Synthesis and Photovoltaic Performance of β-Amino-Substituted Porphyrin Derivatives
Abstract
:1. Introduction
2. Results and Discussion
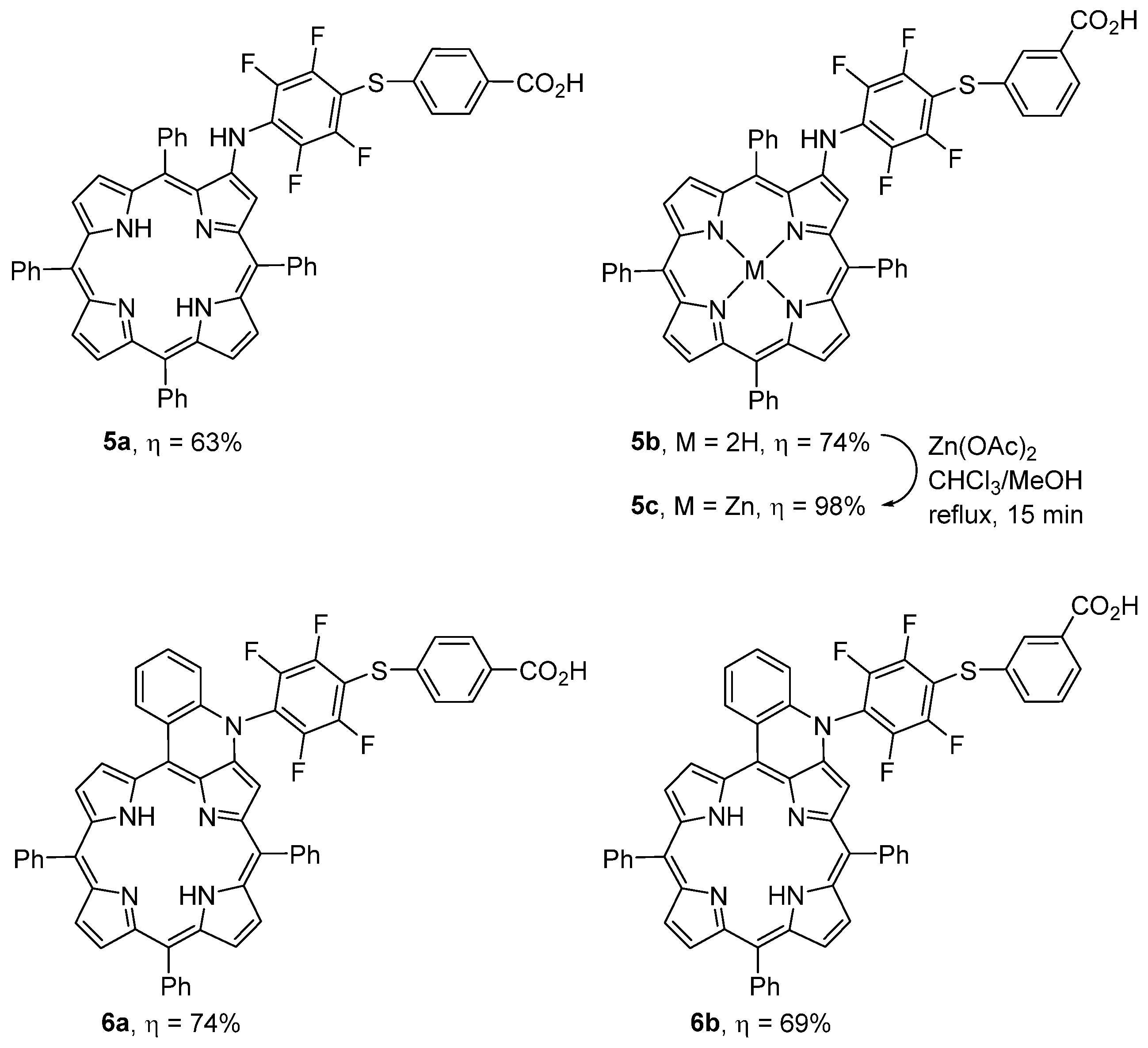
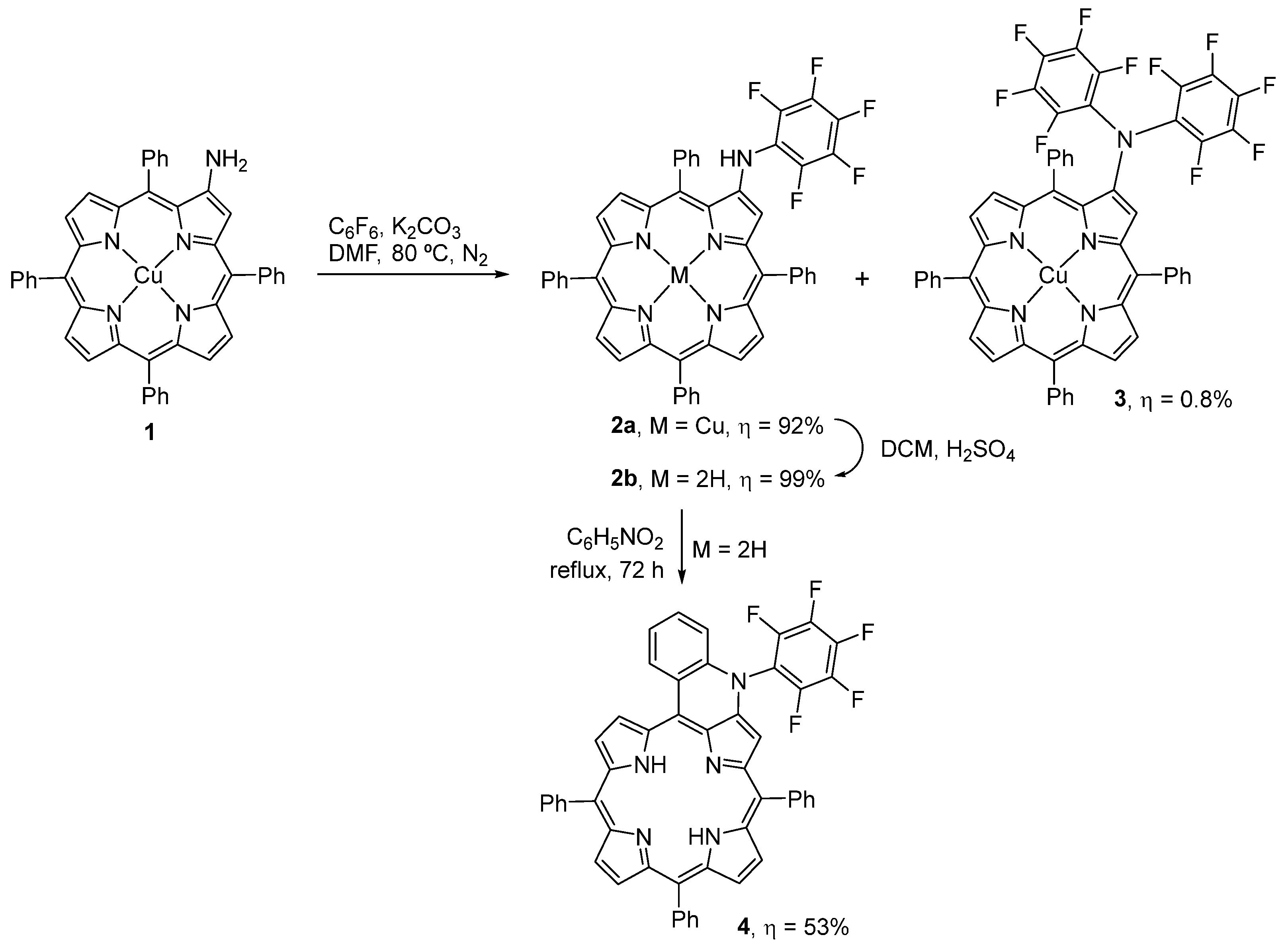
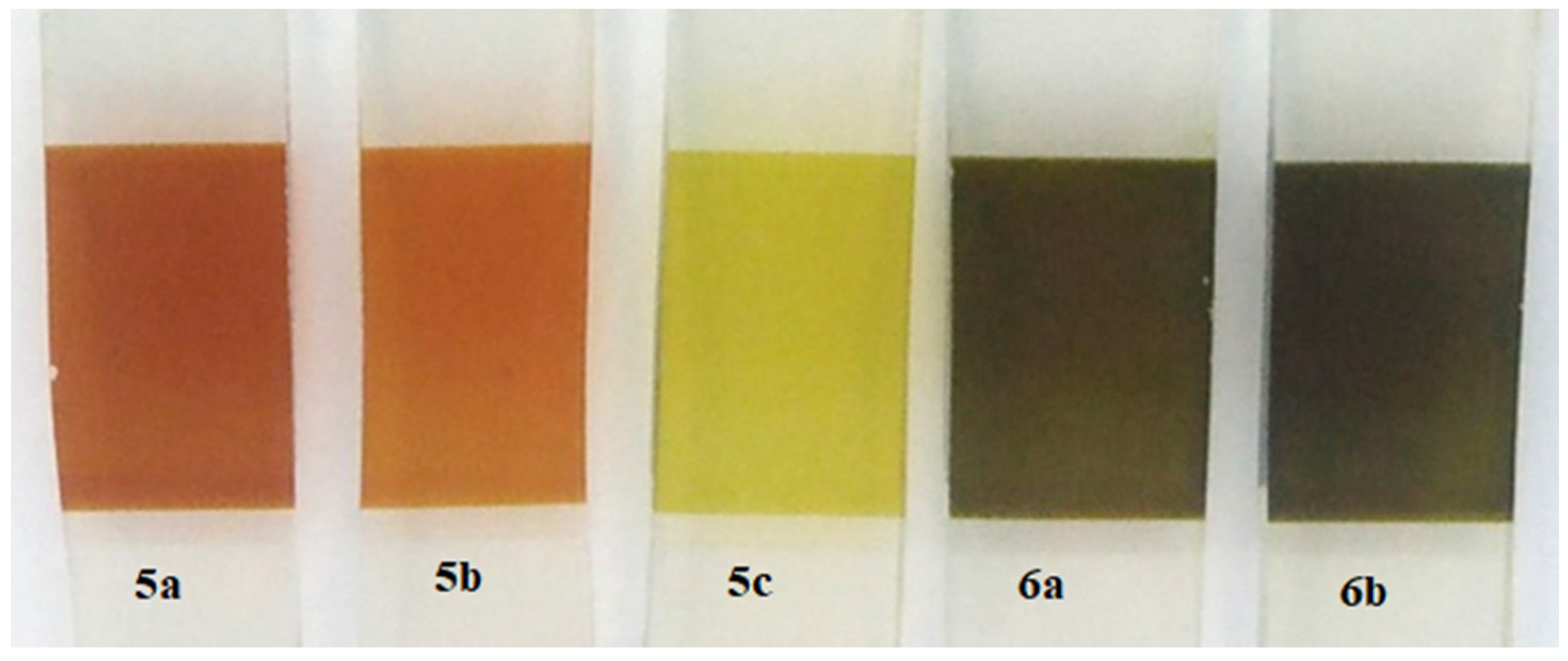
2.1. Synthesis
2.2. Spectroscopic Properties
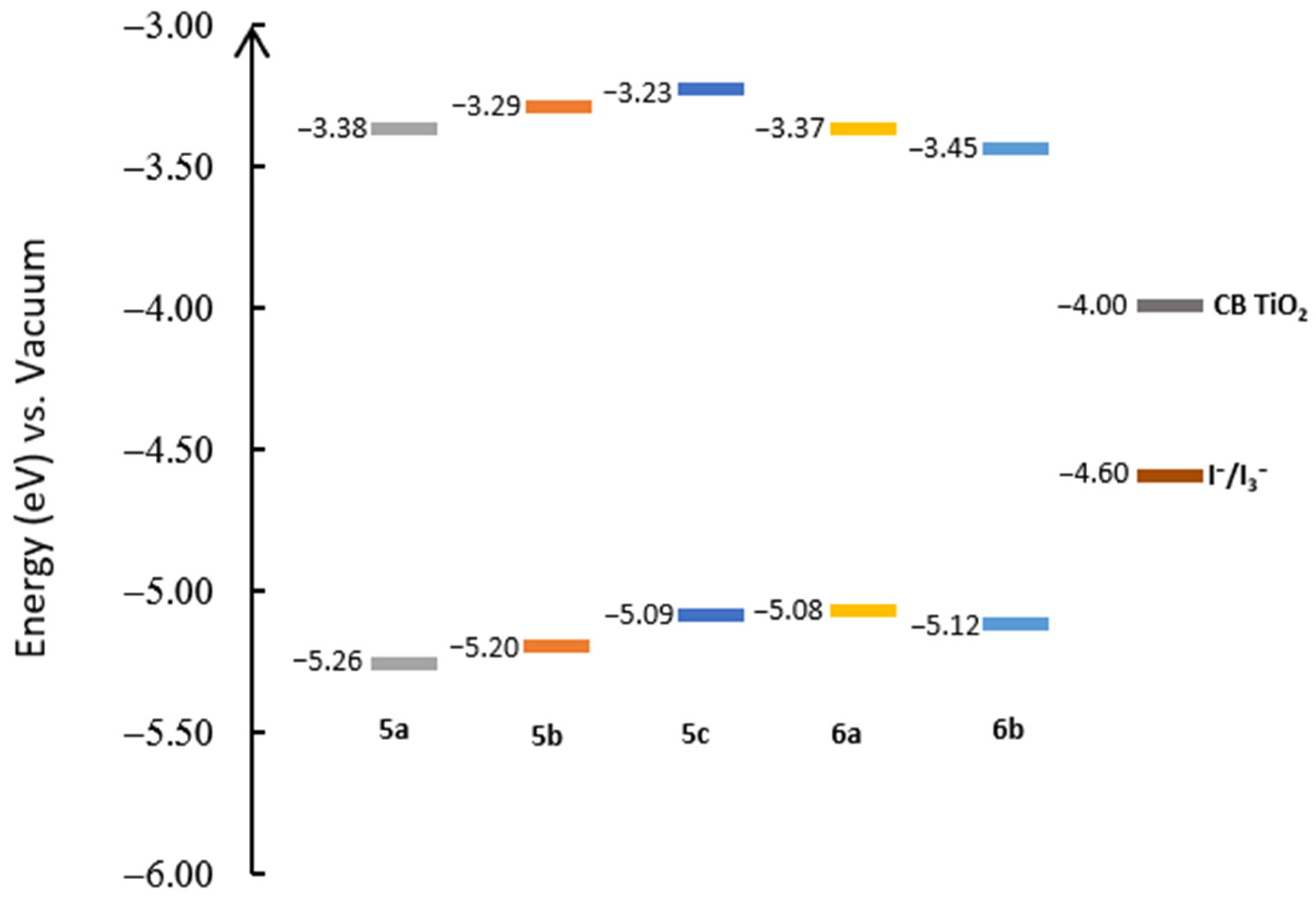
2.3. Electrochemical Characterization
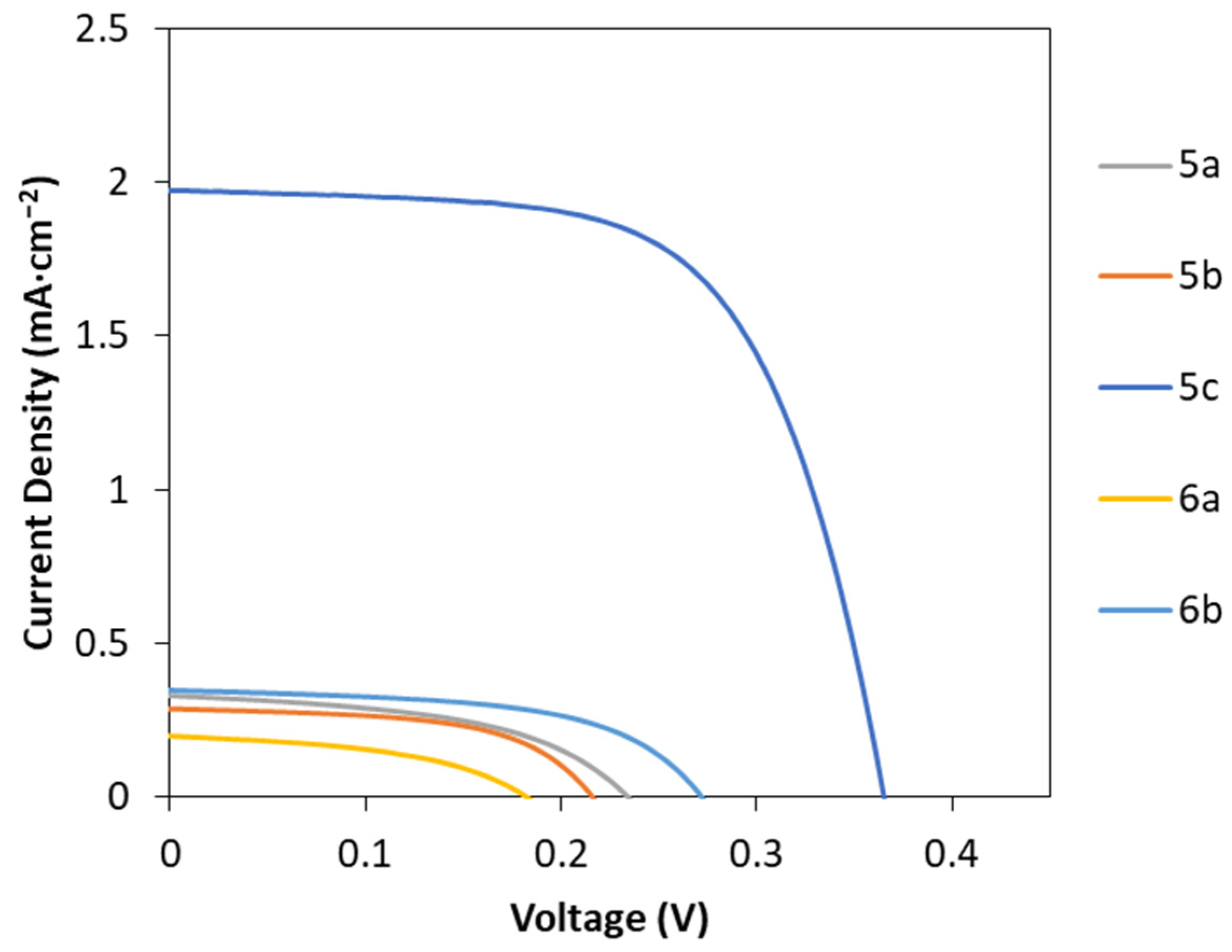
2.4. Fabrication of DSSC Using the Porphyrin Derivatives 5a–c and 6a,b
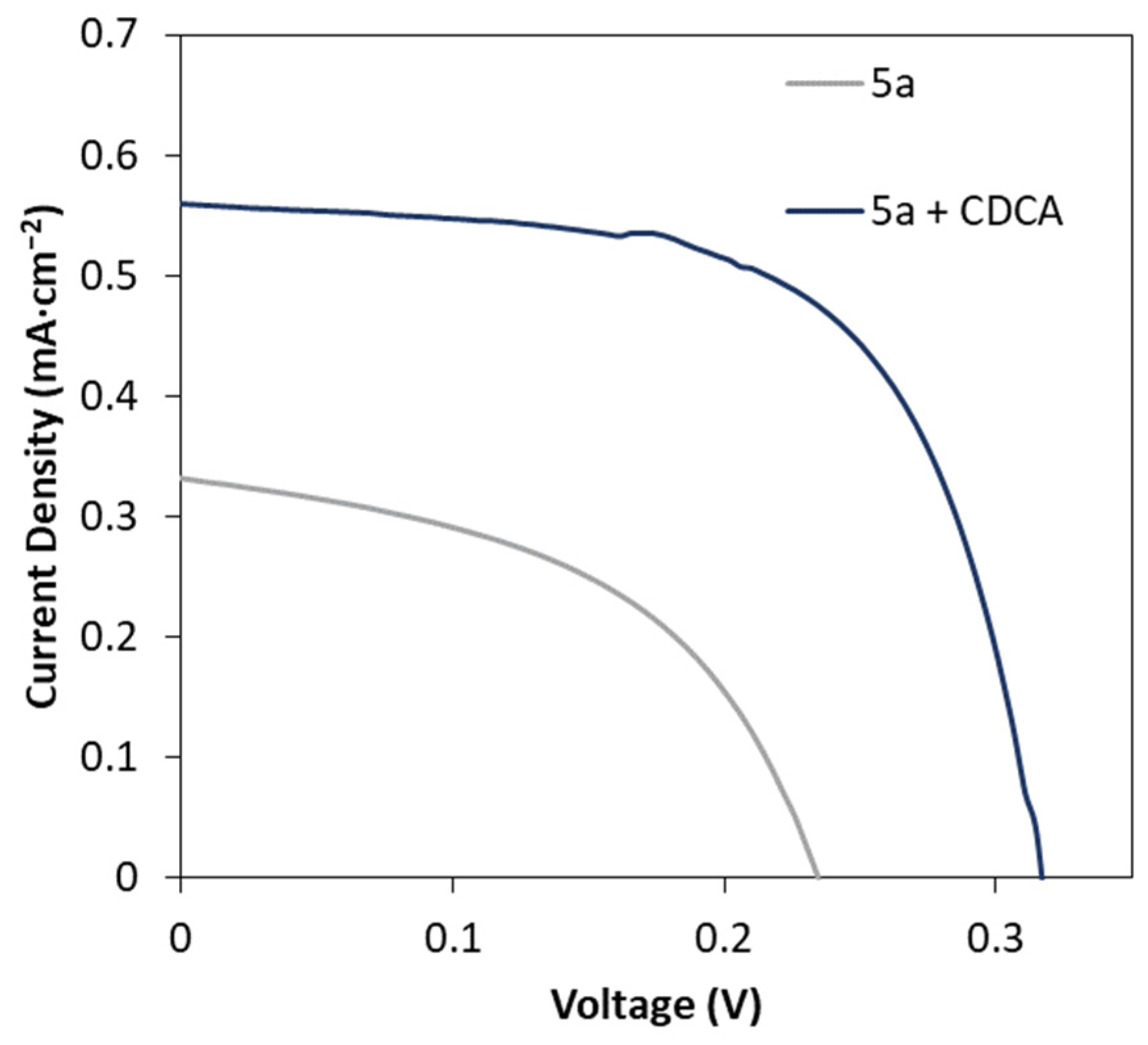
2.5. Effect of CDCA Addition on the DSSC Performance
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Synthesis and Characterization
3.3.1. Synthesis of 2a
3.3.2. Synthesis of 2b
3.3.3. Synthesis of 4
3.3.4. General Procedure for 5a,b and 6a,b
3.3.5. Synthesis of 5c
3.4. Differential Pulse Voltammetry (DPV)
3.5. DSSC Fabrication and Photovoltaic Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higashino, T.; Imahori, H. Porphyrins as Excellent Dyes for Dye-Sensitized Solar Cells: Recent Developments and Insights. Dalt. Trans. 2015, 44, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.-M.; Zhou, H.; Kim, H.K. Rational Design Criteria for D–π–A Structured Organic and Porphyrin Sensitizers for Highly Efficient Dye-Sensitized Solar Cells. J. Mater. Chem. A 2018, 6, 14518–14545. [Google Scholar] [CrossRef]
- Jin, Z.; Masuda, H.; Yamanaka, N.; Minami, M.; Nakamura, T.; Nishikitani, Y. Efficient Electron Transfer Ruthenium Sensitizers for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2009, 113, 2618–2623. [Google Scholar] [CrossRef]
