Ageing and Polypharmacy in Mesenchymal Stromal Cells: Metabolic Impact Assessed by Hyperspectral Imaging of Autofluorescence
Abstract
1. Introduction
2. Results
2.1. Cells from Young and Old Control Mice
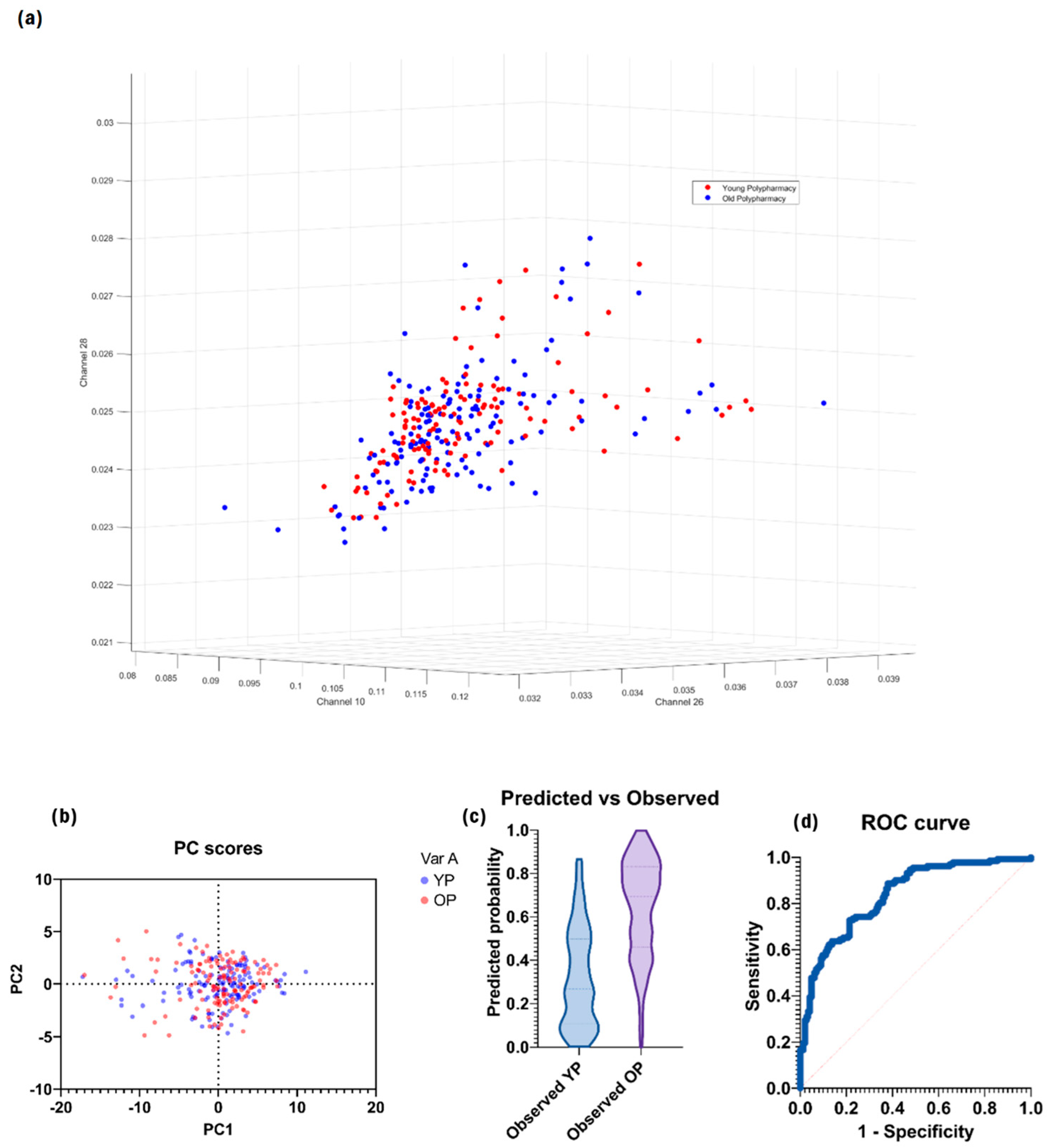
2.2. Cells from Young Polypharmacy-Treated and Old Polypharmacy-Treated Mice
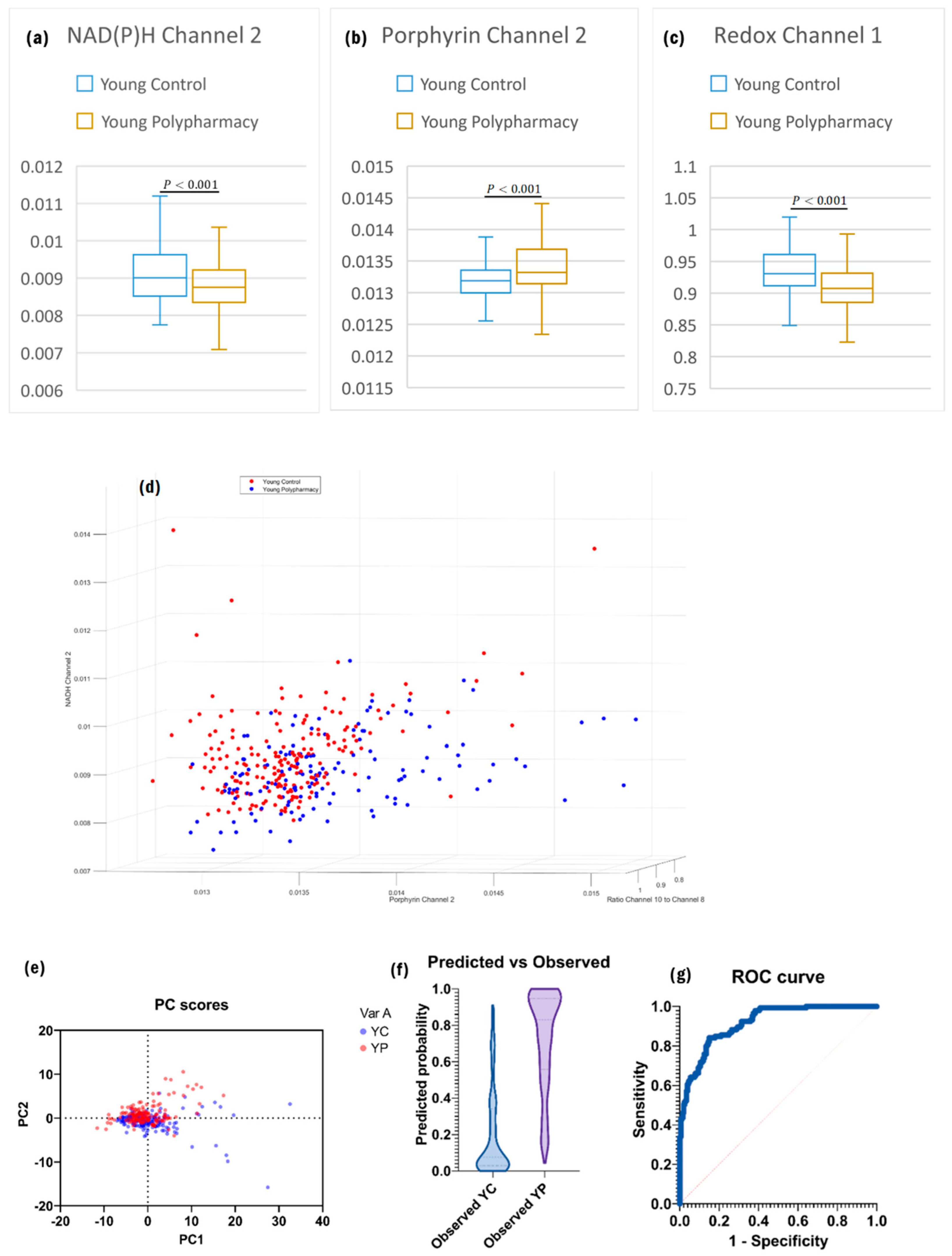
2.3. Cells from Young Control and Young Polypharmacy-Treated Mice
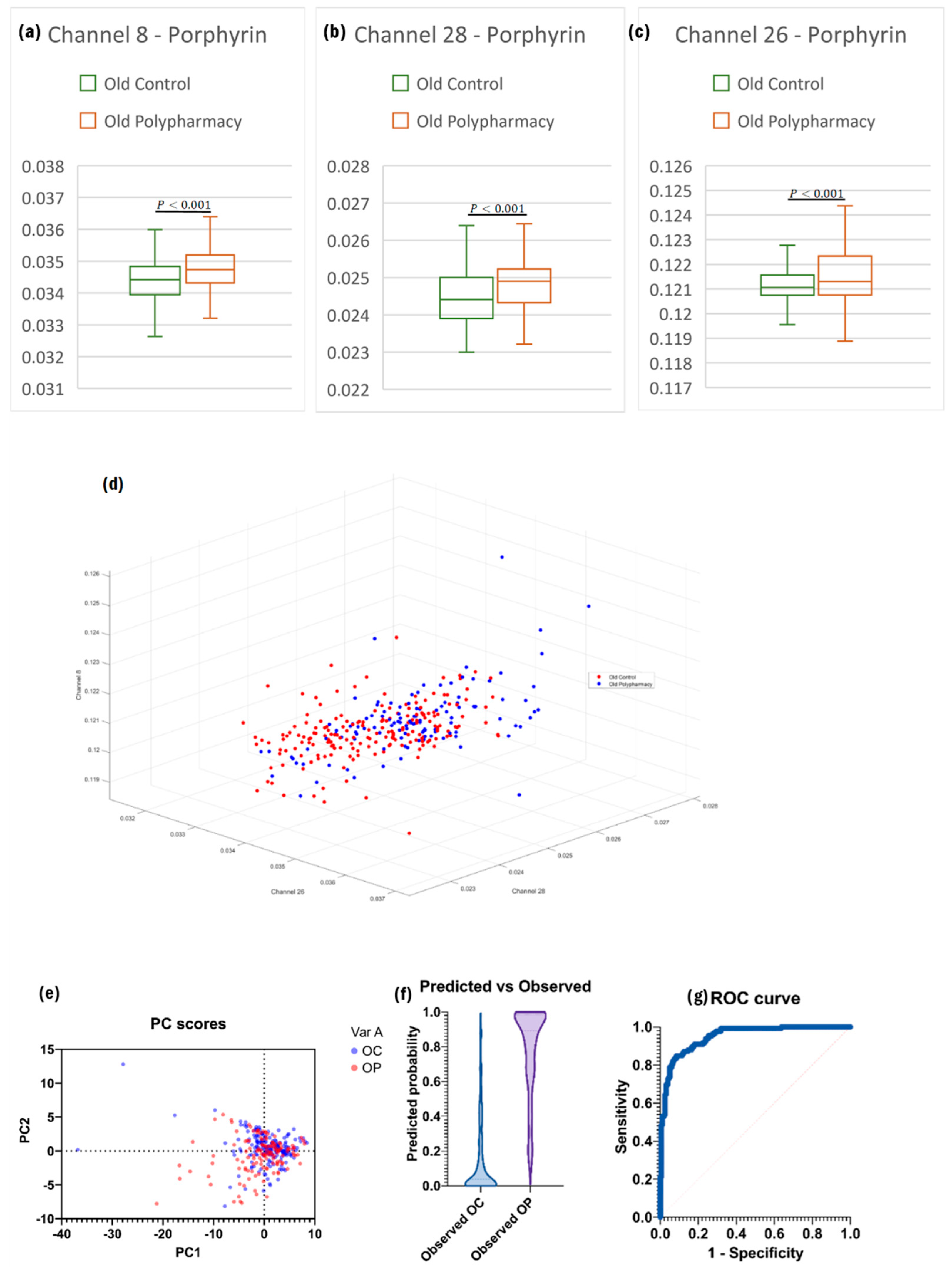
2.4. Cells from Old Control and Old Polypharmacy-Treated Mice
2.5. Cells from Young Polypharmacy-Treated and Old Control Mice
2.6. Cells from Young Control and Old Polypharmacy-Treated Mice
3. Discussion
Conclusions
4. Materials and Methods
4.1. Animal Care and Cell Culture
4.2. Cell Groups and Study Design
4.3. Hyperspectral Imaging
4.4. Image Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajjar, E.R.; Cafiero, A.C.; Hanlon, J.T. Polypharmacy in elderly patients. Am. J. Geriatr. Pharmacother. 2007, 5, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Page, A.T.; Falster, M.O.; Litchfield, M.; Pearson, S.A.; Etherton-Beer, C. Polypharmacy among older Australians, 2006–2017: A population-based study. Med. J. Aust. 2019, 211, 71–75. [Google Scholar] [CrossRef]
- Gnjidic, D.; Hilmer, S.N.; Blyth, F.M.; Naganathan, V.; Cumming, R.G.; Handelsman, D.J.; McLachlan, A.J.; Abernethy, D.R.; Banks, E.; Le Couteur, D.G. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin. Pharmacol. Ther. 2012, 91, 521–528. [Google Scholar] [CrossRef]
- Hilmer, S.N.; Mager, D.E.; Simonsick, E.M.; Ling, S.M.; Windham, B.G.; Harris, T.B.; Shorr, R.I.; Bauer, D.C.; Abernethy, D.R.; Health, A.B.C.S. Drug burden index score and functional decline in older people. Am. J. Med. 2009, 122, 1142–1149.e2. [Google Scholar] [CrossRef]
- Hilmer, S.N.; Gnjidic, D. The effects of polypharmacy in older adults. Clin. Pharmacol. Ther. 2009, 85, 86–88. [Google Scholar] [CrossRef]
