Integrated Transcriptomic and Metabolomic Analysis Reveals the Molecular Regulatory Mechanism of Flavonoid Biosynthesis in Maize Roots under Lead Stress
Abstract
1. Introduction
2. Results
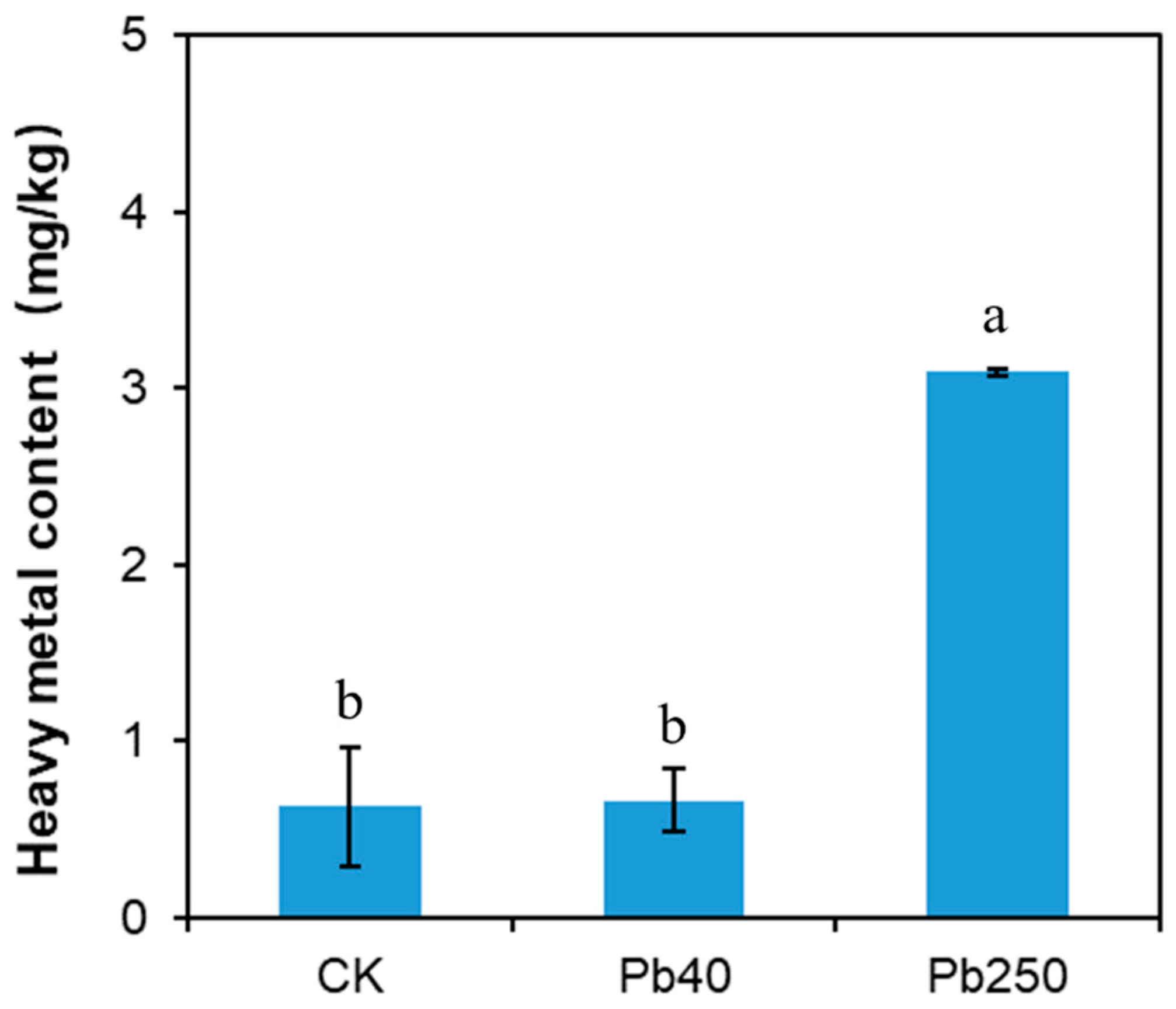
2.1. Pb Accumulation in Maize Roots
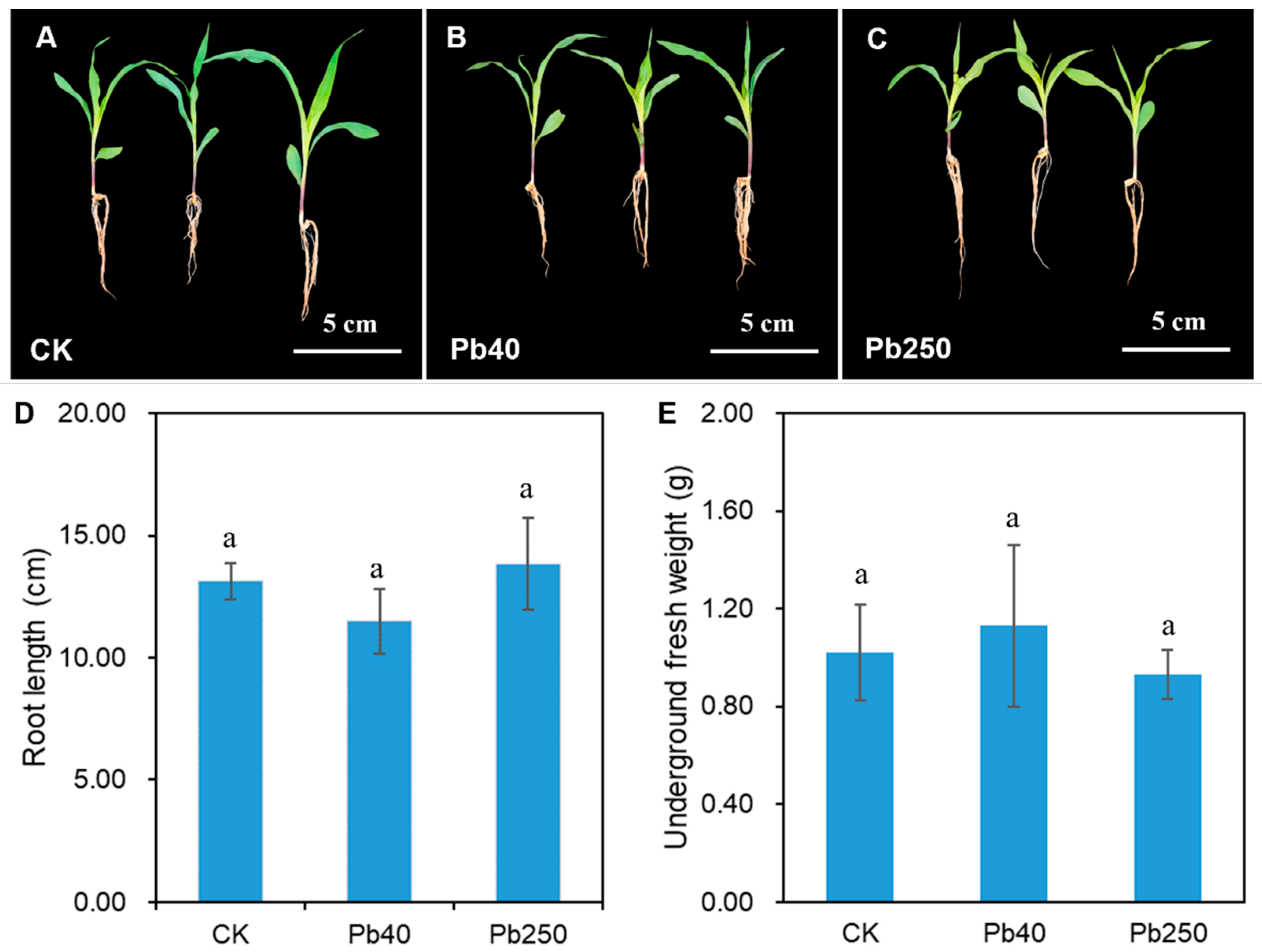
2.2. Phenotypic Characterization of Maize Roots
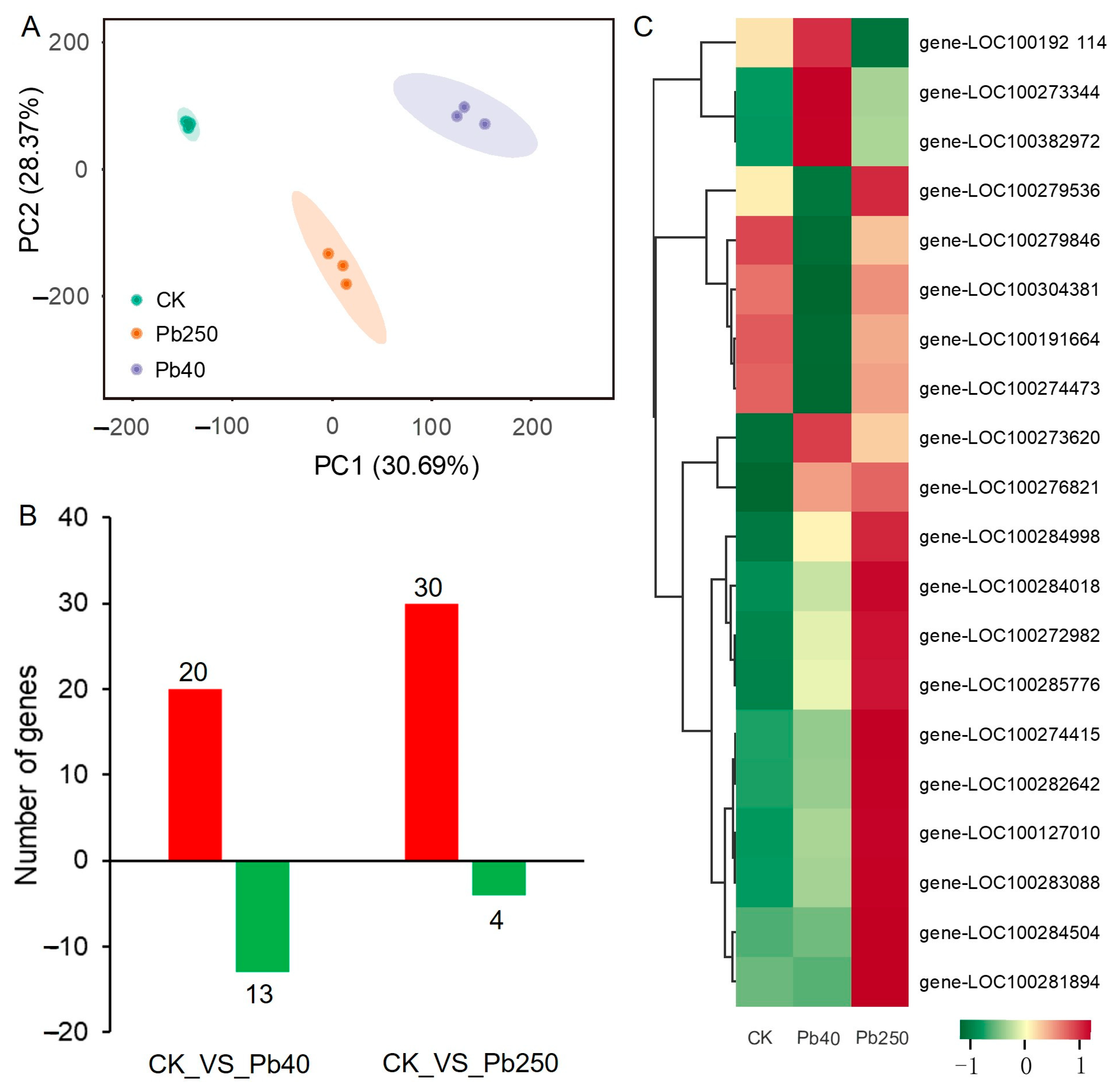
2.3. Transcriptomic Analysis of Maize Root Flavonoids
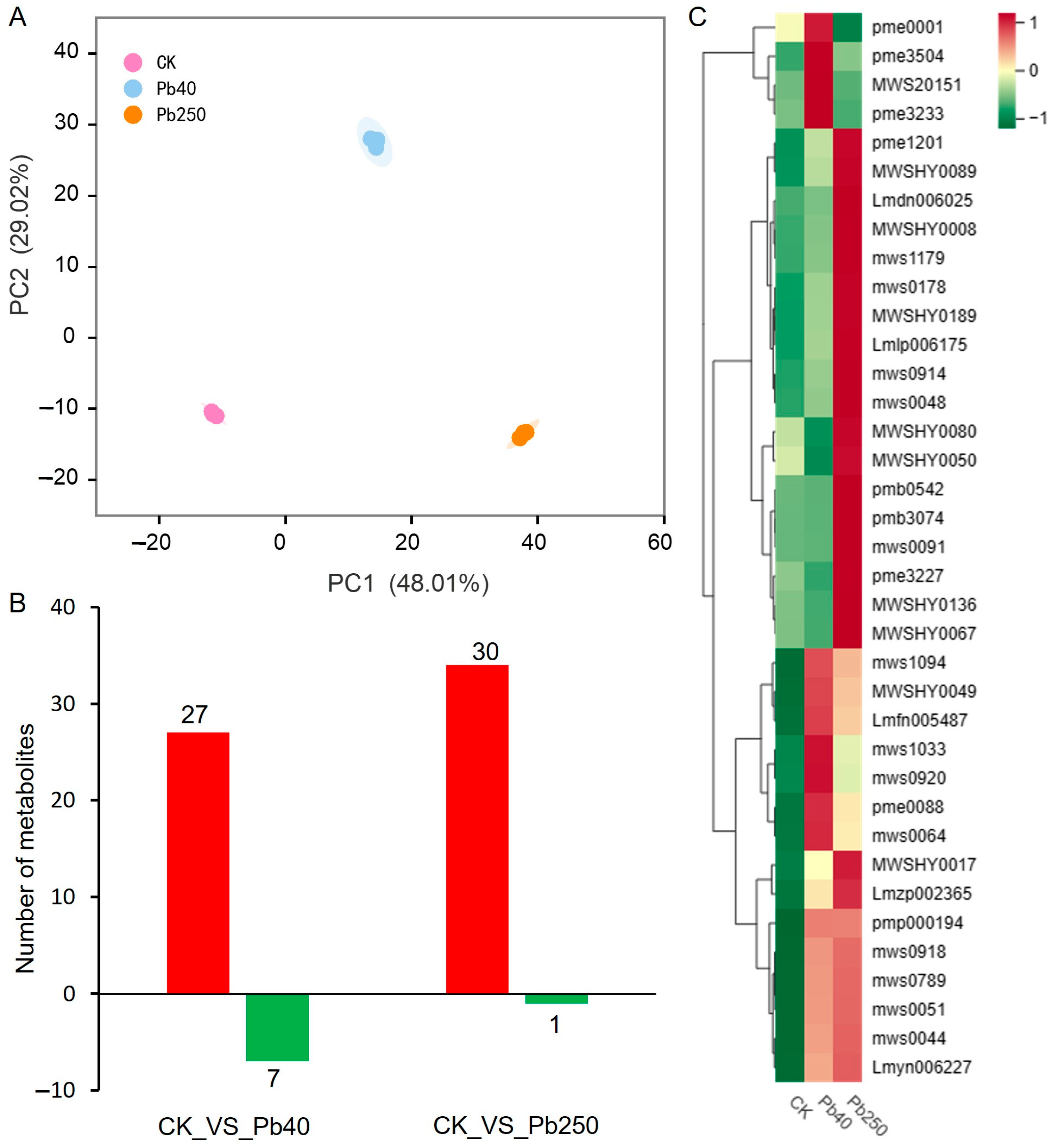
2.4. Metabolomic Analysis of Maize Roots
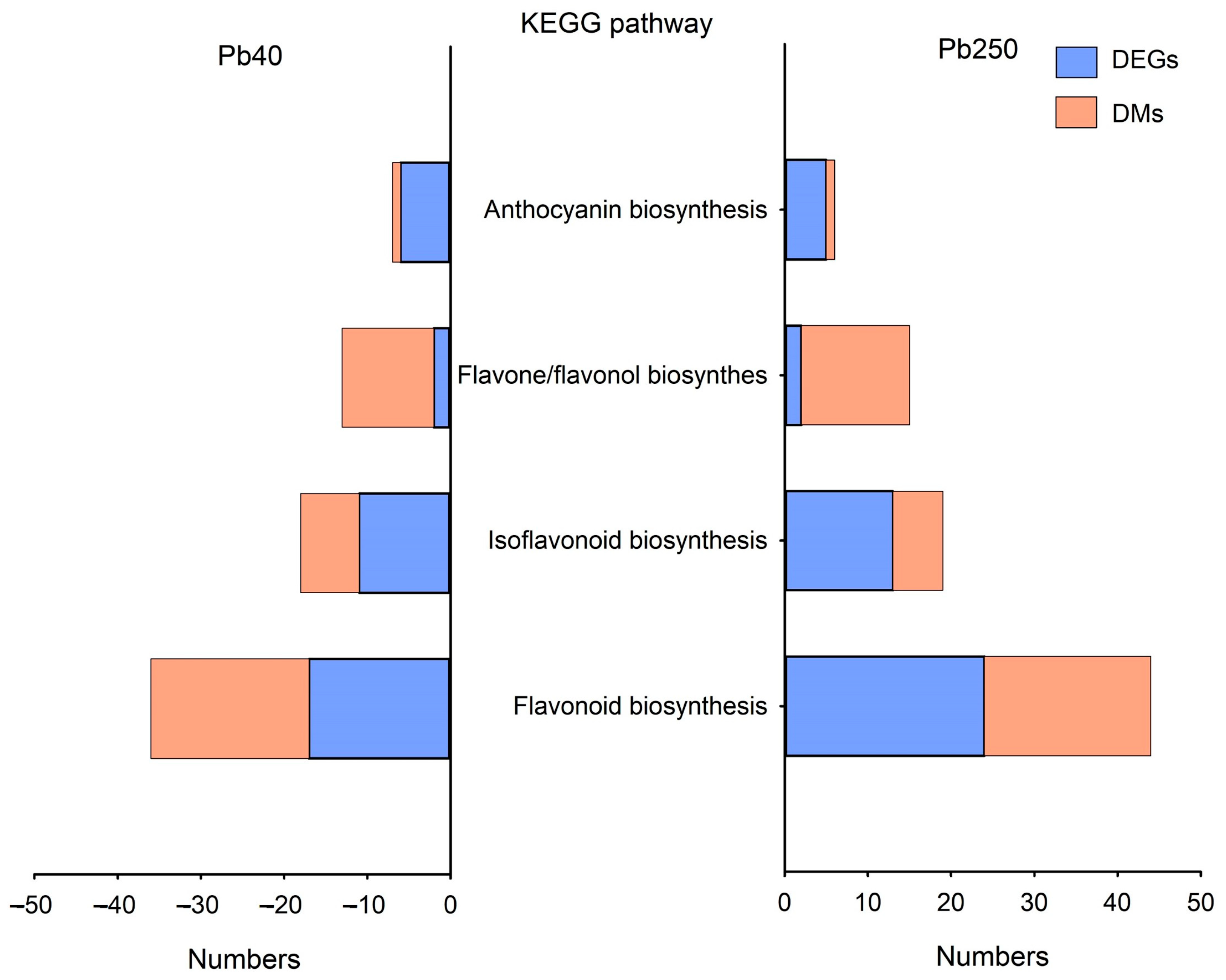
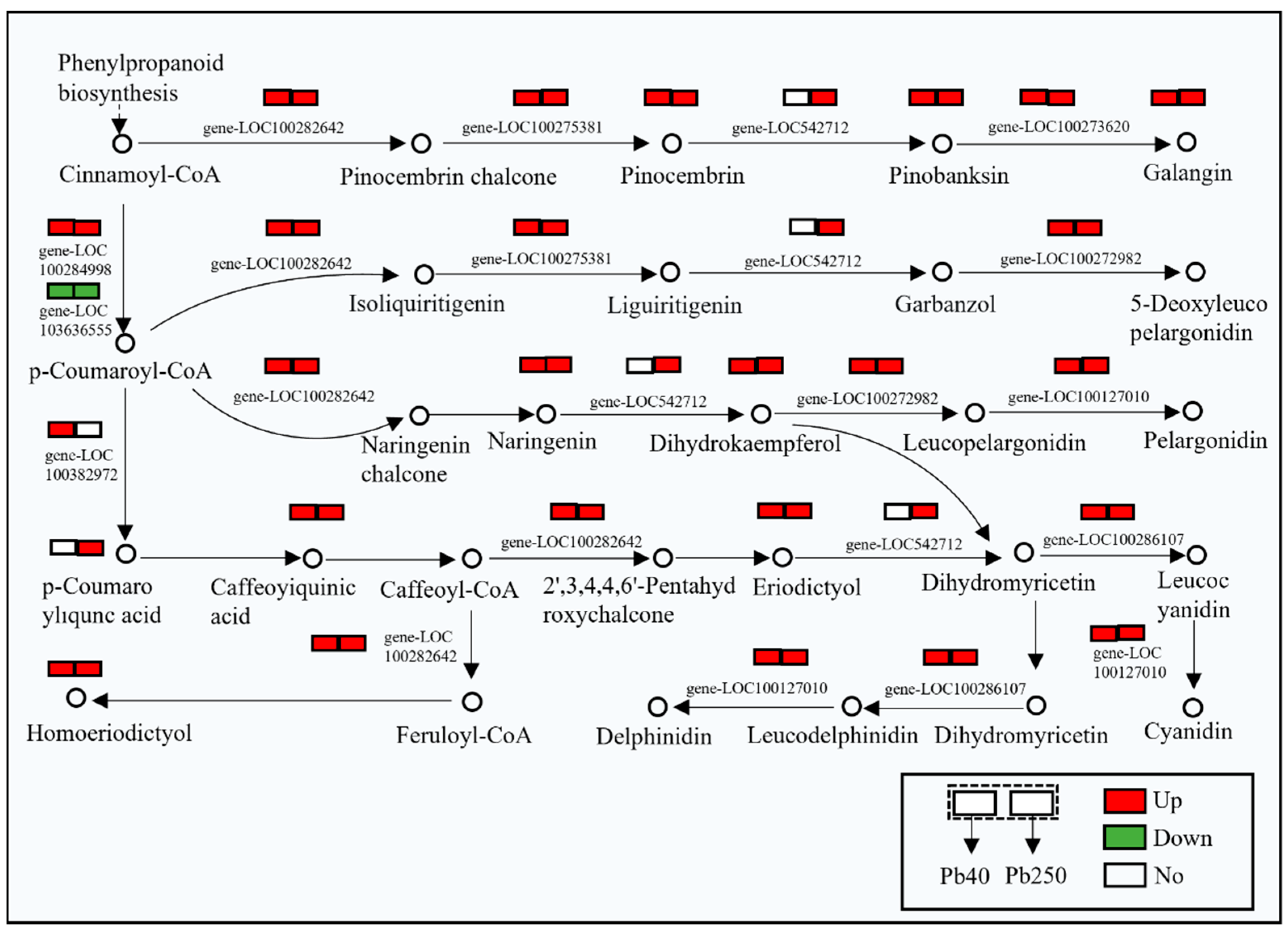
2.5. Pathway Enrichment of Flavonoid-Related Genes and Metabolites
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Determination of the Pb Concentration in Maize Roots
4.3. Characterization of Maize Root Morphology
4.4. Transcriptomic Analysis of Maize Roots
4.5. Metabolomic Analysis of Maize Roots
4.6. Validation of Gene Expression
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Brugliera, F.; Chandler, S. Recent progress of flower colour modification by biotechnology. Int. J. Mol. Sci. 2009, 10, 5350–5369. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Nakayama, T. Achievements and perspectives in biochemistry concerning anthocyanin modification for blue flower coloration. Plant Cell Physiol. 2015, 56, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.; Yoshioka, S.; Kishimoto, S.; Nakayama, M.; Douzono, M.; Tanaka, Y.; Aida, R. Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism. Sci. Adv. 2017, 3, e1602785. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, M.; Dong, H.; Liu, W.; Guo, L.; Wang, X. A spatially-resolved approach to visualize the distribution and biosynthesis of flavones in Scutellaria baicalensis Georgi. J. Pharm. Biomed. Anal. 2020, 179, 113014. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Jia, K.; Liao, K.; Liu, L.; Fan, G.; Zhang, S.; Wang, Y. Metabolomic and transcriptomice analyses of flavonoid biosynthesis in apricot fruits. Front. Plant Sci. 2023, 14, 1210309. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, R.; Galis, I.; Tanakamaru, S. Farinose flavonoids are associated with high freezing tolerance in fairy primrose (Primula malacoides) plants. J. Integr. Plant Biol. 2014, 56, 181–188. [Google Scholar] [CrossRef]
- Meng, D.; Dong, B.; Niu, L.; Song, Z.; Wang, L.; Amin, R.; Cao, H.; Li, H.; Yang, Q.; Fu, Y. The pigeon pea CcCIPK14-CcCBL1 pair positively modulates drought tolerance by enhancing flavonoid biosynthesis. Plant J. 2021, 106, 1278–1297. [Google Scholar] [CrossRef]
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic Acid-Induced Reactive Oxygen Species Are Modulated by Flavonols to Control Stomata Aperture. Plant Physiol. 2017, 175, 1807–1825. [Google Scholar] [CrossRef]
- Ghitti, E.; Rolli, E.; Crotti, E.; Borin, S. Flavonoids Are Intra- and Inter-Kingdom Modulator Signals. Microorganisms 2022, 10, 2479. [Google Scholar] [CrossRef]
- Hassan, A.; Parveen, A.; Hussain, S.; Hussain, I.; Rasheed, R. Investigating the role of different maize (Zea mays L.) cultivars by studying morpho-physiological attributes in chromium-stressed environment. Environ. Sci. Pollut. Res. 2022, 29, 72886–72897. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Sharma, A.; Badola, P.K.; Gautam, H.; Trivedi, P.K. Heterologous expression of Arabidopsis miR858 modulates biosynthesis of secondary metabolites and affects drought tolerance in tobacco. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 152, 287–298. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Ji, G. Pb contaminated soil influence on aboveground biomass and bioactive compounds in leaves of mulberry. Int. J. Phytoremediation 2022, 24, 100–1106. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Huang, Z.; Liu, X.; Imran, S.; Peng, L.; Dai, R.; Deng, Y. Environmental materials for remediation of soils contaminated with lead and cadmium using maize (Zea mays L.) growth as a bioindicator. Environ. Sci. Pollut. Res. 2016, 23, 6168–6178. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Naeem, M.; Akram, A.; Ashraf, M.Y.; Ahmad, K.S.; Zulfiqar, B.; Sardar, H.; Shabbir, R.N.; Majeed, S.; Shehzad, M.A.; et al. Seed priming with KNO(3) mediates biochemical processes to inhibit lead toxicity in maize (Zea mays L.). J. Agric. Food Chem. 2017, 97, 4780–4789. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pitalúa, D.; Hernández-Orduña, M.G.; Martínez-Escalante, G.A.; Lagunes-Gómez, I. Influence of Lead (Pb) and Its Relationship with the pH of Water on the Growth of Creole Maize (Zea mays L.). Agriculture 2022, 12, 749. [Google Scholar] [CrossRef]
- Guo, Z.; Yuan, X.; Li, L.; Zeng, M.; Yang, J.; Tang, H.; Duan, C. Genome-Wide Analysis of the ATP-Binding Cassette (ABC) Transporter Family in Zea mays L. and Its Response to Heavy Metal Stresses. Int. J. Mol. Sci. 2022, 23, 2109. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Yuan, X.; Yuan, M.; Huang, L.; Wang, S.; Liu, C.; Duan, C. Effects of heavy metals on stomata in plants: A review. Int. J. Mol. Sci. 2023, 24, 9302. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-B.; Li, Y.-H.; Shu, X.-C.; Pu, Y.-T.; Wang, X.-J.; Wang, T.; Wang, Z. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xiao, Z.; Wang, Y.; Wang, J.; Zhai, R.; Lin-Wang, K.; Espley, R.; Ma, F.; Li, P. Competition between anthocyanin and kaempferol glycosides biosynthesis affects pollen tube growth and seed set of Malus. Hortic. Res. 2021, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Guidi, L.; Pardossi, A.; Tattini, M.; Gould, K.S. Photoprotection by foliar anthocyanins mitigates effects of boron toxicity in sweet basil (Ocimum basilicum). Planta 2014, 240, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; Al-Amri, S.M. Exogenous melatonin-mediated modulation of arsenic tolerance with improved accretion of secondary metabolite production, activating antioxidant capacity and improved chloroplast ultrastructure in rosemary herb. Ecotoxicol. Environ. Saf. 2019, 180, 333–347. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, X.N.; Zhang, L.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J. Hazard. Mater. 2021, 403, 123922. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Tong, K.; Zhu, G.; Zhang, X.; Liu, X.; Si, Y. Surface spraying of anthocyanin through antioxidant defense and subcellular sequestration to decrease Cd accumulation in rice (Oryza sativa L.) grains in a lead-zinc mine area. Environ. Geochem. Health 2021, 43, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Yang, Y. Anthocyanin-mediated arsenic tolerance in plants. Environ. Pollut. 