Arginine Regulates Skeletal Muscle Fiber Type Formation via mTOR Signaling Pathway
Abstract
1. Introduction
2. Results
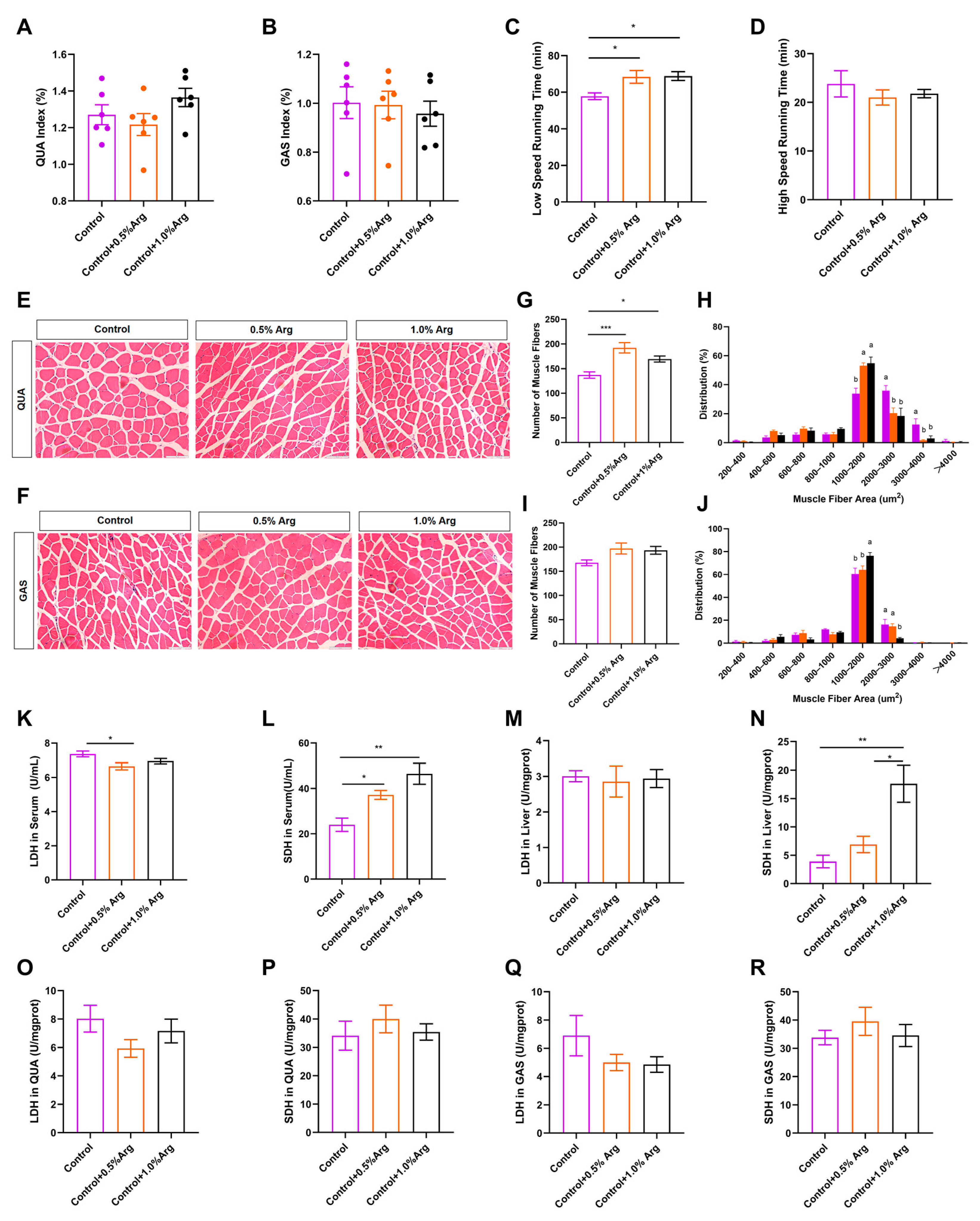
2.1. Growth Performance and Organ Index
2.2. Exogenous Arg Improves Endurance Exercise Ability and Shifts Skeletal Muscle Fiber Size Distribution
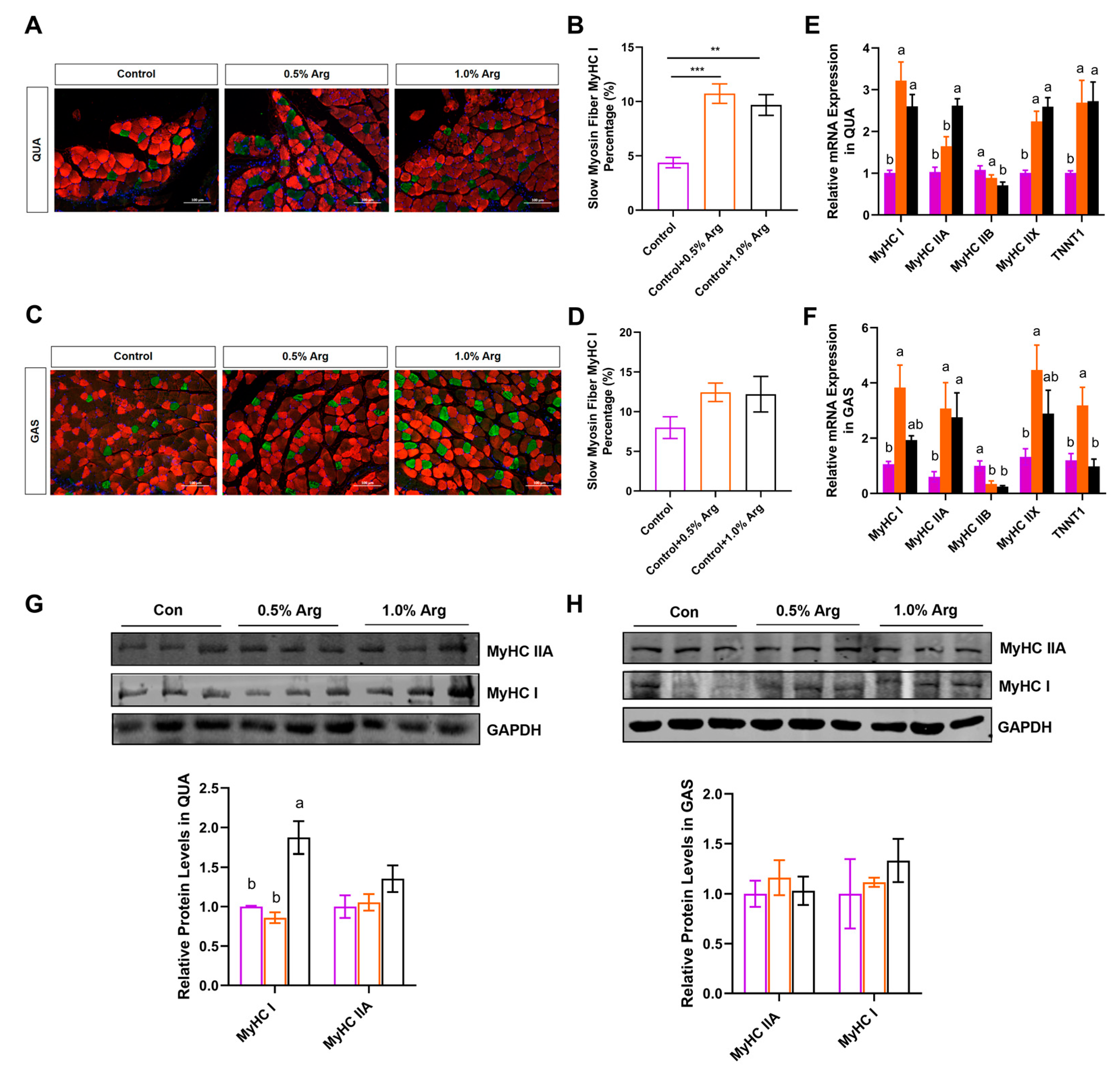
2.3. Arg Increases Proportion of Slow-Twitch Fibers In Vivo
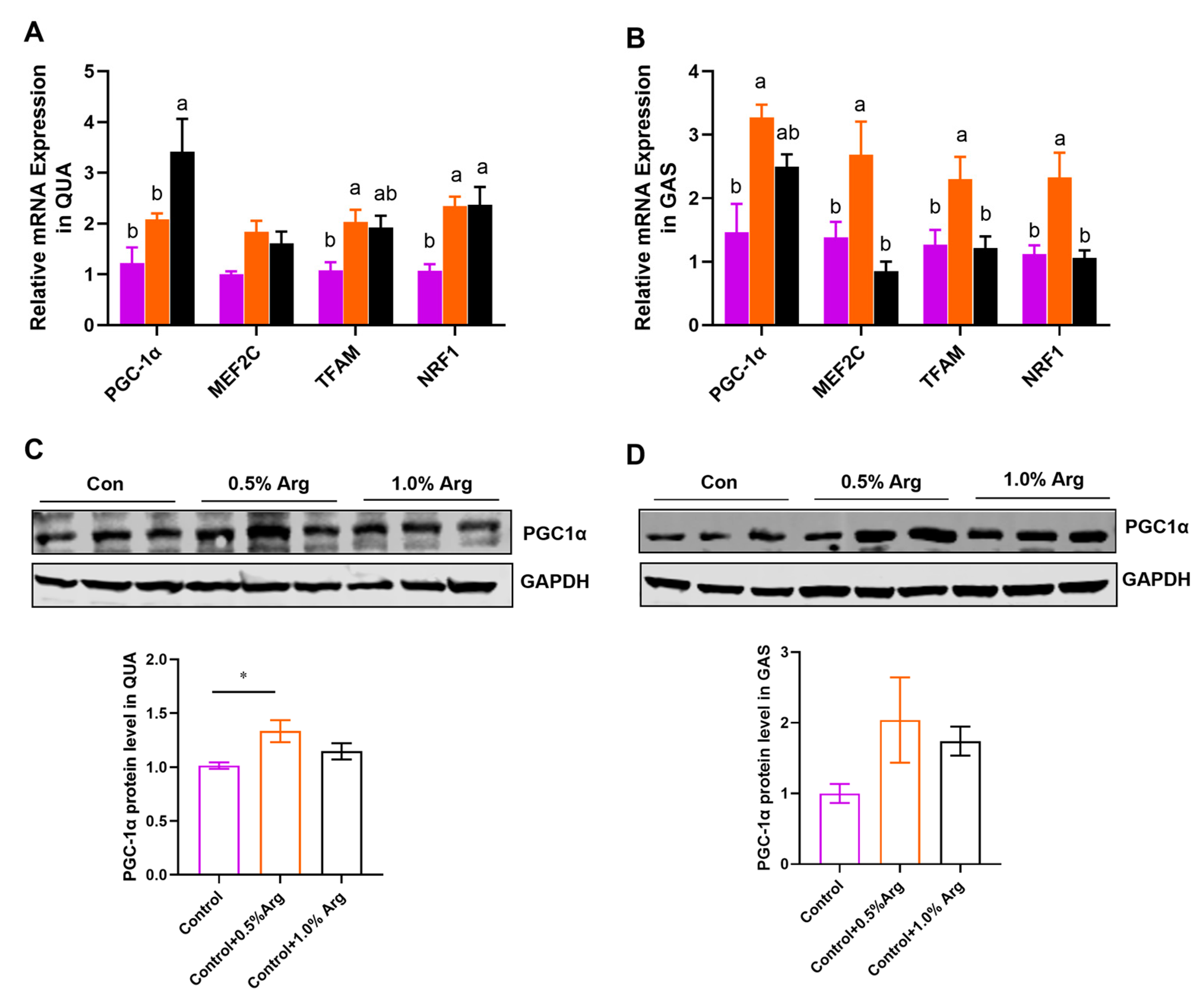
2.4. Arg Promotes Mitochondrial Biogenesis in Skeletal Muscle
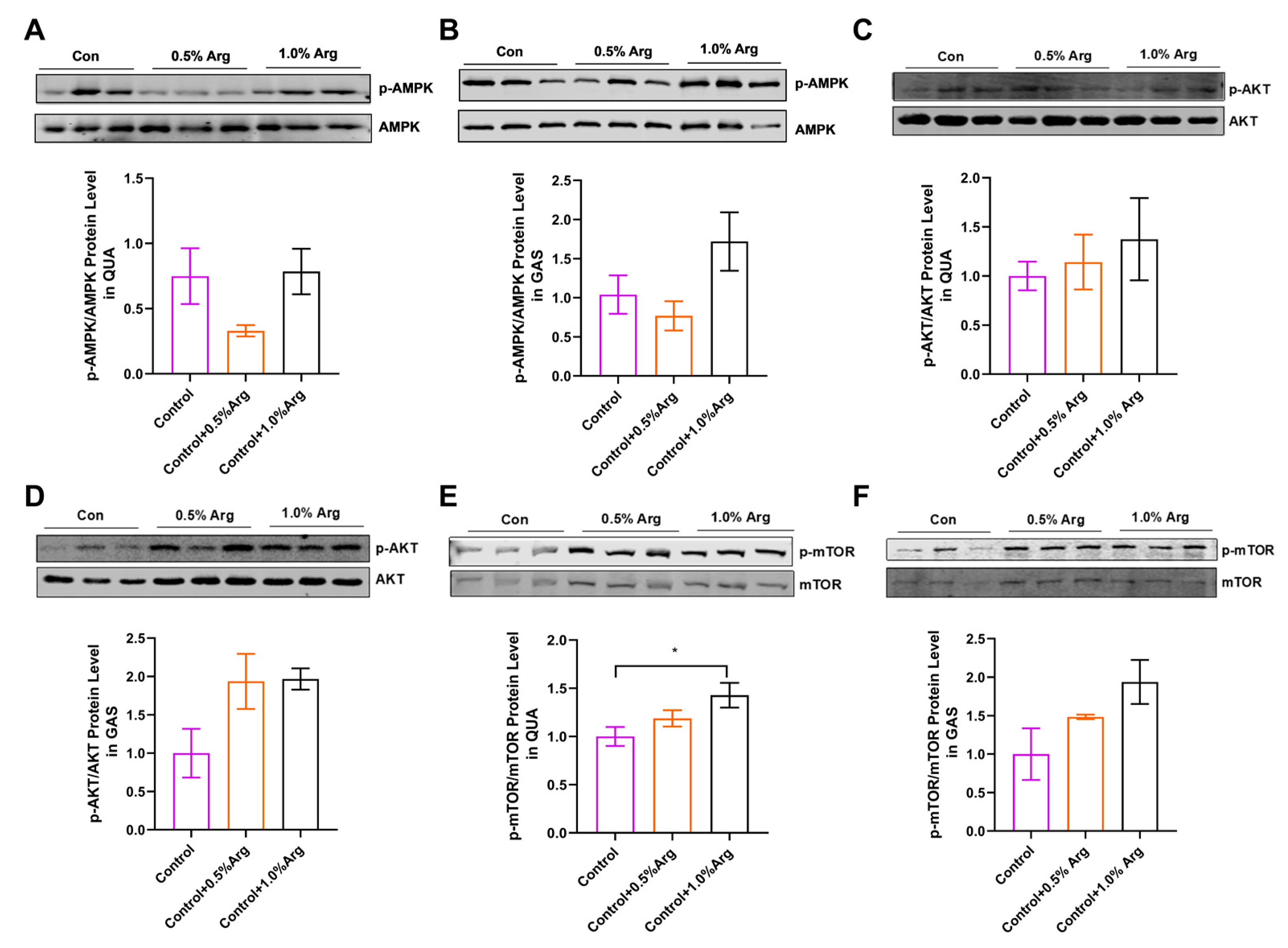
2.5. Arg Activates mTOR Signaling Pathway in Skeletal Muscle of Mice
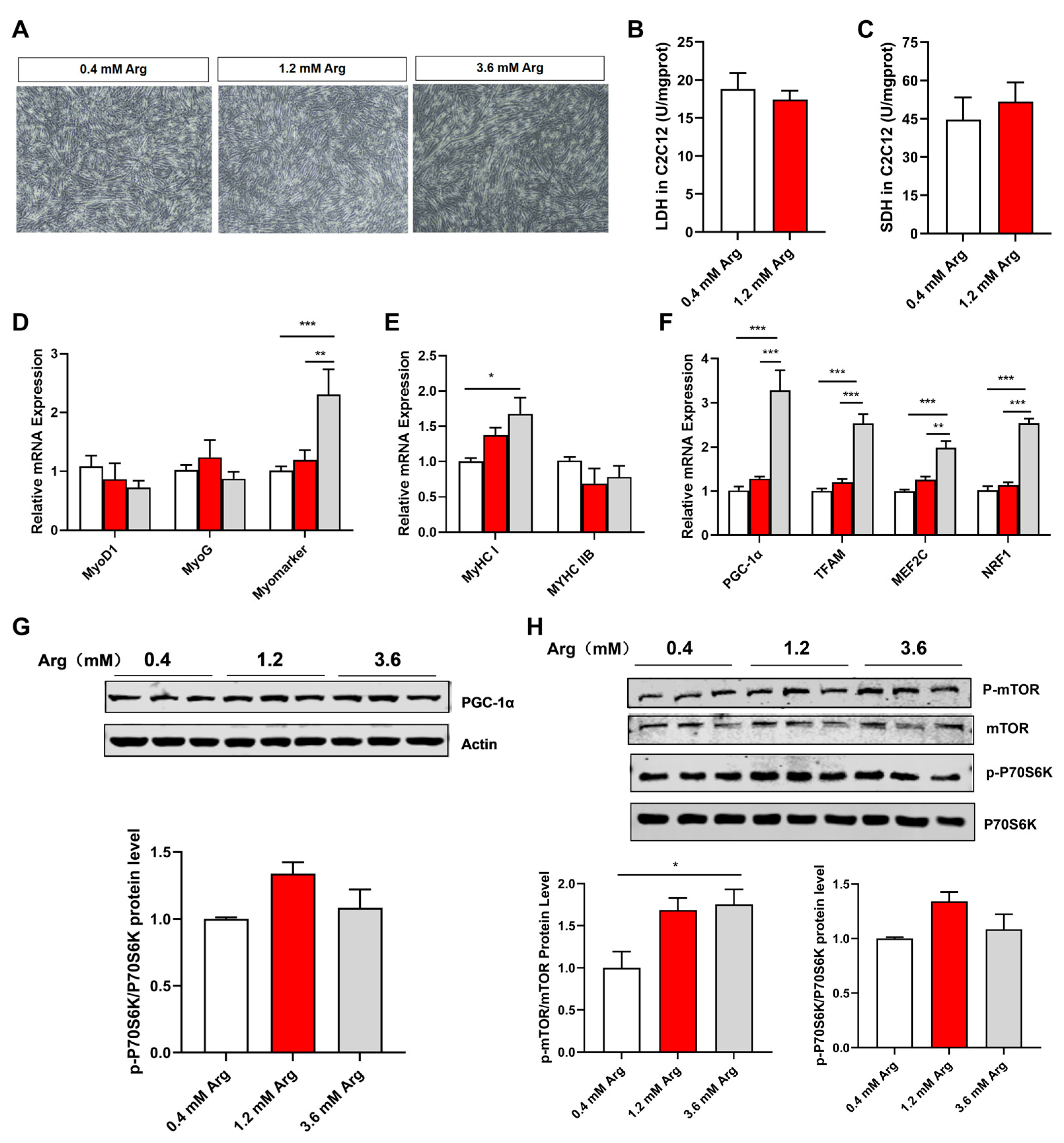
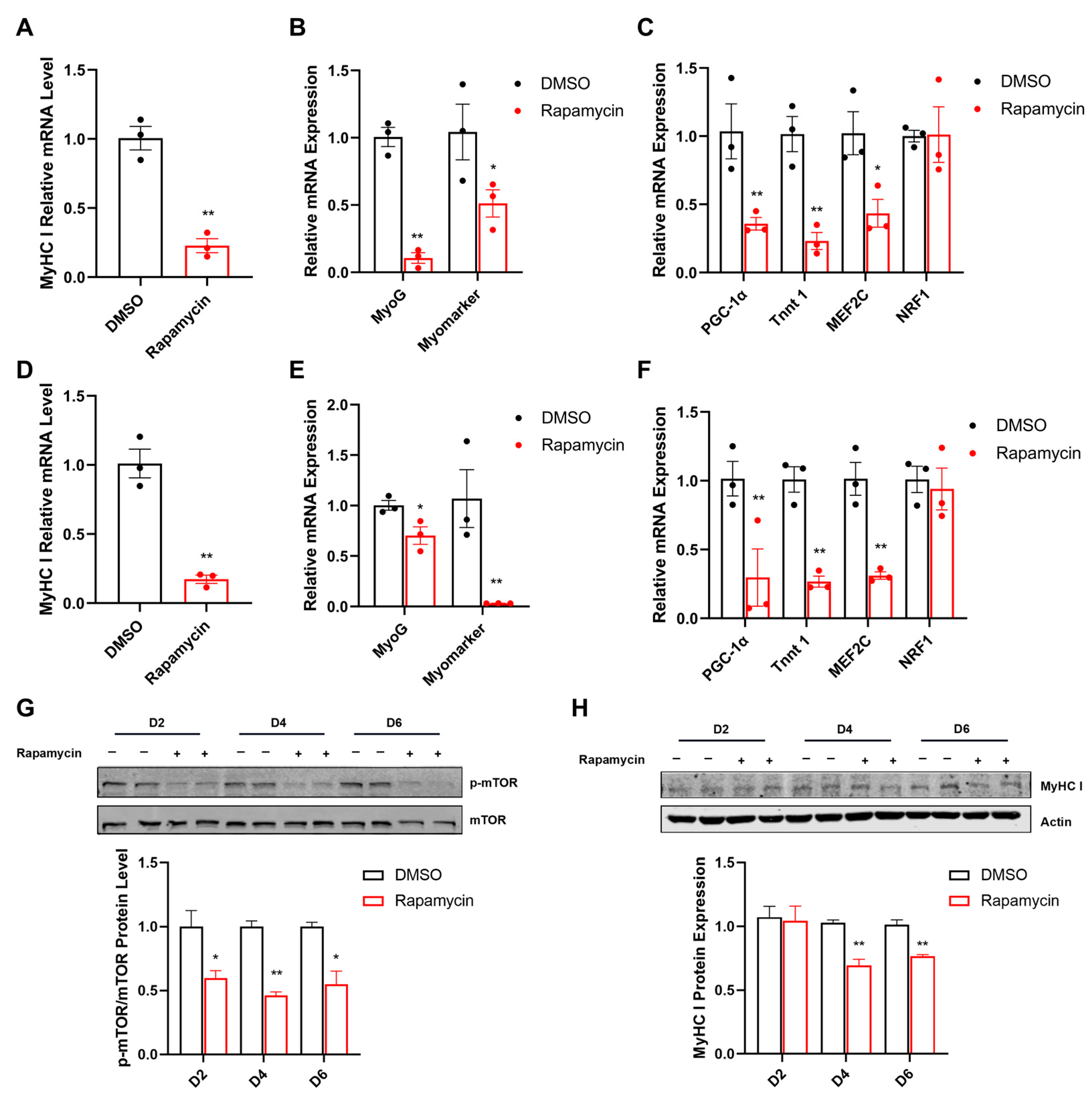
2.6. Arg Affects Fiber Type and Mitochondrial Function via mTOR Signaling Pathway in C2C12 Myotubes
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Strength and Exercise Endurance
4.3. Cell Culture
4.4. Enzyme Activities Assay
4.5. RNA Extraction, Reverse Transcript, and qRT-PCR
4.6. Western Blot Assay
4.7. Histological Analysis
4.8. Immunofluorescence Staining
4.9. Data and Statistical Analysis
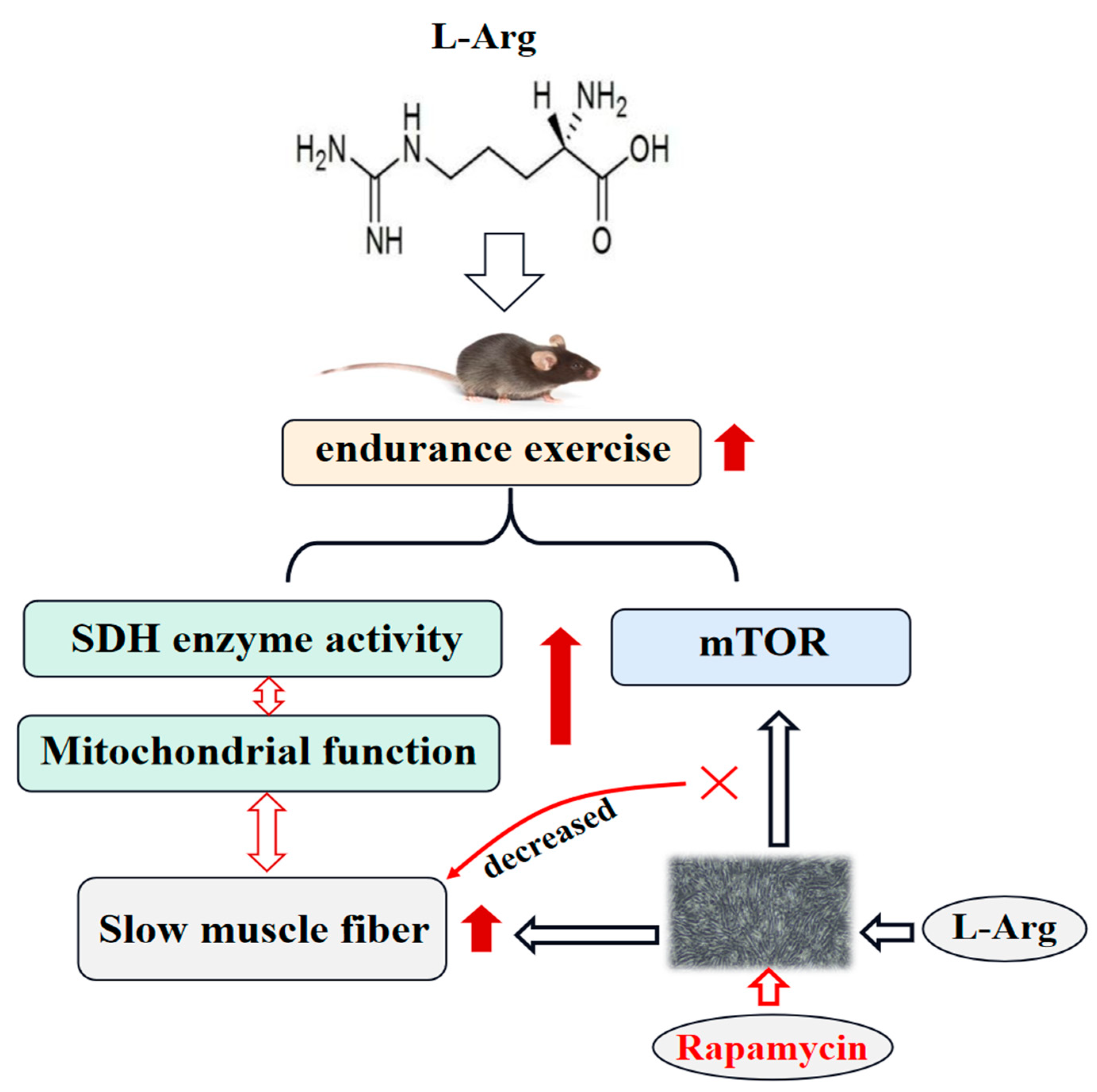
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int. J. Mol. Sci. 2020, 21, 1830. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, T.J.; Nimphius, S.; Bellon, C.R.; Stone, M.H. The Importance of Muscular Strength: Training Considerations. Sports Med. 2018, 48, 765–785. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Weng, K.; Gu, T.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021, 100, 101264. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.L.; Fu, C.Y.; Wang, J.; Chen, S.Y.; Yao, J.; Lai, S.J. Expression of MyHC genes, composition of muscle fiber type and their association with intramuscular fat, tenderness in skeletal muscle of Simmental hybrids. Mol. Biol. Rep. 2014, 41, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; He, J.; Chen, H.; Zheng, P.; Yu, J.; Luo, Y.; et al. Effects of dietary grape seed proanthocyanidin extract supplementation on meat quality, muscle fiber characteristics and antioxidant capacity of finishing pigs. Food Chem. 2022, 367, 130781. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; da Costa, N.; Blackley, R.; Southwood, O.; Evans, G.; Plastow, G.; Wood, J.D.; Richardson, R.I. Relationships of myosin heavy chain fibre types to meat quality traits in traditional and modern pigs. Meat Sci. 2003, 64, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef]
- Zhao, Y.; Cholewa, J.; Shang, H.; Yang, Y.; Ding, X.; Liu, S.; Xia, Z.; Zanchi, N.E.; Wang, Q. Exercise May Promote Skeletal Muscle Hypertrophy via Enhancing Leucine-Sensing: Preliminary Evidence. Front. Physiol. 2021, 12, 741038. [Google Scholar] [CrossRef]
- Chen, X.; Luo, X.; Chen, D.; Yu, B.; He, J.; Huang, Z. Arginine promotes porcine type I muscle fibres formation through improvement of mitochondrial biogenesis. Br. J. Nutr. 2020, 123, 499–507. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.Q.; Yuan, Y.X.; Xu, P.W.; Zhang, C.; Li, F.; Wang, L.N.; Yin, C.; Zhang, L.; Cai, X.C.; et al. Succinate induces skeletal muscle fiber remodeling via SUNCR1 signaling. EMBO Rep. 2019, 20, e47892. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, S.; Song, H.; Traore, S.S.; Li, J.; Raubenheimer, D.; Cui, Z.; Kou, G. Naringin Promotes Skeletal Muscle Fiber Remodeling by the AdipoR1-APPL1-AMPK Signaling Pathway. J. Agric. Food Chem. 2021, 69, 11890–11899. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.D.; Wu, G.; Bazer, F.W.; Park, J.C.; Shinzato, I.; Kim, S.W. Dietary L-arginine supplementation enhances the reproductive performance of gilts. J. Nutr. 2007, 137, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lin, Y.; Jiang, Z.; Zheng, C.; Zhou, G.; Yu, D.; Cao, T.; Wang, J.; Chen, F. Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids 2010, 38, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.M.; El-Senousey, H.K.; Yang, X.J.; Yao, J.H. Dietary L-arginine supplementation reduces abdominal fat content by modulating lipid metabolism in broiler chickens. Animal 2013, 7, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Y.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. Arginine promotes skeletal muscle fiber type transformation from fast-twitch to slow-twitch via Sirt1/AMPK pathway. J. Nutr. Biochem. 2018, 61, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Harachi, M.; Masui, K.; Okamura, Y.; Tsukui, R.; Mischel, P.S.; Shibata, N. mTOR Complexes as a Nutrient Sensor for Driving Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3267. [Google Scholar] [CrossRef] [PubMed]
- He, T.; He, L.; Gao, E.; Hu, J.; Zang, J.; Wang, C.; Zhao, J.; Ma, X. Fat deposition deficiency is critical for the high mortality of pre-weanling newborn piglets. J. Anim. Sci. Biotechnol. 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, N.; Feng, Y.; Zhou, M.; Li, H.; Zhang, X.; Ma, X. From probiotics to postbiotics: Concepts and applications. Anim. Res. One Health 2023, 1, 92–114. [Google Scholar] [CrossRef]
- Morita, M.; Prudent, J.; Basu, K.; Goyon, V.; Katsumura, S.; Hulea, L.; Pearl, D.; Siddiqui, N.; Strack, S.; McGuirk, S.; et al. mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1. Mol. Cell 2017, 67, 922–935.e5. [Google Scholar] [CrossRef]
- Ma, N.; Chen, X.; Johnston, L.J.; Ma, X. Gut microbiota-stem cell niche crosstalk: A new territory for maintaining intestinal homeostasis. iMeta 2022, 1, e54. [Google Scholar] [CrossRef]