- Jella, T.; Srikanth, M.; Bolligarla, R.; Soujanya, Y.; Singh, S.P.; Giribabu, L. Benzimidazole-Functionalized Ancillary Ligands for Heteroleptic Ru(II) Complexes: Synthesis, Characterization and Dye-Sensitized Solar Cell Applications. Dalt. Trans. 2015, 44, 14697–14706. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.-L.; Li, S.-S.; Peng, Z.-J.; Zhao, J.; Gong, B.-Q.; Guan, M.-Y.; Zheng, H.-G.; Xia, F.-F. Efficient Phenothiazine-Ruthenium Sensitizers with High Open-Circuit Voltage (Voc) for High Performance Dye-Sensitized Solar Cells. Dye. Pigment. 2020, 180, 108454. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W. Organic Sensitizers from D–π–A to D–A–π–A: Effect of the Internal Electron-Withdrawing Units on Molecular Absorption, Energy Levels and Photovoltaic Performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Wang, W.; Gurzadyan, G.G.; Li, J.; Li, X.; An, J.; Yu, Z.; Wang, H.; Cai, B.; et al. 13.6% Efficient Organic Dye-Sensitized Solar Cells by Minimizing Energy Losses of the Excited State. ACS Energy Lett. 2019, 4, 943–951. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, X.; Giordano, F.; Hu, Y.; Hua, J.; Zakeeruddin, S.M.; Tian, H.; Grätzel, M. Significance of π-Bridge Contribution in Pyrido[3,4-b]Pyrazine Featured D-A-π-A Organic Dyes for Dye-Sensitized Solar Cells. Mater. Chem. Front. 2017, 1, 181–189. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, S.; Shen, Z.; Mao, L.; Ding, Y.; Ren, D.; Eickemeyer, F.T.; Pfeifer, L.; Cao, D.; Xu, W.; et al. Carbazol-Phenyl-Phenothiazine-Based Sensitizers for Dye-Sensitized Solar Cells. J. Mater. Chem. A 2021, 9, 26311–26322. [Google Scholar] [CrossRef]