2022, 292, 118475. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, B.J.; Chung, J.O.; Kim, H.N.; Kim, E.H.; Jung, S.; Lee, H.; Lee, S.J.; Hong, Y.S. Metabolomic unveiling of a diverse range of green tea (Camellia sinensis) metabolites dependent on geography. Food Chem. 2015, 174, 452–459. [Google Scholar] [CrossRef]
- Medina, S.; Vicente, R.; Amador, A.; Araus, J.L. Interactive Effects of Elevated [CO(2)] and Water Stress on Physiological Traits and Gene Expression during Vegetative Growth in Four Durum Wheat Genotypes. Front. Plant Sci. 2016, 7, 1738. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2015, 6, 1143. [Google Scholar] [CrossRef]
- Nakashima, K.; Ito, Y.; Yamaguchi-Shinozaki, K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009, 149, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Manzi, M.; Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.L.; Liu, Z.W.; Luo, X.G. A metabolomic, transcriptomic profiling, and mineral nutrient metabolism study of the phytotoxicity mechanism of uranium. J. Hazard. Mater. 2020, 386, 121437. [Google Scholar] [CrossRef]
- Lei, Z.; Zijie, Z.; Shengzuo, F.; Yang, L.; Xulan, S. Integrative analysis of metabolome and transcriptome reveals molecular regulatory mechanism of flavonoid biosynthesis in Cyclocarya paliurus under salt stress. Ind. Crops Prod. 2021, 70, 170. [Google Scholar] [CrossRef]
- Yating, S.; Guoyun, Z.; Ning, C.; Jianguo, Z.; Caiyun, H. Metabolomic and transcriptomic analyses provide insights into the flavonoid biosynthesis in sea buckthorn (Hippophae rhamnoides L.). LWT-Food Sci. Technol. 2023, 187, 115276. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, B.; Dong, W.; Li, J.; Wang, D.; Liu, Z.; Gao, C. Comparative transcriptomic and metabolomic analyses provide insights into the responses to NaCl and Cd stress in Tamarix hispida. Sci. Total Environ. 2023, 884, 163889. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the study of the function and mechanism of the action of flavonoids in plants under environmental stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.J.; Zhang, F.J.; Zhang, G.Z.; Jiang, X.Y.; Yu, H.M.; Hou, B.K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NtMYB4 and NtCHS1 Are Critical Factors in the Regulation of Flavonoid Biosynthesis and Are Involved in Salinity Responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.H.; Li, W.; Niu, C.F.; Wei, W.; Hu, Y.; Han, J.Q.; Lu, X.; Tao, J.J.; Jin, M.; Qin, H.; et al. A class B heat shock factor selected for during soybean domestication contributes to salt tolerance by promoting flavonoid biosynthesis. New Phytol. 2020, 225, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Ashraf, M.; Tabassam, Q.; Hussain, M.; Firdous, H. Lead (Pb)-induced regulation of growth, photosynthesis, and mineral nutrition in maize (Zea mays L.) plants at early growth stages. Biol. Trace Elem. Res. 2011, 144, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, L.; Panozzo, A.; Radhouane, L.; Cortivo, C.D.; Barion, G.; Vamerali, T. Root Characteristics and Metal Uptake of Maize (Zea mays L.) under Extreme Soil Contamination. Agronomy 2021, 11, 178. [Google Scholar] [CrossRef]
