio-Responsive Nanoparticles for Complete Tumor Microenvironment Modulation via TGF-β Pathway Inhibition and TAF Reduction. ACS Appl. Mater. Interfaces 2021, 13, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Chen, I.X.; Tong, R.; Ng, M.R.; Martin, J.D.; Naxerova, K.; Wu, M.W.; Huang, P.; Boucher, Y.; Kohane, D.S.; et al. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 10674–10680. [Google Scholar] [CrossRef]
- Ji, T.; Zhao, Y.; Ding, Y.; Wang, J.; Zhao, R.; Lang, J.; Qin, H.; Liu, X.; Shi, J.; Tao, N.; et al. Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer-Associated Fibroblast Activation. Angew. Chem. Int. Ed. Engl. 2016, 55, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, F.; Tan, Y.; Hong, Y.; Meng, T.; Liu, Y.; Dai, S.; Qiu, G.; Yuan, H.; Hu, F. Reversing activity of cancer associated fibroblast for staged glycolipid micelles against internal breast tumor cells. Theranostics 2019, 9, 6764–6779. [Google Scholar] [CrossRef] [PubMed]
- Cun, X.; Chen, J.; Li, M.; He, X.; Tang, X.; Guo, R.; Deng, M.; Li, M.; Zhang, Z.; He, Q. Tumor-Associated Fibroblast-Targeted Regulation and Deep Tumor Delivery of Chemotherapeutic Drugs with a Multifunctional Size-Switchable Nanoparticle. ACS Appl. Mater. Interfaces 2019, 11, 39545–39559. [Google Scholar] [CrossRef]
- Li, M.; Shi, K.; Tang, X.; Wei, J.; Cun, X.; Long, Y.; Zhang, Z.; He, Q. Synergistic tumor microenvironment targeting and blood-brain barrier penetration via a pH-responsive dual-ligand strategy for enhanced breast cancer and brain metastasis therapy. Nanomedicine 2018, 14, 1833–1843. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, T.; Guo, M.; Liu, Y.; Liu, C.; Chen, Y.; Qu, D. Mild-heat-inducible sequentially released liposomal complex remodels the tumor microenvironment and reinforces anti-breast-cancer therapy. Biomater. Sci. 2020, 8, 3916–3925. [Google Scholar] [CrossRef]
- Zhen, Z.; Tang, W.; Wang, M.; Zhou, S.; Wang, H.; Wu, Z.; Hao, Z.; Li, Z.; Liu, L.; Xie, J. Protein Nanocage Mediated Fibroblast-Activation Protein Targeted Photoimmunotherapy To Enhance Cytotoxic T Cell Infiltration and Tumor Control. Nano Lett. 2017, 17, 862–869. [Google Scholar] [CrossRef]
- Cheng, Y.; Zou, J.; He, M.; Hou, X.; Wang, H.; Xu, J.; Yuan, Z.; Lan, M.; Yang, Y.; Chen, X.; et al. Spatiotemporally controlled Pseudomonas exotoxin transgene system combined with multifunctional nanoparticles for breast cancer antimetastatic therapy. J. Control. Release 2024, 367, 167–183. [Google Scholar] [CrossRef]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Gungabeesoon, J.; Gort-Freitas, N.A.; Kiss, M.; Bolli, E.; Messemaker, M.; Siwicki, M.; Hicham, M.; Bill, R.; Koch, P.; Cianciaruso, C.; et al. A neutrophil response linked to tumor control in immunotherapy. Cell 2023, 186, 1448–1464. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ge, Z.; Toh, K.; Liu, X.; Dirisala, A.; Ke, W.; Wen, P.; Zhou, H.; Wang, Z.; Xiao, S.; et al. Enzymatically Transformable Polymersome-Based Nanotherapeutics to Eliminate Minimal Relapsable Cancer. Adv. Mater. 2021, 33, e2105254. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Rowinsky, E.K. The ErbB receptor family: A therapeutic target for cancer. Trends Mol. Med. 2002, 8, S19–S26. [Google Scholar] [CrossRef] [PubMed]
- Gandullo-Sánchez, L.; Ocaña, A.; Pandiella, A. HER3 in cancer: From the bench to the bedside. J. Exp. Clin. Cancer Res. 2022, 41, 310. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.J.; Castel, P.; Radosevic-Robin, N.; Elkabets, M.; Auricchio, N.; Aceto, N.; Weitsman, G.; Barber, P.; Vojnovic, B.; Ellis, H.; et al. Antagonism of EGFR and HER3 enhances the response to inhibitors of the PI3K-Akt pathway in triple-negative breast cancer. Sci. Signal. 2014, 7, ra29. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.; Vinogradov, S.V.; Morrissey, P.; Chernin, M.; Ahmed, M.M. Curcumin-encapsulating Nanogels as an Effective Anti-cancer Formulation for Intracellular Uptake. Mol. Cell Pharmacol. 2015, 7, 25–40. [Google Scholar] [PubMed]