- Zeng, K.; Tong, Z.; Ma, L.; Zhu, W.H.; Wu, W.; Xie, Y.; Xie, Y. Molecular Engineering Strategies for Fabricating Efficient Porphyrin-Based Dye-Sensitized Solar Cells. Energy Environ. Sci. 2020, 13, 1617–1657. [Google Scholar] [CrossRef]
- Sekaran, B.; Misra, R. β-Pyrrole Functionalized Porphyrins: Synthesis, Electronic Properties, and Applications in Sensing and DSSC. Coord. Chem. Rev. 2022, 453, 214312. [Google Scholar] [CrossRef]
- Ramasamy, S.; Bhagavathiachari, M.; Suthanthiraraj, S.A.; Pichai, M. Mini Review on the Molecular Engineering of Photosensitizer: Current Status and Prospects of Metal-Free/Porphyrin Frameworks at the Interface of Dye-Sensitized Solar Cells. Dye. Pigment. 2022, 203, 110380. [Google Scholar] [CrossRef]
- Hu, Y.; Alsaleh, A.; Trinh, O.; D’Souza, F.; Wang, H. β-Functionalized Push–Pull opp-Dibenzoporphyrins as Sensitizers for Dye-Sensitized Solar Cells: The Push Group Effect. J. Mater. Chem. A 2021, 9, 27692–27700. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Nguyen, V.S.; Wei, T.; Yeh, C. Double Fence Porphyrins That Are Compatible with Cobalt(II/III) Electrolyte for High-Efficiency Dye-Sensitized Solar Cells. Angew. Chemie Int. Ed. 2021, 60, 4886–4893. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, B.; Chang, S.; Chen, T. Ruthenium-Based Photosensitizers for Dye-Sensitized Solar Cells. In Organometallics and Related Molecules for Energy Conversion; Green Chemistry and Sustainable Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 91–114. [Google Scholar] [CrossRef]