- Hilmer, S.N.; Mager, D.E.; Simonsick, E.M.; Cao, Y.; Ling, S.M.; Windham, B.G.; Harris, T.B.; Hanlon, J.T.; Rubin, S.M.; Shorr, R.I.; et al. A drug burden index to define the functional burden of medications in older people. Arch. Intern. Med. 2007, 167, 781–787. [Google Scholar] [CrossRef]
- Gemikonakli, G.; Mach, J.; Tran, T.; Wu, H.; Hilmer, S.N. Probing polypharmacy, ageing and sex effects on physical function using different tests. Fundam. Clin. Pharmacol. 2024, 38, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Mach, J.; Gnjidic, D.; Naganathan, V.; Blyth, F.M.; Waite, L.M.; Handelsman, D.J.; Le Couteur, D.G.; Hilmer, S.N. Comparing Effects of Polypharmacy on Inflammatory Profiles in Older Adults and Mice: Implications for Translational Aging Research. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1295–1303. [Google Scholar] [CrossRef]
- Mach, J.; Gemikonakli, G.; Logan, C.; Vander Wyk, B.; Allore, H.; Ekambareshwar, S.; Kane, A.E.; Howlett, S.E.; de Cabo, R.; Le Couteur, D.G.; et al. Chronic Polypharmacy with Increasing Drug Burden Index Exacerbates Frailty and Impairs Physical Function, with Effects Attenuated by Deprescribing, in Aged Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1010–1018. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Liang, H.; Tang, D.; Huang, M.; Zhao, J.; Yang, X.; Liu, Y.; Shu, L.; Wang, J.; et al. Efficacy of mesenchymal stem cell transplantation therapy for type 1 and type 2 diabetes mellitus: A meta-analysis. Stem Cell Res. Ther. 2021, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Mabotuwana, N.S.; Rech, L.; Lim, J.; Hardy, S.A.; Murtha, L.A.; Rainer, P.P.; Boyle, A.J. Paracrine Factors Released by Stem Cells of Mesenchymal Origin and their Effects in Cardiovascular Disease: A Systematic Review of Pre-clinical Studies. Stem Cell Rev. Rep. 2022, 18, 2606–2628. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yu, G.; Yang, K.; Xiang, W.; Li, J.; Chen, H. Efficacy and Safety of Mesenchymal Stem Cell Transplantation in the Treatment of Autoimmune Diseases (Rheumatoid Arthritis, Systemic Lupus Erythematosus, Inflammatory Bowel Disease, Multiple Sclerosis, and Ankylosing Spondylitis): A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Stem Cells Int. 2022, 2022, 9463314. [Google Scholar] [PubMed]