- Luo, L.; Xu, F.; Peng, H.; Luo, Y.; Tian, X.; Battaglia, G.; Zhang, H.; Gong, Q.; Gu, Z.; Luo, K. Stimuli-responsive polymeric pro-drug-based nanomedicine delivering nifuroxazide and doxorubicin against primary breast cancer and pulmonary metastasis. J. Control. Release 2020, 318, 124–135. [Google Scholar] [CrossRef]
- Dias, A.M.M.; Courteau, A.; Bellaye, P.S.; Kohli, E.; Oudot, A.; Doulain, P.E.; Petitot, C.; Walker, P.M.; Decréau, R.; Collin, B. Superparamagnetic Iron Oxide Nanoparticles for Immunotherapy of Cancers through Macrophages and Magnetic Hyperthermia. Pharmaceutics 2022, 14, 2388. [Google Scholar] [CrossRef]
- Farcas, C.G.; Dehelean, C.; Pinzaru, I.A.; Mioc, M.; Socoliuc, V.; Moaca, E.A.; Avram, S.; Ghiulai, R.; Coricovac, D.; Pavel, I.; et al. Thermosensitive Betulinic Acid-Loaded Magnetoliposomes: A Promising Antitumor Potential for Highly Aggressive Human Breast Adenocarcinoma Cells Under Hyperthermic Conditions. Int. J. Nanomed. 2020, 15, 8175–8200. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Jeon, S.; Yoon, H.Y.; Shim, M.K.; Ko, H.; Min, J.; Na, J.H.; Chang, H.; Han, H.; Kim, J.H.; et al. Effects of tumor microenvironments on targeted delivery of glycol chitosan nanoparticles. J. Control. Release 2017, 267, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Burgess, D.J. Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharm. Sin. B 2020, 10, 2110–2124. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef] [PubMed]
- Thakor, P.; Bhavana, V.; Sharma, R.; Srivastava, S.; Singh, S.B.; Mehra, N.K. Polymer-drug conjugates: Recent advances and future perspectives. Drug Discov. 2020, 25, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Swetha, K.L.; Maravajjala, K.S.; Li, S.D.; Singh, M.S.; Roy, A. Breaking the niche: Multidimensional nanotherapeutics for tumor microenvironment modulation. Drug Deliv. Transl. Res. 2023, 13, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, R.; Liu, G.; Kang, Y.; Wu, J. Redox-Responsive Self-Assembled Nanoparticles for Cancer Therapy. Adv. Healthc. Mater. 2020, 9, e2000605. [Google Scholar] [CrossRef]
- Rahimkhoei, V.; Alzaidy, A.H.; Abed, M.J.; Rashki, S.; Salavati-Niasari, M. Advances in inorganic nanoparticles-based drug delivery in targeted breast cancer theranostics. Adv. Colloid Interface Sci. 2024, 329, 103204. [Google Scholar] [CrossRef]
- Pulaski, B.A.; Ostrand-Rosenberg, S. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol. 2001. Chapter 20: Unit 20.2. [Google Scholar] [CrossRef]
- Yang, L.; Yong, L.; Zhu, X.; Feng, Y.; Fu, Y.; Kong, D.; Lu, W.; Zhou, T.Y. Disease progression model of 4T1 metastatic breast cancer. J. Pharmacokinet. Pharmacodyn. 2020, 47, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Vakhshiteh, F.; Bagheri, Z.; Soleimani, M.; Ahvaraki, A.; Pournemat, P.; Alavi, S.E.; Madjd, Z. Heterotypic tumor spheroids: A platform for nanomedicine evaluation. J. Nanobiotechnol. 2023, 21, 249. [Google Scholar] [CrossRef] [PubMed]
| Cancer Antigen | Antibody Targets | References |
|---|---|---|
| HER2 | CD3 × HER2 mPEG × HER2 CD47 × HER2 | [51,52,53] |
| HER3 | CD3 × HER3 HER2 × HER3 EFGR × HER3 | [51,54,55] |
| p95HER2 | CD3 × p95HER2 | [56] |
| Trop-2 | CD3 × Trop-2 | [57] |
| CEACAM-5 | CD3 × CEACAM-5 | [57] |
| EphA10 | CD3 × EphA10 | [58] |
| EpCAM | CD3 × EpCAM | [59,60] |
| p-Cadherin | CD3 × p-Cadherin | [61] |
| EGFR | CD3 × EGFR mPEG × EGFR | [62,63] |
| Notch | EFGR × notch | [64] |
| Mesothelin | CD16 × mesothelin | [65] |
| Muc1 | CD16 × Muc1 | [66] |
| CTLA-4 | PD-1 × CTLA-4 | [67] |
| LAG-3 | PD-1 × LAG-3 | [68] |