- Fukunaga, T.; Mori, S.; Omura, T.; Noda, Y.; Fujita, Y.; Ohsawa, I.; Shigemoto, K. Muscle fiber type specific alterations of mitochondrial respiratory function and morphology in aged female mice. Biochem. Biophys. Res. Commun. 2021, 540, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, H.; Cao, X.; Yang, D.; Yan, Y.; Lu, J.; Wang, X.; Wang, H. Endurance Exercise-Induced Fgf21 Promotes Skeletal Muscle Fiber Conversion through TGF-beta1 and p38 MAPK Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 11401. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, X.; Qiu, K.; He, L.; Wang, Y.; Yin, J. Arginine promotes myogenic differentiation and myotube formation through the elevation of cytoplasmic calcium concentration. Anim. Nutr. 2021, 7, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Q.; Zhang, X.; Zhu, X.; Dong, X.; Shen, L.; Zhang, S.; Niu, L.; Chen, L.; Zhang, M.; et al. L-Arginine Alleviates LPS-Induced Oxidative Stress and Apoptosis via Activating SIRT1-AKT-Nrf2 and SIRT1-FOXO3a Signaling Pathways in C2C12 Myotube Cells. Antioxidants 2021, 10, 1957. [Google Scholar] [CrossRef] [PubMed]
- Yazdanabadi, F.I.; Moghaddam, G.H.; Nematollahi, A.; Daghighkia, H.; Sarir, H. Effect of arginine supplementation on growth performance, lipid profile, and inflammatory responses of broiler chicks challenged with coccidiosis. Prev. Vet. Med. 2020, 180, 105031. [Google Scholar] [CrossRef] [PubMed]
- Filho, S.T.S.; lima, E.M.d.C.; de Oliveira, D.H.; de Abreu, M.L.T.; Rosa, P.V.; de Laurentiz, A.C.; Naves, L.d.P.; Rodrigues, P.B. Supplemental L-arginine improves feed conversion and modulates lipid metabolism in male and female broilers from 29 to 42 days of age. Animal 2021, 15, 100120. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-Arginine Modulates Glucose and Lipid Metabolism in Obesity and Diabetes. Curr. Protein Pept. Sci. 2017, 18, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.J.; Jiang, Q.Y.; Zhang, T.; Yin, Y.L.; Li, F.N.; Deng, J.P.; Wu, G.Y.; Kong, X.F. Dietary supplementation with arginine and glutamic acid modifies growth performance, carcass traits, and meat quality in growing-finishing pigs. J. Anim. Sci. 2017, 95, 2680–2689. [Google Scholar] [CrossRef]
- Schiaffino, S. Muscle fiber type diversity revealed by anti-myosin heavy chain antibodies. FEBS J. 2018, 285, 3688–3694. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.; Yan, J.; Chen, M.; Zhu, M.; Tang, Y.; Liu, S.; Tang, Z. A comprehensive epigenome atlas reveals DNA methylation regulating skeletal muscle development. Nucleic Acids Res. 2021, 49, 1313–1329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; He, J.; Yu, J.; Chen, J.; Chen, D. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef]
- Lee, S.H.; Joo, S.T.; Ryu, Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010, 86, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Hammelman, J.E.; Bowker, B.C.; Grant, A.L.; Forrest, J.C.; Schinckel, A.P.; Gerrard, D.E. Early postmortem electrical stimulation simulates PSE pork development. Meat Sci. 2003, 63, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kojima-Yuasa, A.; Tadano, H.; Mizuno, A.; Kon, A.; Norikura, T. Ursolic acid improves the indoxyl sulfate-induced impairment of mitochondrial biogenesis in C2C12 cells. Nutr. Res. Pract. 2022, 16, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Lezza, A.M. Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions: Useful insights from aging and calorie restriction studies. Mitochondrion 2015, 25, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Wang, J.; Zhang, D.; Wu, H.; Li, W.; Wei, H.; Ta, N.; Fan, Y.; Liu, Y.; et al. Mitophagy receptor FUNDC1 is regulated by PGC-1alpha/NRF1 to fine tune mitochondrial homeostasis. EMBO Rep. 2021, 22, e50629. [Google Scholar] [CrossRef]