- Campbell, J.M.; Mahbub, S.; Habibalahi, A.; Paton, S.; Gronthos, S.; Goldys, E. Ageing human bone marrow mesenchymal stem cells have depleted NAD(P)H and distinct multispectral autofluorescence. Geroscience 2021, 43, 859–868. [Google Scholar] [CrossRef]
- Al-Azab, M.; Safi, M.; Idiiatullina, E.; Al-Shaebi, F.; Zaky, M.Y. Aging of mesenchymal stem cell: Machinery, markers, and strategies of fighting. Cell Mol. Biol. Lett. 2022, 27, 69. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Mach, J.; Gemikonakli, G.; Tran, T.; Allore, H.; Gnjidic, D.; Howlett, S.E.; de Cabo, R.; Le Couteur, D.G.; Hilmer, S.N. Polypharmacy Results in Functional Impairment in Mice: Novel Insights Into Age and Sex Interactions. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Paulose-Ram, R.; Burt, V.L.; Kit, B.K. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. NCHS Data Brief 2014, 177, 1–8. [Google Scholar]
- Rüdesheim, S.; Wojtyniak, J.G.; Selzer, D.; Hanke, N.; Mahfoud, F.; Schwab, M.; Lehr, T. Physiologically Based Pharmacokinetic Modeling of Metoprolol Enantiomers and α-Hydroxymetoprolol to Describe CYP2D6 Drug-Gene Interactions. Pharmaceutics 2020, 12, 1200. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Perry, C.M.; Scott, L.J. Oxybutynin extended-release: A review of its use in the management of overactive bladder. Drugs 2004, 64, 885–912. [Google Scholar] [CrossRef]
- Berterame, S.; Erthal, J.; Thomas, J.; Fellner, S.; Vosse, B.; Clare, P.; Hao, W.; Johnson, D.T.; Mohar, A.; Pavadia, J.; et al. Use of and barriers to access to opioid analgesics: A worldwide, regional, and national study. Lancet 2016, 387, 1644–1656. [Google Scholar] [CrossRef]
