Mapping the Development of Human Spermatogenesis Using Transcriptomics-Based Data: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Literature Search
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction and Interpretation
3. Results and Discussion
3.1. Fetal Testis
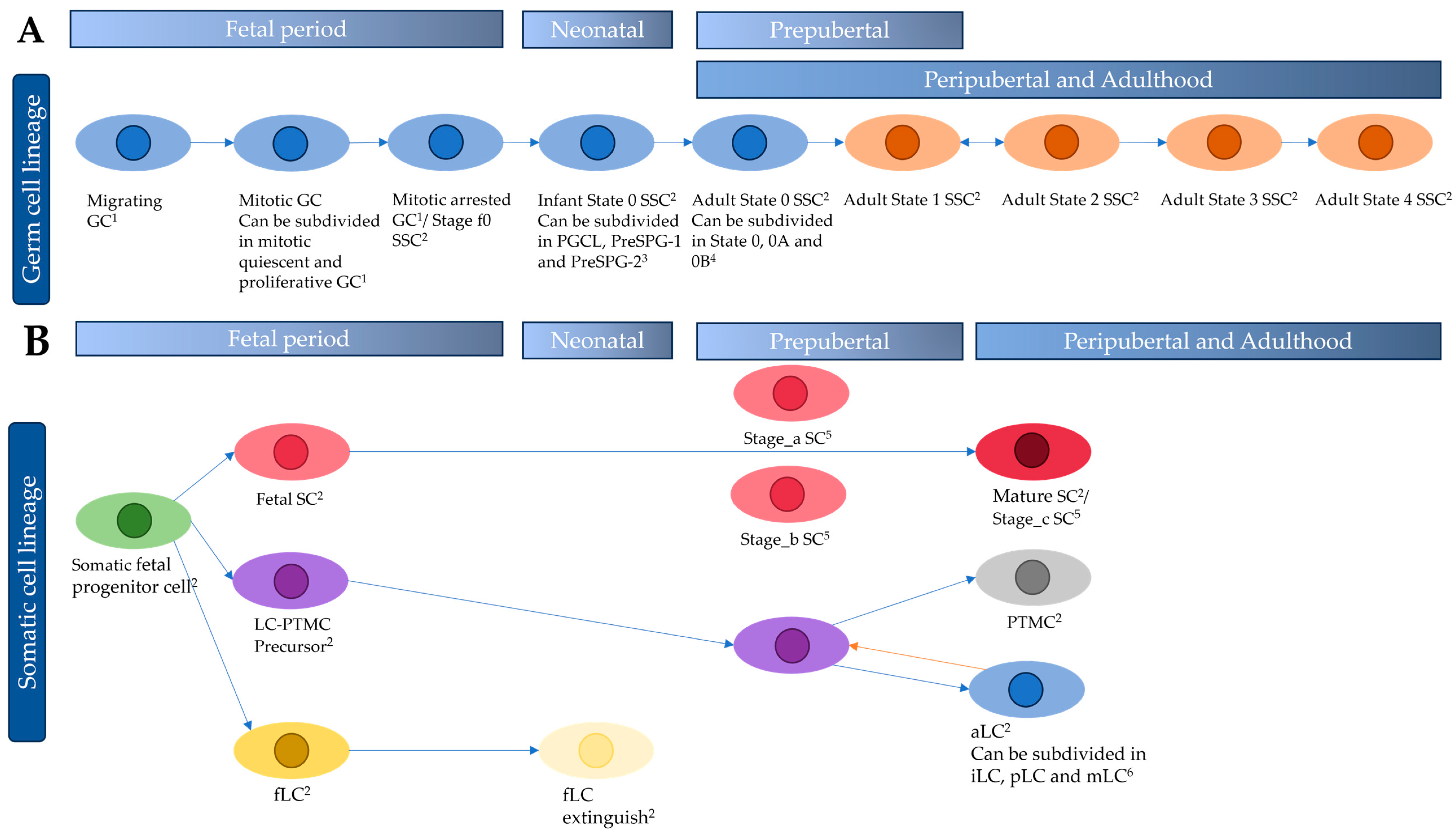
3.1.1. The Germ Cell Lineage
| Developmental Stage | Author | Year of Publication | Number of Participants | Age | GSE Number | Other Database | Reference |
|---|---|---|---|---|---|---|---|
| Prenatal | F. Guo et al. | 2015 | 15 | 4–19 WPC | GSE63818 | [32] | |
| Li et al. | 2017 | 12 | 4–25 WPC | GSE86146 | [30] | ||
| Chitiashvili et al. | 2020 | 5 | 6–16 WPC | GSE143356 | http://germline.mcdb.ucla.edu (accessed on 26 March 2024) | [33] | |
| J. Guo et al. | 2021 | 6 | 6, 7, 8, 12, 15, 16 WPC | GSE143356 | [31] | ||
| Garcia-Alonso et al. | 2022 | 22 | 6–21 WPC | https://www.reproductivecellatlas.org/ (accessed on 26 March 2024) | [28] | ||
| R. Wang et al. | 2022 | 9 | 6–23 WPC | [29] | |||
| Neonatal | Sohni et al. | 2019 | 2 | 2, 7 days | GSE124263 | [34] | |
| J. Guo et al. | 2021 | 1 | 5 months | GSE161617 | [31] | ||
| Prepubertal | J. Guo et al. | 2018 | 2 | 1 year | GSE120508 | [38] | |
| J. Guo et al. | 2020 | 2 | 7, 11 years | GSE134144 | https://humantestisatlas.shinyapps.io/humantestisatlas1/ (accessed on 26 March 2024) | [36] | |
| Zhao et al. | 2020 | 4 | 2, 5, 8, 11 years | GSE149512 | [37] | ||
| Voigt et al. | 2022 | 3 | 1, 2, 7 years | GSE196819 | [35] | ||
| Peripubertal | J. Guo et al. | 2020 | 2 | 13, 14 years | GSE134144 | [36] | |
| Adult | J. Guo et al. | 2017 | 5 | adult | GSE92280 | [39] | |
| Neuhaus et al. | 2017 | 5 | adult | GSE91063 | [40] | ||
| J. Guo et al. | 2018 | 3 | 17, 24, 25 years | GSE120508 | [38] | ||
| Hermann et al. | 2018 | 7 | 37, 38, 34, 36, 49, 43, 43 years | GSE108977, GSE109037 | https://data.mendeley.com/datasets/kxd5f8vpt4/1 (accessed on 26 March 2024) | [41] | |
| M. Wang et al. | 2018 | 1 | 30 years | GSE106487 | [42] | ||
| Sohni et al. | 2019 | 2 | 37, 42 years | GSE124263 | [34] | ||
| Chen et al. | 2020 | 1 | 36 years | GSE144085 | [43] | ||
| Shami et al. | 2020 | 4 | 20–40 years | GSE142585 | [44] | ||
| B. Xia et al. | 2020 | 2 | 40, 45 years | GSE125372 | [45] | ||
| Zhao et al. | 2020 | 6 | 17, 23, 25, 28, 28, 31 years | GSE149512 | [37] | ||
| Alfano et al. | 2021 | 1 | 37 years | GSE154535 | [46] | ||
| Di Persio et al. | 2021 | 3 | 31, 33, 55 years | GSE153947 | [47] | ||
| Mahyari et al. | 2021 | 4 | adult | GSE169062 | https://github.com/eisascience/HISTA and https://doi.org/10.5281/zenodo.4433041 (accessed on 26 March 2024) | [48] | |
| Nie et al. | 2022 | 4 | 17–22 years | GSE182786 | [49] | ||
| K. Xia et al. | 2022 | 3 | 28, 24, 31 years | [5] | |||