- Prima, E.C.; Nugroho, H.S.; Nugraha; Refantero, G.; Panatarani, C.; Yuliarto, B. Performance of the Dye-Sensitized Quasi-Solid State Solar Cell with Combined Anthocyanin-Ruthenium Photosensitizer. RSC Adv. 2020, 10, 36873–36886. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-Sensitized Solar Cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Song, H.; Liu, Q.; Xie, Y. Porphyrin-Sensitized Solar Cells: Systematic Molecular Optimization, Coadsorption and Cosensitization. Chem. Commun. 2018, 54, 1811–1824. [Google Scholar] [CrossRef]
- Di Carlo, G.; Biroli, A.O.; Tessore, F.; Caramori, S.; Pizzotti, M. β-Substituted Zn(II) Porphyrins as Dyes for DSSC: A Possible Approach to Photovoltaic Windows. Coord. Chem. Rev. 2018, 358, 153–177. [Google Scholar] [CrossRef]
- Bhuse, D.V.; Bhuse, V.M.; Bhagat, P.R. Ant-like Small Molecule Metal-Free Dimeric Porphyrin Sensitizer for True Energy-Generating DSSC with 9.3% Efficiency. J. Mater. Sci. Mater. Electron. 2022, 33, 14305–14322. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-Sensitized Solar Cells with 13% Efficiency Achieved through the Molecular Engineering of Porphyrin Sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Carella, A.; Borbone, F.; Centore, R. Research Progress on Photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Diau, E.W.G. Porphyrin-Sensitized Solar Cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Hombrecher, H.K.; Gherdan, V.M.; Ohm, S.; Cavaleiro, J.A.S.; da Graça, M.; Neves, P.M.S.; de Fátima Condesso, M. Synthesis and Electrochemical Investigation of β-Alkyloxy Substituted meso-Tetraphenylporphyrins. Tetrahedron 1993, 49, 8569–8578. [Google Scholar] [CrossRef]