- Lalji, H.M.; McGrogan, A.; Bailey, S.J. An analysis of antidepressant prescribing trends in England 2015–2019. J. Affect. Disord. Rep. 2021, 6, 100205. [Google Scholar] [CrossRef]
- Campbell, J.M.; Walters, S.N.; Habibalahi, A.; Mahbub, S.B.; Anwer, A.G.; Handley, S.; Grey, S.T.; Goldys, E.M. Pancreatic Islet Viability Assessment Using Hyperspectral Imaging of Autofluorescence. Cells 2023, 12, 2302. [Google Scholar] [CrossRef]
- Croce, A.C.; Ferrigno, A.; Bottiroli, G.; Vairetti, M. Autofluorescence-based optical biopsy: An effective diagnostic tool in hepatology. Liver Int. 2018, 38, 1160–1174. [Google Scholar] [CrossRef]
- Campbell, J.M.; Habibalahi, A.; Mahbub, S.; Gosnell, M.; Anwer, A.G.; Paton, S.; Gronthos, S.; Goldys, E. Non-destructive, label free identification of cell cycle phase in cancer cells by multispectral microscopy of autofluorescence. BMC Cancer 2019, 19, 1242. [Google Scholar] [CrossRef]
- Campbell, J.M.; Mahbub, S.B.; Bertoldo, M.J.; Habibalahi, A.; Goss, D.M.; Ledger, W.L.; Gilchrist, R.B.; Wu, L.E.; Goldys, E.M. Multispectral autofluorescence characteristics of reproductive aging in old and young mouse oocytes. Biogerontology 2022, 23, 237–249. [Google Scholar] [CrossRef]
- Habibalahi, A.; Moghari, M.D.; Campbell, J.M.; Anwer, A.G.; Mahbub, S.B.; Gosnell, M.; Saad, S.; Pollock, C.; Goldys, E.M. Non-invasive real-time imaging of reactive oxygen species (ROS) using auto-fluorescence multispectral imaging technique: A novel tool for redox biology. Redox Biol. 2020, 34, 101561. [Google Scholar] [CrossRef]
- Tan, T.C.Y.; Mahbub, S.B.; Campbell, J.M.; Habibalahi, A.; Campugan, C.A.; Rose, R.D.; Chow, D.J.X.; Mustafa, S.; Goldys, E.M.; Dunning, K.R. Non-invasive, label-free optical analysis to detect aneuploidy within the inner cell mass of the preimplantation embryo. Hum. Reprod. 2021, 37, 14–29. [Google Scholar] [CrossRef]
- Khezrian, M.; McNeil, C.J.; Myint, P.K.; Murray, A.D. The association between polypharmacy and late life deficits in cognitive, physical and emotional capability: A cohort study. Int. J. Clin. Pharm. 2019, 41, 251–257. [Google Scholar] [CrossRef]