| Gui et al. | 2024 | 2 | 22, 30 years | Data available on request | [50] | ||
| Elderly | M. Wang et al. | 2018 | 1 | 60 years | GSE106487 | [42] | |
| Nie et al. | 2022 | 8 | 62–76 years | GSE182786 | [49] | ||
| K. Xia et al. | 2022 | 3 | 61, 70, 87 years | [5] | |||
| Gui et al. | 2024 | 2 | 80, 90 years | Data available on request | [50] |
3.1.2. The Somatic Cell Lineage
3.2. Neonatal Testis
3.2.1. The Germ Cell Lineage
3.2.2. The Somatic Cell Lineage
3.2.3. Cell Interaction
3.3. Prepubertal and Peripubertal Testis
3.3.1. The Germ Cell Lineage
3.3.2. The Somatic Cell Lineage
3.4. Adult Testis
3.4.1. The Germ Cell Lineage
3.4.2. The Somatic Cell Lineage
3.5. The Aged Testis
3.5.1. The Germ Cell Lineage
3.5.2. The Somatic Cell Lineage
3.6. Testicular Cell Culture
| Author | Year of Publication | Age of Cultured Cells | Culture Time | Addition of | Reference |
|---|---|---|---|---|---|
| Tan et al. | 2020 | 32 and 37 years old | 14 days | MK-2206 HCL | [61] |
| Liebich et al. | 2022 | 41–48 years old | 24 h | FCS | [62] |
| R. Wang et al. | 2022 | 7 and 15 WPC | 24 h | LDN-19318 | [29] |
3.7. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aLC | Adult Leydig cell |
| BER | Base-excision repair |
| BM | Basal membrane |
| BMP | Bone morphogenetic protein |
| BTB | Blood–testis barrier |
| M-CSF, CSF1 | Colony stimulating factor 1 |
| EC | Endothelial cell |
| ECM | Extra cellular matrix |
| FCS | Fetal calf serum |
| FGF | Fibroblast growth factor |
| fLC | Fetal Leydig cell |
| GC | Germ Cell |
| GDNF | Glial cell line-derived neurotrophic factor |
| GEO | Gene Expression Omnibus |
| GO | Gene Ontology |
| GSE | GEO series accession number |
| iLC | Immature Leydig cell |
| ITT | Immature testicular tissue |
| IVM | In vitro maturation |
| LAMA | Laminin alpha |
| LC | Leydig cell |
| LC-PTMC | Leydig-peritubular myoid cell |
| mLC | Mature Leydig cell |
| OA | Obstructive azoospermia |
| OXPHOS | Oxidative phosphorylation |
| pLC | Progenitor Leydig cell |
| PreSPG | Prespermatogonia |
| PTMC | Peritubular myoid cell |
| RA | Retinoic acid |
| SC | Sertoli cell |
| scRNA-seq | Single-cell RNA-sequencing |
| SSC | Spermatogonial stem cell |
| State f0 | Fetal State 0 |
| WPC | Weeks post-conception |
Appendix A
Appendix B
Appendix C
References
- Dunleavy, J.E.M.; O’Bryan, M.K.; Stanton, P.G.; O’Donnell, L. The cytoskeleton in spermatogenesis. Reproduction 2019, 157, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S. Spermatogenesis. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Robinson, M.; Sparanese, S.; Witherspoon, L.; Flannigan, R. Human in vitro spermatogenesis as a regenerative therapy—Where do we stand? Nat. Rev. Urol. 2023, 20, 461–479. [Google Scholar] [CrossRef]