- Summermatter, S.; Thurnheer, R.; Santos, G.; Mosca, B.; Baum, O.; Treves, S.; Hoppeler, H.; Zorzato, F.; Handschin, C. Remodeling of calcium handling in skeletal muscle through PGC-1alpha: Impact on force, fatigability, and fiber type. Am. J. Physiol. Cell Physiol. 2012, 302, C88–C99. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Wu, W.; Hou, L.; Chen, H.; Zuo, B.; Xiong, Y.; Yang, J. Skeletal Muscle-Specific Overexpression of PGC-1alpha Induces Fiber-Type Conversion through Enhanced Mitochondrial Respiration and Fatty Acid Oxidation in Mice and Pigs. Int. J. Biol. Sci. 2017, 13, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiang, L.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. Leucine regulates slow-twitch muscle fibers expression and mitochondrial function by Sirt1/AMPK signaling in porcine skeletal muscle satellite cells. Anim. Sci. J. 2019, 90, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, N.; Song, P.; He, T.; Levesque, C.; Bai, Y.; Zhang, A.; Ma, X. Grape Seed Proanthocyanidin Affects Lipid Metabolism via Changing Gut Microflora and Enhancing Propionate Production in Weaned Pigs. J. Nutr. 2019, 149, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Han, M.; Li, D.; Hu, S.; Gilbreath, K.R.; Bazer, F.W.; Wu, G. L-Arginine promotes protein synthesis and cell growth in brown adipocyte precursor cells via the mTOR signal pathway. Amino Acids 2017, 49, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, K.; Sun, L.; Jiao, H.; Zhou, Y.; Li, H.; Wang, X.; Zhao, J.; Lin, H. L-Arginine/nitric oxide regulates skeletal muscle development via muscle fibre-specific nitric oxide/mTOR pathway in chickens. Anim. Nutr. 2022, 10, 68–85. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Jiang, C.; Ma, G.; Huang, Y.; Zhang, H.; Lai, Y.; Wang, M.; Ahmed, T.; Lin, R.; et al. mTORC1-PGC1 axis regulates mitochondrial remodeling during reprogramming. FEBS J. 2020, 287, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Summer, R.; Shaghaghi, H.; Schriner, D.; Roque, W.; Sales, D.; Cuevas-Mora, K.; Desai, V.; Bhushan, A.; Ramirez, M.I.; Romero, F. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L1049–L1060. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, L.; Wu, W.; Liu, Z.; Huang, Y.; Yang, L.; Luo, Q.; Chen, J.; Hou, Y.; Song, G. Exercise protects proliferative muscle satellite cells against exhaustion via the Igfbp7-Akt-mTOR axis. Theranostics 2020, 10, 6448–6466. [Google Scholar] [CrossRef]
- Mishra, P.; Varuzhanyan, G.; Pham, A.H.; Chan, D.C. Mitochondrial Dynamics is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization. Cell Metab. 2015, 22, 1033–1044. [Google Scholar] [CrossRef]
- El Assar, M.; Alvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodriguez-Manas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Sato, Y.; Obeng, K.A.; Yoshizawa, F. Acute oral administration of L-leucine upregulates slow-fiber- and mitochondria-related genes in skeletal muscle of rats. Nutr. Res. 2018, 57, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Edman, S.; Soderlund, K.; Moberg, M.; Apro, W.; Blomstrand, E. mTORC1 Signaling in Individual Human Muscle Fibers Following Resistance Exercise in Combination with Intake of Essential Amino Acids. Front. Nutr. 2019, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Seko, D.; Ogawa, S.; Li, T.S.; Taimura, A.; Ono, Y. mu-Crystallin controls muscle function through thyroid hormone action. FASEB J. 2016, 30, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
| Target Genes | Primer Sequence (5′ to 3′) |