- Huizer-Pajkos, A.; Kane, A.E.; Howlett, S.E.; Mach, J.; Mitchell, S.J.; de Cabo, R.; Le Couteur, D.G.; Hilmer, S.N. Adverse Geriatric Outcomes Secondary to Polypharmacy in a Mouse Model: The Influence of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 571–577. [Google Scholar] [CrossRef]
- Tahoun, M.; Gee, C.T.; McCoy, V.E.; Sander, P.M.; Muller, C.E. Chemistry of porphyrins in fossil plants and animals. RSC Adv. 2021, 11, 7552–7563. [Google Scholar] [CrossRef]
- Udartseva, O.O.; Andreeva, E.R.; Buravkova, L.B. Accumulation and elimination of photosens and protoporphyrin IX by different types of mesenchymal cells. Bull. Exp. Biol. Med. 2013, 155, 568–571. [Google Scholar] [CrossRef]
- Kao, C.C.; Yang, Z.Y.; Chen, W.L. Association between protoporphyrin IX and sarcopenia: A cross sectional study. BMC Geriatr. 2021, 21, 384. [Google Scholar] [CrossRef]
- Adavallan, K.; Gurushankar, K.; Nazeer, S.S.; Gohulkumar, M.; Jayasree, R.S.; Krishnakumar, N. Optical redox ratio using endogenous fluorescence to assess the metabolic changes associated with treatment response of bioconjugated gold nanoparticles in streptozotocin-induced diabetic rats. Laser Phys. Lett. 2017, 14, 065901. [Google Scholar] [CrossRef]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The Good, the Bad, and the Ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267–275. [Google Scholar] [CrossRef]
- Saheera, S.; Potnuri, A.G.; Nair, R.R. Modulation of cardiac stem cell characteristics by metoprolol in hypertensive heart disease. Hypertens. Res. 2018, 41, 253–262. [Google Scholar] [CrossRef]
- Verdi, J.; Mortazavi-Tabatabaei, S.A.; Sharif, S.; Verdi, H.; Shoae-Hassani, A. Citalopram increases the differentiation efficacy of bone marrow mesenchymal stem cells into neuronal-like cells. Neural Regen. Res. 2014, 9, 845–850. [Google Scholar]