- Xia, K.; He, S.; Luo, P.; Dong, L.; Gao, F.; Chen, X.; Ye, Y.; Gao, Y.; Ma, Y.; Zhang, Y.; et al. Transcriptomic landscape and potential therapeutic targets for human testicular aging revealed by single-cell RNA sequencing. bioRxiv 2022, 12, 519976. [Google Scholar] [CrossRef]
- Stukenborg, J.B.; Jahnukainen, K.; Hutka, M.; Mitchell, R.T. Cancer treatment in childhood and testicular function: The importance of the somatic environment. Endocr. Connect. 2018, 7, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; Kanbar, M. In Vitro Spermatogenesis. In Female and Male Fertility Preservation; Grynberg, M., Patrizio, P., Eds.; Springer: Cham, Switzerland, 2022; pp. 587–607. [Google Scholar] [CrossRef]
- Steinberger, A.; Dighe, R.R.; Díaz, J. Testicular peptides and their endocrine and paracrine functions. Arch. Biol. Med. Exp. 1984, 17, 267–271. [Google Scholar] [PubMed]
- Medrano, J.V.; Vilanova-Perez, T.; Fornes-Ferrer, V.; Navarro-Gomezlechon, A.; Martinez-Triguero, M.L.; Garcia, S.; Gomez-Chacon, J.; Povo, I.; Pellicer, A.; Andres, M.M.; et al. Influence of temperature, serum, and gonadotropin supplementation in short- and long-term organotypic culture of human immature testicular tissue. Fertil. Steril. 2018, 110, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.; Caldeira-Brant, A.L.; Chu, T.; Abdalla, S.; Orwig, K.E. Human immature testicular tissue organ culture: A step towards fertility preservation and restoration. Front. Endocrinol. 2023, 14, 1242263. [Google Scholar] [CrossRef]
- de Michele, F.; Poels, J.; Weerens, L.; Petit, C.; Evrard, Z.; Ambroise, J.; Gruson, D.; Wyns, C. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum. Reprod. 2017, 32, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Portela, J.M.D.; de Winter-Korver, C.M.; van Daalen, S.K.M.; Meißner, A.; de Melker, A.A.; Repping, S.; van Pelt, A.M.M. Assessment of fresh and cryopreserved testicular tissues from (pre)pubertal boys during organ culture as a strategy for in vitro spermatogenesis. Hum. Reprod. 2019, 34, 2443–2455. [Google Scholar] [CrossRef]
- Roulet, V.; Denis, H.; Staub, C.; Le Tortorec, A.; Delaleu, B.; Satie, A.P.; Patard, J.J.; Jegou, B.; Dejucq-Rainsford, N. Human testis in organotypic culture: Application for basic or clinical research. Hum. Reprod. 2006, 21, 1564–1575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perrard, M.H.; Sereni, N.; Schluth-Bolard, C.; Blondet, A.; Giscard d’Estaing, S.; Plotton, I.; Morel-Journel, N.; Lejeune, H.; David, L.; Durand, P. Complete Human and Rat Ex Vivo Spermatogenesis from Fresh or Frozen Testicular Tissue. Biol. Reprod. 2016, 95, 89. [Google Scholar] [CrossRef] [PubMed]
- de Michele, F.; Poels, J.; Vermeulen, M.; Ambroise, J.; Gruson, D.; Guiot, Y.; Wyns, C. Haploid Germ Cells Generated in Organotypic Culture of Testicular Tissue From Prepubertal Boys. Front. Physiol. 2018, 9, 1413. [Google Scholar] [CrossRef]