- Costa, J.I.T.; Farinha, A.S.F.; Neves, M.G.P.M.S.; Tomé, A.C. An Easy Access to Porphyrin Triads and Their Supramolecular Interaction with a Pyridyl [60]Fulleropyrrolidine. Dye. Pigment. 2016, 135, 163–168. [Google Scholar] [CrossRef]
- Pereira, A.M.V.M.; Alonso, C.M.A.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.S.; Paz, F.A.A.; Cavaleiro, J.A.S. A New Synthetic Approach to N-Arylquinolino[2,3,4-at]Porphyrins from β-Arylaminoporphyrins. J. Org. Chem. 2008, 73, 7353–7356. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.A.D.F.; Moura, N.M.M.; Figueira, F.; Ferreira, R.I.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Silvestre, A.J.D.; Freire, C.S.R.; Tomé, J.P.C.; et al. New Materials Based on Cationic Porphyrins Conjugated to Chitosan or Titanium Dioxide: Synthesis, Characterization and Antimicrobial Efficacy. Int. J. Mol. Sci. 2019, 20, 2522. [Google Scholar] [CrossRef] [PubMed]
- Diskin-Posner, Y.; Patra, G.K.; Goldberg, I. Supramolecular Assembly of Metalloporphyrins in Crystals by Axial Coordination through Amine Ligands. J. Chem. Soc. Dalt. Trans. 2001, 2, 2775–2782. [Google Scholar] [CrossRef]
- Bravin, C.; Badetti, E.; Licini, G.; Zonta, C. Tris(2-Pyridylmethyl)Amines as Emerging Scaffold in Supramolecular Chemistry. Coord. Chem. Rev. 2021, 427, 213558. [Google Scholar] [CrossRef]
- Arnaut, L.G. Design of Porphyrin-Based Photosensitizers for Photodynamic Therapy. Adv. Inorg. Chem. 2011, 63, 187–233. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.S.M.; Castro, M.C.R.; Pereira, A.I.; Mendes, A.; Serpa, C.; Pina, J.; Justino, L.L.G.; Burrows, H.D.; Raposo, M.M.M. Optical and Photovoltaic Properties of Thieno[3,2-b]thiophene-Based Push-Pull Organic Dyes with Different Anchoring Groups for Dye-Sensitized Solar Cells. ACS Omega 2017, 2, 9268–9279. [Google Scholar] [CrossRef]
- Hart, A.S.; KC, C.B.; Gobeze, H.B.; Sequeira, L.R.; D’Souza, F. Porphyrin-Sensitized Solar Cells: Effect of Carboxyl Anchor Group Orientation on the Cell Performance. ACS Appl. Mater. Interfaces 2013, 5, 5314–5323. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, R.; Martín-Gomis, L.; Fernández-Lázaro, F.; Zakavi, S.; Sastre-Santos, Á. Effect of the Number of Anchoring and Electron-Donating Groups on the Efficiency of Free-Base- and Zn-Porphyrin-Sensitized Solar Cells. Materials 2019, 12, 650. [Google Scholar] [CrossRef]
- Zou, J.; Tang, Y.; Baryshnikov, G.; Yang, Z.; Mao, R.; Feng, W.; Guan, J.; Li, C.; Xie, Y. Porphyrins Containing a Tetraphenylethylene-Substituted Phenothiazine Donor for Fabricating Efficient Dye Sensitized Solar Cells with High Photovoltages. J. Mater. Chem. A 2022, 10, 1320–1328. [Google Scholar] [CrossRef]
- Kurumisawa, Y.; Higashino, T.; Nimura, S.; Tsuji, Y.; Iiyama, H.; Imahori, H. Renaissance of Fused Porphyrins: Substituted Methylene-Bridged Thiophene-Fused Strategy for High-Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2019, 141, 9910–9919. [Google Scholar] [CrossRef]
- Xie, M.; Liu, J.; Dai, L.; Peng, H.; Xie, Y. Advances and Prospects of Porphyrin Derivatives in the Energy Field. RSC Adv. 2023, 13, 24699–24730. [Google Scholar] [CrossRef]