| TGFβ | PD-L1 × TGFβ | [69,70] |
| Payloads or Associated Agents | Nanocarrier | Function | References |
|---|---|---|---|
| Doxorubicin | cleavable PEG chains covering the folate–modified liposome | target tumor cells and M2-TAMs; induce ICD at tumor sites; activate effector T cells (combined with CpG immune adjuvant); reduce M2-TAMs; promote maturation of DCs; | [91] |
| Doxorubicin | poly(lactic-co-glycolic) acid NPs functionalized by acid-sensitive sheddable PEGylation and mannose modification | reduce TAM population and density in tumor tissues | [92] |
| Doxorubicin, mitomycin C | iRGD peptide (internalizing Arg-Gly-Asp peptide mimetic) functionalized terpolymer and poly(methacrylic acid)- polysorbate 80-grafted starch-lipid NP | cross intact blood–brain barrier; enhance cellular uptake, cytotoxicity and drug delivery; selective targetability to human TNBC cells and murine macrophages | [93] |
| Doxorubicin and zymosan | NP complex composed of pegylated polyethylenimine and zymosan and Dox | enhance cellular uptake; induce apoptosis; induce secretion of proinflammatory cytokine; modify the biodistribution of DOX; reversed TAMs polarization from M2 to M1 phenotype; anti- angiogenic effect | [94] |
| Macrophage migration inhibitory factor-siRNA | glucan -based NPs | reduce tumor cell proliferation and enhance apoptosis; reduce the number of MIF at tumor; antitumor and anti-metastasis; increase CD4+ T cells infiltration | [95] |
| siCCR2 | siCCR2-encapsulated cationic polymeric NP | block monocytes recruitment, reduce TAMs abundance in tumor tissues; reverse tumor immune suppression; enhance the antitumor effect of chemotherapy | [96] |
| VEGF siRNA (siVEGF) and PIGF siRNA (siPIGF) | PEG = MT/PC/siVEGF/siPIGF NPs a novel dual-stage pH-sensitive carrier composed of cationic polyethylene glycol (PEG) and mannose modified trimethyl chitosan conjugate (PEG = MT), and an anionic poly- (allylamine hydrochloride)-citraconic anhydride (PAH-Cit, PC) | inhibit the proliferation of BCs; reverse TAMs polarization from M2 to M1 phenotype; inhibit BC lung metastasis; anti-angiogenic effect | [97] |
| Hydrazinocurcumin | RR-11a-coupled liposomal NPs | suppress STAT3 activity; reverse TAMs polarization from M2 to M1 phenotype | [98] |
| Metformin | Hollow mesoporous manganese dioxide NPs coated with macrophage membranes | reverse TAMs polarization from M2 to M1 phenotype; target TAMs; suppress tumor growth | [99] |
| Zoledronic acid (ZOL) | Asn-Gly-Arg and PEG2000 modified liposomes | inhibit tumor growth; reduce TAMs; inhibit tumor angiogenesis | [100] |
| Payloads or Associated Agents | Nanocarrier | Function | References |
|---|---|---|---|
| Cytosine-phosphate-guanine (CpG) | 3-aminopropyltriethoxysilane-modified Fe3O4 NPs | suppress the metastasis of BC to the lungs; increases infiltrating lymphocytes in tumors; stimulate humoral immune response | [106] |
| Macrophage Inflammatory Protein 3 Beta (MIP-3β) | NP complex composed of 1,2-Dioleoyl-3-trimethylammonium-propane, folic acid modified poly (ethylene glycol)-b-poly(ε-caprolactone) and methoxy poly (ethylene glycol)-poly(lactide) | activate CD8+ T-lymphocytes; induce DCs maturation; inhibit M2 polarization; suppress angiogenesis; suppress tumor growth and metastasis | [107] |
| Ganoderma lucidumpolysaccharide | Ganoderma lucidumpolysaccharide contained gold nanocomposites | induce DCs maturation; reverse the decline of CD4+ T cells and CD8+ T cells population; stimulate T cells proliferation; suppress tumor growth and metastasis | [108] |
| CD73 specific siRNA | CD73-specific siRNA-loaded chitosanlactate NPs | inhibit the expression of CD 73; reduce tumor growth rate; decrease Treg, MDSCs and TAMs, enhance CTL function; enhance the secretion of Th1 frequency and inflammatory cytokine network; antitumor and anti-metastasis; | [109] |