- Aden, N.L.; Bleeke, M.; Kordes, U.R.; Brunne, B.; Holstermann, B.; Biemann, R.; Ceglarek, U.; Soave, A.; Salzbrunn, A.; Schneider, S.W.; et al. Germ Cell Maintenance and Sustained Testosterone and Precursor Hormone Production in Human Prepubertal Testis Organ Culture with Tissues from Boys 7 Years+ under Conditions from Adult Testicular Tissue. Cells 2023, 12, 415. [Google Scholar] [CrossRef]
- Wyns, C.; Curaba, M.; Petit, S.; Vanabelle, B.; Laurent, P.; Wese, J.F.; Donnez, J. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum. Reprod. 2011, 26, 737–747. [Google Scholar] [CrossRef]
- Wyns, C.; Kanbar, M.; Giudice, M.G.; Poels, J. Fertility preservation for prepubertal boys: Lessons learned from the past and update on remaining challenges towards clinical translation. Hum. Reprod. Update 2021, 27, 433–459. [Google Scholar] [CrossRef]
- Cilio, S.; Fallara, G.; Stanghellini, M.T.L.; Ciceri, F.; Montorsi, F.; Lunghi, F.; Salonia, A. Impact of Hydroxyurea to Treat Haematological Disorders on Male Fertility: Two Case Reports and a Systematic Review. World J. Men’s Health 2024, 42, 531–542. [Google Scholar] [CrossRef]
- Wisniewski, A.B.; Batista, R.L.; Costa, E.M.F.; Finlayson, C.; Sircili, M.H.P.; Dénes, F.T.; Domenice, S.; Mendonca, B.B. Management of 46,XY Differences/Disorders of Sex Development (DSD) Throughout Life. Endocr. Rev. 2019, 40, 1547–1572. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Yokonishi, T.; Kubota, Y.; Inoue, K.; Ogonuki, N.; Matoba, S.; Ogura, A.; Ogawa, T. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat. Commun. 2011, 2, 472. [Google Scholar] [CrossRef]
- Matsumura, T.; Katagiri, K.; Yao, T.; Ishikawa-Yamauchi, Y.; Nagata, S.; Hashimoto, K.; Sato, T.; Kimura, H.; Shinohara, T.; Sanbo, M.; et al. Generation of rat offspring using spermatids produced through in vitro spermatogenesis. Sci. Rep. 2023, 13, 12105. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, L.; Cheng, Q.; Diao, F.; Zeng, Q.; Yang, X.; Wu, Y.; Zhang, H.; Huang, M.; Chen, J.; et al. In vitro testicular organogenesis from human fetal gonads produces fertilization-competent spermatids. Cell Res. 2020, 30, 244–255. [Google Scholar] [CrossRef]
- Abofoul-Azab, M.; Lunenfeld, E.; Levitas, E.; Zeadna, A.; Younis, J.S.; Bar-Ami, S.; Huleihel, M. Identification of Premeiotic, Meiotic, and Postmeiotic Cells in Testicular Biopsies Without Sperm from Sertoli Cell-Only Syndrome Patients. Int. J. Mol. Sci. 2019, 20, 470. [Google Scholar] [CrossRef]
- Single-Cell RNA-Sequencing Based Data of the Human Testis: A Scoping Review towards Mapping the Development of Spermatogenesis. Available online: https://osf.io/8xrbv/ (accessed on 25 March 2024).