- Zhang, Y.; Higashino, T.; Imahori, H. Molecular Designs, Synthetic Strategies, and Properties for Porphyrins as Sensitizers in Dye-Sensitized Solar Cells. J. Mater. Chem. A 2023, 11, 12659–12680. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals, 7th ed.; Elsevier: Oxford, UK, 2013. [Google Scholar]

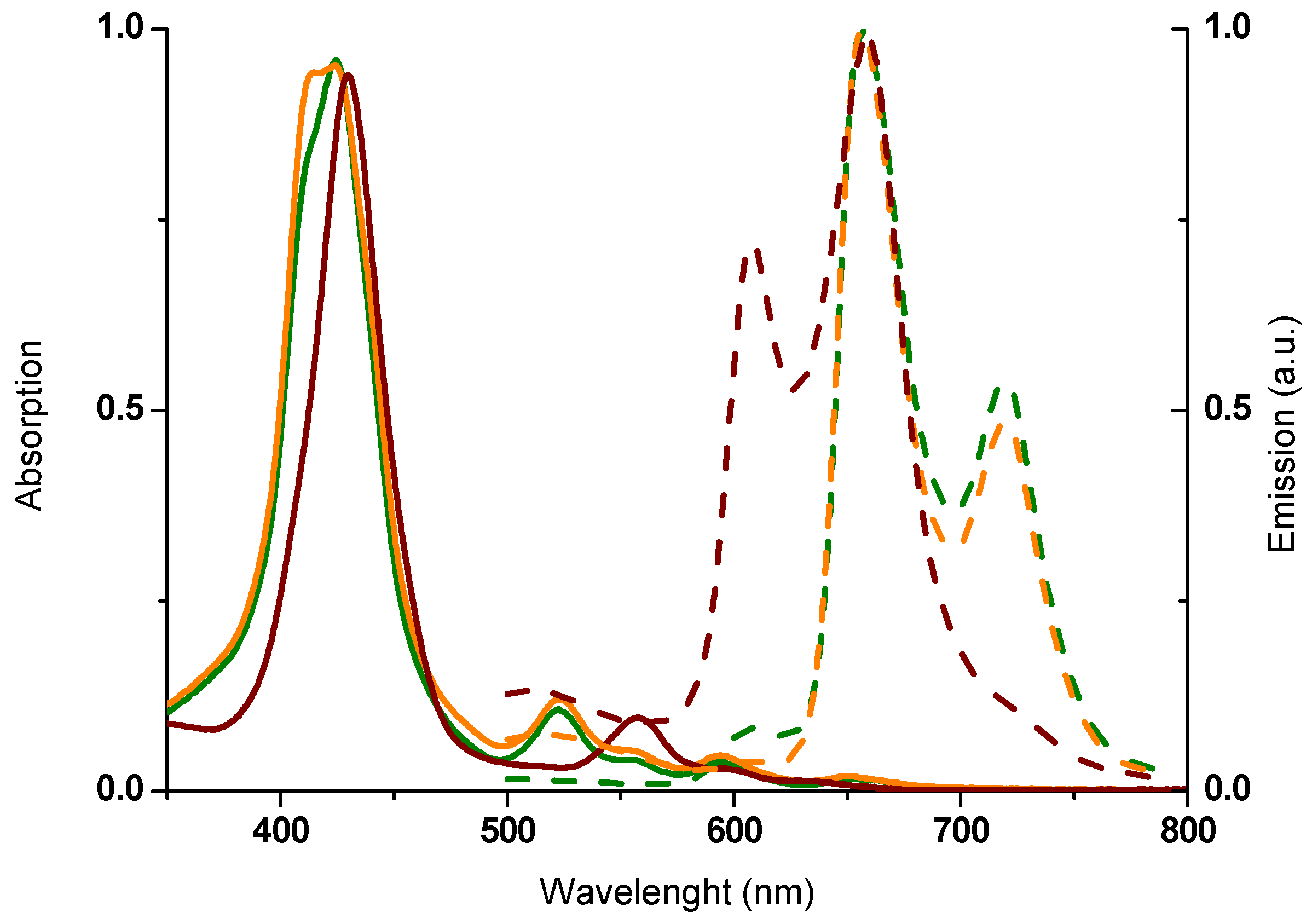
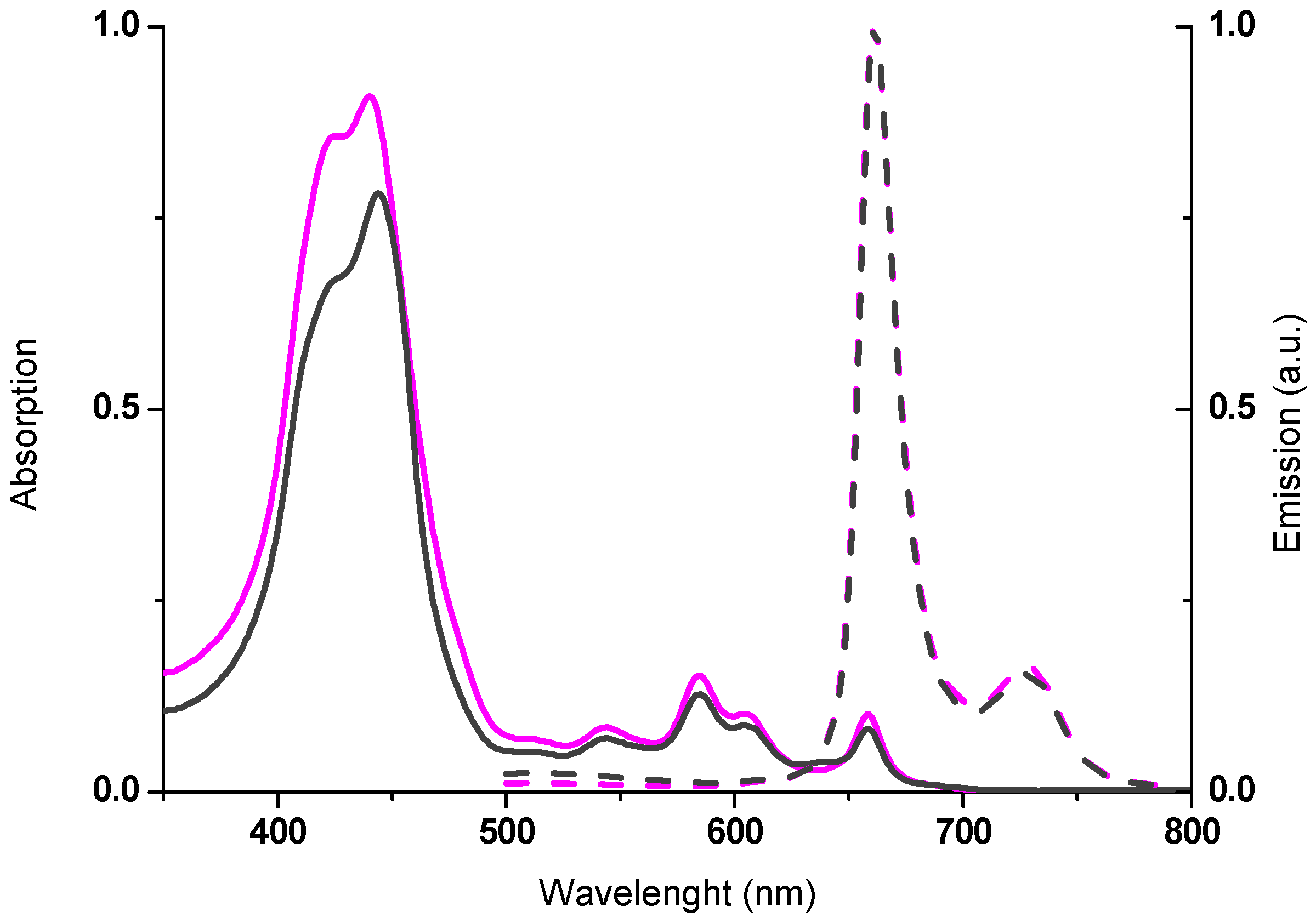

| Compounds | λmax,abs (nm) a | log ε (M−1 cm−1) | λmax,em (nm) b | Stokes Shift (cm−1) c |
|---|---|---|---|---|
| 5a | 424 | 5.26 | 655 719 | 94 |
| 523 | 4.31 | |||
| 554 | 3.89 | |||
| 594 | 3.86 | |||
| 651 | 3.48 | |||
| 5b | 415 | 5.03 | 657 | 164 |
| 424 | 5.03719 | |||
| 522 | 4.13 | |||
| 550 | 3.79 | |||
| 594 | 3.72 | |||
| 650 | 3.35 | |||
| 5c | 430 | 4.74 | 608 | — |
| 558 | 3.73 | |||
| 592 sh | 3.22 | 658 | ||
| 629 sh | 2.87 | |||
| 6a | 425 | 4.46 | 661 | 69 |
| 440 | 4.48 | |||
| 544 | 3.52 | |||
| 584 | 3.74 | 727 | ||
| 605 | 3.59 | |||
| 658 | 3.59 | |||
| 6b | 424 | 4.43 | 661 | 69 |
| 444 | 4.50 | |||
| 544 | 3.46 | |||
| 585 | 3.71 | 728 | ||
| 605 | 3.54 | |||
| 658 | 3.52 |
| Dye | Eox SCE (V) | HOMO vs. Vacuum (eV) | Ered SCE (V) | LUMO vs. Vacuum (eV) | Band Gap (eV) |
|---|---|---|---|---|---|
| 5a | 0.819 | −5.26 | −1.063 | −3.38 | 1.88 |
| 5b | 0.761 | −5.20 | −1.151 | −3.29 | 1.91 |
| 5c | 0.649 | −5.09 | −1.210 | −3.23 | 1.86 |
| 6a | 0.638 | −5.08 | −1.068 | −3.37 | 1.71 |
| 6b | 0.677 | −5.12 | −0.995 | −3.45 | 1.67 |
| Compound | Voc (mV) | Jsc (mA cm−2) | FF | PCE (%) |
|---|---|---|---|---|
| 5a | 213 ± 4 | 0.30 ± 0.02 | 0.41 ± 0.08 | 0.03 ± 0.01 |
| 5b | 206 ± 25 | 0.23 ± 0.03 | 0.43 ± 0.12 | 0.02 ± 0.01 |