- Gupta, P.; Kumar, V.; Usmani, Z.; Rani, R.; Chandra, A.; Gupta, V.K. A comparative evaluation towards the potential of Klebsiella sp. and Enterobacter sp. in plant growth promotion, oxidative stress tolerance and chromium uptake in Helianthus annuus (L.). J. Hazard. Mater. 2019, 377, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.; Zhao, C.; Fang, Z.; Jiao, P. Transcriptomic and metabolomic analysis reveals the role of CoA in the salt tolerance of Zygophyllum spp. BMC Plant Biol. 2020, 20, 9. [Google Scholar] [CrossRef]
| Gene ID | Control | Pb40 | Pb250 |
|---|---|---|---|
| LOC100286107 | 0.30 ± 0.10 | 0.31 ± 0.09 * | 5.54 ± 0.44 ** |
| LOC100275381 | 1.19 ± 0.05 | 3.94 ± 2.12 ** | 0.70 ± 0.03 ** |
| LOC100285776 | 0.78 ± 0.27 | 0.69 ± 0.32 | 5.27 ± 3.02 ** |
| LOC542712 | 17.39 ± 0.52 | 20.24 ± 1.39 | 5.0 ± 2.17 ** |
| LOC100192114 | 0.07 ± 0.01 | 0.22 ± 0.10 * | 1.23 ± 0.08 ** |
| Pb40 | Pb250 | ||
|---|---|---|---|
| GO_ID | GO_Description | Number of Enriched Genes | Number of Enriched Genes |
| GO:0009813 | flavonoid biosynthetic process | 12 | 12 |
| GO:0016758 | transferase activity | 9 | 11 |
| GO:0005506 | iron ion binding | 12 | 11 |
| GO:0008152 | metabolic process | 8 | 10 |
| GO:0009718 | anthocyanin-containing compound biosynthetic process | 9 | 10 |
| GO:0046872 | metal ion binding | 7 | 7 |
| GO:0050734 | hydroxycinnamoyltransferase activity | 4 | 7 |
| GO:0016021 | integral component of membrane | 7 | 7 |
| GO:0031418 | L-ascorbic acid binding | 4 | 4 |
| GO:0050662 | coenzyme binding | 4 | 4 |
| GO:0010023 | proanthocyanidin biosynthetic process | 3 | 3 |
| GO:0045430 | chalcone isomerase activity | 3 | 3 |
| GO:0050589 | leucocyanidin oxygenase activity | 3 | 3 |
| GO:0045552 | dihydrokaempferol 4-reductase activity | 3 | 3 |