- Tang, F.; Barbacioru, C.; Wang, Y.; Nordman, E.; Lee, C.; Xu, N.; Wang, X.; Bodeau, J.; Tuch, B.B.; Siddiqui, A.; et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 2009, 6, 377–382. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Garcia-Alonso, L.; Lorenzi, V.; Mazzeo, C.I.; Alves-Lopes, J.P.; Roberts, K.; Sancho-Serra, C.; Engelbert, J.; Marečková, M.; Gruhn, W.H.; Botting, R.A.; et al. Single-cell roadmap of human gonadal development. Nature 2022, 607, 540–547. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Li, L.; Yang, M.; Yong, J.; Zhai, F.; Wen, L.; Yan, L.; Qiao, J.; Tang, F. Dissecting Human Gonadal Cell Lineage Specification and Sex Determination Using A Single-cell RNA-seq Approach. Genom. Proteom. Bioinform. 2022, 20, 223–245. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.; Yan, L.; Yong, J.; Liu, X.; Hu, Y.; Fan, X.; Wu, X.; Guo, H.; Wang, X.; et al. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell 2017, 20, 858–873.e4. [Google Scholar] [CrossRef]
- Guo, J.; Sosa, E.; Chitiashvili, T.; Nie, X.; Rojas, E.J.; Oliver, E.; Plath, K.; Hotaling, J.M.; Stukenborg, J.B.; Clark, A.T.; et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 2021, 28, 764–778.e4. [Google Scholar] [CrossRef]
- Guo, F.; Yan, L.; Guo, H.; Li, L.; Hu, B.; Zhao, Y.; Yong, J.; Hu, Y.; Wang, X.; Wei, Y.; et al. The Transcriptome and DNA Methylome Landscapes of Human Primordial Germ Cells. Cell 2015, 161, 1437–1452. [Google Scholar] [CrossRef]
- Chitiashvili, T.; Dror, I.; Kim, R.; Hsu, F.M.; Chaudhari, R.; Pandolfi, E.; Chen, D.; Liebscher, S.; Schenke-Layland, K.; Plath, K.; et al. Female human primordial germ cells display X-chromosome dosage compensation despite the absence of X-inactivation. Nat. Cell Biol. 2020, 22, 1436–1446. [Google Scholar] [CrossRef]
- Sohni, A.; Tan, K.; Song, H.W.; Burow, D.; de Rooij, D.G.; Laurent, L.; Hsieh, T.C.; Rabah, R.; Hammoud, S.S.; Vicini, E.; et al. The Neonatal and Adult Human Testis Defined at the Single-Cell Level. Cell Rep. 2019, 26, 1501–1517.e4. [Google Scholar] [CrossRef]
- Voigt, A.L.; Dardari, R.; Su, L.; Lara, N.L.M.; Sinha, S.; Jaffer, A.; Munyoki, S.K.; Alpaugh, W.; Dufour, A.; Biernaskie, J.; et al. Metabolic transitions define spermatogonial stem cell maturation. Hum. Reprod. 2022, 37, 2095–2112. [Google Scholar] [CrossRef]
- Guo, J.; Nie, X.; Giebler, M.; Mlcochova, H.; Wang, Y.; Grow, E.J.; Kim, R.; Tharmalingam, M.; Matilionyte, G.; Lindskog, C.; et al. The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty. Cell Stem Cell 2020, 26, 262–276.e4. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Yao, C.C.; Xing, X.Y.; Jing, T.; Li, P.; Zhu, Z.J.; Yang, C.; Zhai, J.; Tian, R.H.; Chen, H.X.; et al. Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat. Commun. 2020, 11, 5683. [Google Scholar] [CrossRef]
- Guo, J.; Grow, E.J.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Nie, X.; Guo, Y.; Takei, Y.; Yun, J.; Cai, L.; et al. The adult human testis transcriptional cell atlas. Cell Res. 2018, 28, 1141–1157. [Google Scholar] [CrossRef]