| 5c | 361 ± 7 | 1.8 ± 0.1 | 0.56 ± 0.06 | 0.37 ± 0.06 |
| 6a | 185 ± 5 | 0.17 ± 0.03 | 0.42 ± 0.03 | 0.013 ± 0.003 |
| 6b | 280 ± 8 | 0.35 ± 0.01 | 0.49 ± 0.05 | 0.049 ± 0.003 |
| N719 | 427 ± 12 | 15.3 ± 0.5 | 0.57 ± 0.01 | 3.70 ± 0.09 |
| Compound | Voc (mV) | Jsc (mA cm−2) | FF | PCE (%) |
|---|---|---|---|---|
| 5a | 213 ± 4 | 0.30 ± 0.02 | 0.41 ± 0.08 | 0.03 ± 0.01 |
| 5a+ CDCA | 329 ± 7 | 0.53 ± 0.02 | 0.63 ± 0.01 | 0.11 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerqueira, A.F.R.; Pinto, A.L.; Malta, G.; Neves, M.G.P.M.S.; Parola, A.J.; Tomé, A.C. Synthesis and Photovoltaic Performance of β-Amino-Substituted Porphyrin Derivatives. Int. J. Mol. Sci. 2024, 25, 5979. https://doi.org/10.3390/ijms25115979
Cerqueira AFR, Pinto AL, Malta G, Neves MGPMS, Parola AJ, Tomé AC. Synthesis and Photovoltaic Performance of β-Amino-Substituted Porphyrin Derivatives. International Journal of Molecular Sciences. 2024; 25(11):5979. https://doi.org/10.3390/ijms25115979
Chicago/Turabian StyleCerqueira, Ana F. R., Ana Lucia Pinto, Gabriela Malta, Maria G. P. M. S. Neves, A. Jorge Parola, and Augusto C. Tomé. 2024. "Synthesis and Photovoltaic Performance of β-Amino-Substituted Porphyrin Derivatives" International Journal of Molecular Sciences 25, no. 11: 5979. https://doi.org/10.3390/ijms25115979
APA StyleCerqueira, A. F. R., Pinto, A. L., Malta, G., Neves, M. G. P. M. S., Parola, A. J., & Tomé, A. C. (2024). Synthesis and Photovoltaic Performance of β-Amino-Substituted Porphyrin Derivatives. International Journal of Molecular Sciences, 25(11), 5979. https://doi.org/10.3390/ijms25115979