| Resiquimod CR848 and doxorubicin-hyaluronic acid conjugate (HA-DOX) | HA-DOX coated PHIS/R848 NPs | promote DCs maturation and activation; selective effects on breast cancer cells; regulate antitumor immune response by promoting infiltration of T cells and CTLs; | [110] |
| DOX,IL-2,IFN-γ | DC cell-derived nanovesicles | suppress tumor growth and metastasis; adsorb IFN-γ; enhance DCs mature; increase the infiltration of CTLs and activation of NK cells; increase the recruitment of CD45+immune cells and Ly6G+neutrophils | [111] |
| Doxorubicin | highly integrated mesoporous silica NPs | induce DCs maturation; improve drug accumulate in the tumor; induce anticancer immune response | [112] |
| Doxorubicin | Low-dose doxorubicin hydrochloride cancer cell membrane coated calcium carbonate NPs | induce ICD; CRT exposure; promote DCs maturation | [113] |
| Payloads or Associated Agents | Nanocarrier | Function | References |
|---|---|---|---|
| Doxorubicin | Cleavable amphiphilic peptide (containing a TGPA peptide sequence)-NPs | increase the effective drug concentration at the FAP-α-rich tumor sites; facilitate drug penetration through the stromal barrier; possess tumor targeting specificity | [124] |
| Telmisartan, Doxorubicin | Glycolipid polymer-based micelles composed of chitosan and stearic acid | Decrease activity of CAFs; inhibit CAFs secreted cytokines; | [125] |
| Gemcitabine,18β-glycyrrhetinic acid | NPs composed of dendrigraft poly-l-lysine, PEG-PCL, substrate peptide of MMP-2 | regulate the chemoexposed TAFs; deliver drug to the deep region of tumor tissue | [126] |
| Paclitaxel | Liposome co-modified with acid-cleavable folic acid and dNP2 peptide | enhance the uptake and deep penetration in FR-positive tumor cells and FR-negative CAFs; exhibit synergistic TME targeting and blood brain barrier transmigration; accumulate in brain metastatic sites; | [127] |
| Sodium tanshinone IIA sulfonate and celastrol | A gold nanorod-anchored thermo- liposomal complex | normalize the tumor blood vessel; decrease the density of TAFs and collagen; regulate the secretion of cytokines | [128] |
| ZnF16Pc | ZnF16Pc-nanoparticle protein cage conjugated with a FAP- targeted single chain variable fragment | selectively eliminate CAFs; ECM destruction; suppress CXCL12 secretion; enhance CD8+ T cells infiltration | [129] |
| Pseudomonas exotoxin A/anisamide | Poly L lysine-based cationic carrier was coupled with the glutathione-sensitive disulfide bound vitamin E succinate and was covered with a sigma1 receptor and integrin αvβ3 receptor multitargeting | suppress angiogenesis; suppress tumor growth and metastasis; target elimination of CAFs; | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Hu, Q.; Huang, T. Breast Cancer Treatment Strategies Targeting the Tumor Microenvironment: How to Convert “Cold” Tumors to “Hot” Tumors. Int. J. Mol. Sci. 2024, 25, 7208. https://doi.org/10.3390/ijms25137208
Yang L, Hu Q, Huang T. Breast Cancer Treatment Strategies Targeting the Tumor Microenvironment: How to Convert “Cold” Tumors to “Hot” Tumors. International Journal of Molecular Sciences. 2024; 25(13):7208. https://doi.org/10.3390/ijms25137208
Chicago/Turabian StyleYang, Liucui, Qingyi Hu, and Tao Huang. 2024. "Breast Cancer Treatment Strategies Targeting the Tumor Microenvironment: How to Convert “Cold” Tumors to “Hot” Tumors" International Journal of Molecular Sciences 25, no. 13: 7208. https://doi.org/10.3390/ijms25137208
APA StyleYang, L., Hu, Q., & Huang, T. (2024). Breast Cancer Treatment Strategies Targeting the Tumor Microenvironment: How to Convert “Cold” Tumors to “Hot” Tumors. International Journal of Molecular Sciences, 25(13), 7208. https://doi.org/10.3390/ijms25137208