- Guo, J.; Grow, E.J.; Yi, C.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Murphy, P.J.; Wike, C.L.; Carrell, D.T.; Goriely, A.; et al. Chromatin and Single-Cell RNA-Seq Profiling Reveal Dynamic Signaling and Metabolic Transitions during Human Spermatogonial Stem Cell Development. Cell Stem Cell 2017, 21, 533–546.e6. [Google Scholar] [CrossRef]
- Neuhaus, N.; Yoon, J.; Terwort, N.; Kliesch, S.; Seggewiss, J.; Huge, A.; Voss, R.; Schlatt, S.; Grindberg, R.V.; Schöler, H.R. Single-cell gene expression analysis reveals diversity among human spermatogonia. Mol. Hum. Reprod. 2017, 23, 79–90. [Google Scholar] [CrossRef]
- Hermann, B.P.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.N.; Chen, I.C.; Gildersleeve, H.; Lehle, J.D.; Mayo, M.; Westernströer, B.; et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018, 25, 1650–1667.e8. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Chang, G.; Chen, Y.; An, G.; Yan, L.; Gao, S.; Xu, Y.; Cui, Y.; Dong, J.; et al. Single-Cell RNA Sequencing Analysis Reveals Sequential Cell Fate Transition during Human Spermatogenesis. Cell Stem Cell 2018, 23, 599–614.e4. [Google Scholar] [CrossRef]
- Chen, S.; An, G.; Wang, H.; Wu, X.; Ping, P.; Hu, L.; Chen, Y.; Fan, J.; Cheng, C.Y.; Sun, F. Human obstructive (postvasectomy) and nonobstructive azoospermia—Insights from scRNA-Seq and transcriptome analysis. Genes Dis. 2020, 9, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Shami, A.N.; Zheng, X.; Munyoki, S.K.; Ma, Q.; Manske, G.L.; Green, C.D.; Sukhwani, M.; Orwig, K.E.; Li, J.Z.; Hammoud, S.S. Single-Cell RNA Sequencing of Human, Macaque, and Mouse Testes Uncovers Conserved and Divergent Features of Mammalian Spermatogenesis. Dev. Cell 2020, 54, 529–547.e12. [Google Scholar] [CrossRef]
- Xia, B.; Yan, Y.; Baron, M.; Wagner, F.; Barkley, D.; Chiodin, M.; Kim, S.Y.; Keefe, D.L.; Alukal, J.P.; Boeke, J.D.; et al. Widespread Transcriptional Scanning in the Testis Modulates Gene Evolution Rates. Cell 2020, 180, 248–262.e21. [Google Scholar] [CrossRef]
- Alfano, M.; Tascini, A.S.; Pederzoli, F.; Locatelli, I.; Nebuloni, M.; Giannese, F.; Garcia-Manteiga, J.M.; Tonon, G.; Amodio, G.; Gregori, S.; et al. Aging, inflammation and DNA damage in the somatic testicular niche with idiopathic germ cell aplasia. Nat. Commun. 2021, 12, 5205. [Google Scholar] [CrossRef]
- Di Persio, S.; Tekath, T.; Siebert-Kuss, L.M.; Cremers, J.F.; Wistuba, J.; Li, X.; Meyer Zu Hörste, G.; Drexler, H.C.A.; Wyrwoll, M.J.; Tüttelmann, F.; et al. Single-cell RNA-seq unravels alterations of the human spermatogonial stem cell compartment in patients with impaired spermatogenesis. Cell Rep. Med. 2021, 2, 100395. [Google Scholar] [CrossRef]
- Mahyari, E.; Guo, J.; Lima, A.C.; Lewinsohn, D.P.; Stendahl, A.M.; Vigh-Conrad, K.A.; Nie, X.; Nagirnaja, L.; Rockweiler, N.B.; Carrell, D.T.; et al. Comparative single-cell analysis of biopsies clarifies pathogenic mechanisms in Klinefelter syndrome. Am. J. Hum. Genet. 2021, 108, 1924–1945. [Google Scholar] [CrossRef]
- Nie, X.; Munyoki, S.K.; Sukhwani, M.; Schmid, N.; Missel, A.; Emery, B.R.; DonorConnect; Stukenborg, J.B.; Mayerhofer, A.; Orwig, K.E.; et al. Single-cell analysis of human testis aging and correlation with elevated body mass index. Dev. Cell 2022, 57, 1160–1176.e5. [Google Scholar] [CrossRef]