|---|---|
| GAPDH | F: ATGGTGAAGGTCGGAGTGAA R: CGTGGGTGGAATCATACTGG |
| MYHC I | F: GTCAAGGCCAAGATCGTGTC R: CTCCTTCACAGTCACCGTCT |
| MYHC IIA | F: CAGTGTCTAAGGCCAAGGGA R: TCTCATCAAGCTGCCTGGAA |
| MYHC IIB | F: AAGCCTGCCTCCTTCTTCAT R: CAAACACCGATGACTTGGCA |
| MYHC IIX | F: CCAAAGGCAAGGTTGAAGCT R: CAGCCAGCGATGTTGTAGTC |
| PGC-1α | F: GGATATACTTTACGCAGGTCGA R: CGTCTGAGTTGGTATCTAGGTC |
| MEF2C | F: GATCTCCGCGTTCTTATCCC R: CCAATGACTGAGCCGACTG |
| TNNT1 | F: TGGATCCACCAGCTGGAATCAGAA R: GCTGATGCGGTTGTAGAGCACATT |
| NRF1 | F: GTTGCCCAAGTGAATTACTCTG R: TCGTCTGGATGGTCATTTCAC |
| TFAM | F: GTGAGCAAGTATAAAGAGCAGC R: CTGAACGAGGTCTTTTTGGTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Wei, Y.; Feng, Y.; Zhang, S.; Ma, N.; Wang, K.; Tan, P.; Zhao, Y.; Zhao, J.; Ma, X. Arginine Regulates Skeletal Muscle Fiber Type Formation via mTOR Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 6184. https://doi.org/10.3390/ijms25116184
Zhou M, Wei Y, Feng Y, Zhang S, Ma N, Wang K, Tan P, Zhao Y, Zhao J, Ma X. Arginine Regulates Skeletal Muscle Fiber Type Formation via mTOR Signaling Pathway. International Journal of Molecular Sciences. 2024; 25(11):6184. https://doi.org/10.3390/ijms25116184
Chicago/Turabian StyleZhou, Min, Yihan Wei, Yue Feng, Shumin Zhang, Ning Ma, Kaige Wang, Peng Tan, Ying Zhao, Jinbiao Zhao, and Xi Ma. 2024. "Arginine Regulates Skeletal Muscle Fiber Type Formation via mTOR Signaling Pathway" International Journal of Molecular Sciences 25, no. 11: 6184. https://doi.org/10.3390/ijms25116184
APA StyleZhou, M., Wei, Y., Feng, Y., Zhang, S., Ma, N., Wang, K., Tan, P., Zhao, Y., Zhao, J., & Ma, X. (2024). Arginine Regulates Skeletal Muscle Fiber Type Formation via mTOR Signaling Pathway. International Journal of Molecular Sciences, 25(11), 6184. https://doi.org/10.3390/ijms25116184