| GO:0047890 | flavanone 4-reductase activity | 3 | 3 |
| GO:0009753 | response to jasmonic acid | 3 | 3 |
| GO:0033773 | isoflavone 2′-hydroxylase activity | 4 | 3 |
| GO:0016210 | naringenin–chalcone synthase activity | 2 | 2 |
| GO:0016705 | oxidoreductase activity | 2 | 2 |
| GO:0097237 | cellular response to toxic substance | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Yuan, X.; Li, T.; Wang, S.; Yu, Y.; Liu, C.; Duan, C. Integrated Transcriptomic and Metabolomic Analysis Reveals the Molecular Regulatory Mechanism of Flavonoid Biosynthesis in Maize Roots under Lead Stress. Int. J. Mol. Sci. 2024, 25, 6050. https://doi.org/10.3390/ijms25116050
Guo Z, Yuan X, Li T, Wang S, Yu Y, Liu C, Duan C. Integrated Transcriptomic and Metabolomic Analysis Reveals the Molecular Regulatory Mechanism of Flavonoid Biosynthesis in Maize Roots under Lead Stress. International Journal of Molecular Sciences. 2024; 25(11):6050. https://doi.org/10.3390/ijms25116050
Chicago/Turabian StyleGuo, Zhaolai, Xinqi Yuan, Ting Li, Sichen Wang, Yadong Yu, Chang’e Liu, and Changqun Duan. 2024. "Integrated Transcriptomic and Metabolomic Analysis Reveals the Molecular Regulatory Mechanism of Flavonoid Biosynthesis in Maize Roots under Lead Stress" International Journal of Molecular Sciences 25, no. 11: 6050. https://doi.org/10.3390/ijms25116050
APA StyleGuo, Z., Yuan, X., Li, T., Wang, S., Yu, Y., Liu, C., & Duan, C. (2024). Integrated Transcriptomic and Metabolomic Analysis Reveals the Molecular Regulatory Mechanism of Flavonoid Biosynthesis in Maize Roots under Lead Stress. International Journal of Molecular Sciences, 25(11), 6050. https://doi.org/10.3390/ijms25116050