- Gui, Y.; Ma, X.; Xiong, M.; Wen, Y.; Cao, C.; Zhang, L.; Wang, X.; Liu, C.; Zhang, H.; Huang, X.; et al. Transcriptome analysis of meiotic and post-meiotic spermatogenic cells reveals the potential hub genes of aging on the decline of male fertility. Gene 2024, 893, 147883. [Google Scholar] [CrossRef]
- Rey, R.; Josso, N.; Racine, C. Sexual Differentiation. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Hassan, H.; Shanak, S. GOTrapper: A tool to navigate through branches of gene ontology hierarchy. BMC Bioinform. 2019, 20, 20. [Google Scholar] [CrossRef]
- Hung, J.Y.; Yen, M.C.; Jian, S.F.; Wu, C.Y.; Chang, W.A.; Liu, K.T.; Hsu, Y.L.; Chong, I.W.; Kuo, P.L. Secreted protein acidic and rich in cysteine (SPARC) induces cell migration and epithelial mesenchymal transition through WNK1/snail in non-small cell lung cancer. Oncotarget 2017, 8, 63691–63702. [Google Scholar] [CrossRef]
- GEO Overview. Available online: https://www.ncbi.nlm.nih.gov/geo/info/overview.html (accessed on 28 April 2024).
- Cheng, C.; Chen, W.; Jin, H.; Chen, X. A Review of Single-Cell RNA-Seq Annotation, Integration, and Cell-Cell Communication. Cells 2023, 12, 1970. [Google Scholar] [CrossRef]
- Rossi, P.; Dolci, S. Paracrine mechanisms involved in the control of early stages of Mammalian spermatogenesis. Front. Endocrinol. 2013, 4, 181. [Google Scholar] [CrossRef]
- Suzuki, T. Overview of single-cell RNA sequencing analysis and its application to spermatogenesis research. Reprod. Med. Biol. 2023, 22, e12502. [Google Scholar] [CrossRef]
- Mäkelä, J.-A.; Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testis Development. Endocr. Rev. 2018, 40, 857–905. [Google Scholar] [CrossRef]
- La Manno, G.; Soldatov, R.; Zeisel, A.; Braun, E.; Hochgerner, H.; Petukhov, V.; Lidschreiber, K.; Kastriti, M.E.; Lönnerberg, P.; Furlan, A.; et al. RNA velocity of single cells. Nature 2018, 560, 494–498. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Tan, K.; Song, H.W.; Thompson, M.; Munyoki, S.; Sukhwani, M.; Hsieh, T.C.; Orwig, K.E.; Wilkinson, M.F. Transcriptome profiling reveals signaling conditions dictating human spermatogonia fate in vitro. Proc. Natl. Acad. Sci. USA 2020, 117, 17832–17841. [Google Scholar] [CrossRef]
- Liebich, A.; Schmid, N.; Koupourtidou, C.; Herrmann, C.; Dietrich, K.G.; Welter, H.; Ninkovic, J.; Mayerhofer, A. The Molecular Signature of Human Testicular Peritubular Cells Revealed by Single-Cell Analysis. Cells 2022, 11, 3685. [Google Scholar] [CrossRef]
- Clermont, Y. Renewal of spermatogonia in man. Am. J. Anat. 1966, 118, 509–524. [Google Scholar] [CrossRef]
- Clermont, Y. Spermatogenesis in man. A study of the spermatogonial population. Fertil. Steril. 1966, 17, 705–721. [Google Scholar] [CrossRef]
- Di Persio, S.; Neuhaus, N. Human spermatogonial stem cells and their niche in male (in)fertility: Novel concepts from single-cell RNA-sequencing. Hum. Reprod. 2023, 38, 1–13. [Google Scholar] [CrossRef]
- Fayomi, A.P.; Orwig, K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018, 29, 207–214. [Google Scholar] [CrossRef]
- Jan, S.Z.; Vormer, T.L.; Jongejan, A.; Röling, M.D.; Silber, S.J.; de Rooij, D.G.; Hamer, G.; Repping, S.; van Pelt, A.M.M. Unraveling transcriptome dynamics in human spermatogenesis. Development 2017, 144, 3659–3673. [Google Scholar] [CrossRef]
- Roosen-Runge, E.C.; Marberger, E.; Nelson, W.O. Quantitative investigations on human testicular biopsies. II. Infertility and other conditions. Fertil. Steril. 1957, 8, 203–219. [Google Scholar] [CrossRef]
- Voigt, A.L.; Dardari, R.; Lara, N.L.M.; He, T.; Steele, H.; Dufour, A.; Orwig, K.E.; Dobrinski, I. Multiomics approach to profiling Sertoli cell maturation during development of the spermatogonial stem cell niche. Mol. Hum. Reprod. 2023, 29, gaad004. [Google Scholar] [CrossRef]
- Voigt, A.L.; de Lima e Martins Lara, N.; Dobrinski, I. Comparing the adult and pre-pubertal testis: Metabolic transitions and the change in the spermatogonial stem cell metabolic microenvironment. Andrology 2023, 11, 1132–1146. [Google Scholar] [CrossRef]
- Kurek, M.; Åkesson, E.; Yoshihara, M.; Oliver, E.; Cui, Y.; Becker, M.; Alves-Lopes, J.P.; Bjarnason, R.; Romerius, P.; Sundin, M.; et al. Spermatogonia Loss Correlates with LAMA 1 Expression in Human Prepubertal Testes Stored for Fertility Preservation. Cells 2021, 10, 241. [Google Scholar] [CrossRef]
- The Gene Ontology Resource. Available online: https://geneontology.org/ (accessed on 11 May 2024).
- Wosnitzer, M.S.; Goldstein, M. Obstructive azoospermia. Urol. Clin. N. Am. 2014, 41, 83–95. [Google Scholar] [CrossRef]
- Raleigh, D.; O’Donnell, L.; Southwick, G.J.; de Kretser, D.M.; McLachlan, R.I. Stereological analysis of the human testis after vasectomy indicates impairment of spermatogenic efficiency with increasing obstructive interval. Fertil. Steril. 2004, 81, 1595–1603. [Google Scholar] [CrossRef]
- McVicar, C.M.; O’Neill, D.A.; McClure, N.; Clements, B.; McCullough, S.; Lewis, S.E.M. Effects of vasectomy on spermatogenesis and fertility outcome after testicular sperm extraction combined with ICSI. Hum. Reprod. 2005, 20, 2795–2800. [Google Scholar] [CrossRef][Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaspen, L.; Kanbar, M.; Wyns, C. Mapping the Development of Human Spermatogenesis Using Transcriptomics-Based Data: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 6925. https://doi.org/10.3390/ijms25136925
Kwaspen L, Kanbar M, Wyns C. Mapping the Development of Human Spermatogenesis Using Transcriptomics-Based Data: A Scoping Review. International Journal of Molecular Sciences. 2024; 25(13):6925. https://doi.org/10.3390/ijms25136925
Chicago/Turabian StyleKwaspen, Lena, Marc Kanbar, and Christine Wyns. 2024. "Mapping the Development of Human Spermatogenesis Using Transcriptomics-Based Data: A Scoping Review" International Journal of Molecular Sciences 25, no. 13: 6925. https://doi.org/10.3390/ijms25136925
APA StyleKwaspen, L., Kanbar, M., & Wyns, C. (2024). Mapping the Development of Human Spermatogenesis Using Transcriptomics-Based Data: A Scoping Review. International Journal of Molecular Sciences, 25(13), 6925. https://doi.org/10.3390/ijms25136925