- Nantavisai, S.; Rodprasert, W.; Pathanachai, K.; Wikran, P.; Kitcharoenthaworn, P.; Smithiwong, S.; Archasappawat, S.; Sawangmak, C. Simvastatin enhances proliferation and pluripotent gene expression by canine bone marrow-derived mesenchymal stem cells (cBM-MSCs) in vitro. Heliyon 2019, 5, e02663. [Google Scholar] [CrossRef]
- Zanette, D.L.; Lorenzi, J.C.; Panepucci, R.A.; Palma, P.V.; Dos Santos, D.F.; Prata, K.L.; Silva, W.A., Jr. Simvastatin modulates mesenchymal stromal cell proliferation and gene expression. PLoS ONE 2015, 10, e0120137. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: Implications for further clinical uses. Cell Mol. Life Sci. 2021, 78, 447–467. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef]
- Quek, H.; Page, A.; Potter, K.; Etherton-Beer, C. Deprescribing considerations for older people in general practice. Aust. J. General. Pract. 2023, 52, 173–180. [Google Scholar] [CrossRef]
- Mawson, S.; Keen, C.; Skilbeck, J.; Ross, H.; Smith, L.; Dixey, J.; Walters, S.J.; Simpson, R.; Greenfield, D.M.; Snowden, J.A. Feasibility and benefits of a structured prehabilitation programme prior to autologous stem cell transplantation (ASCT) in patients with myeloma; a prospective feasibility study. Physiotherapy 2021, 113, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.; Feyer, J.; Limbach, M.; Pahl, A.; Stoelzel, F.; Beck, H.; Wegner, A.; Rosenberger, F.; Dreger, P.; Luft, T.; et al. Prehabilitative high-intensity interval training and resistance exercise in patients prior allogeneic stem cell transplantation. Sci. Rep. 2023, 13, 22069. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Yang, F.; Ning, G.; Zhen, L.; Wu, L.; Zheng, Y.; Zhang, Q.; Lin, D.; Xie, C.; et al. Nicotinamide mononucleotide promotes osteogenesis and reduces adipogenesis by regulating mesenchymal stromal cells via the SIRT1 pathway in aged bone marrow. Cell Death Dis. 2019, 10, 336. [Google Scholar] [CrossRef]
- Huang, S.; Xu, L.; Sun, Y.; Wu, T.; Wang, K.; Li, G. An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. J. Orthop. Transl. 2015, 3, 26–33. [Google Scholar] [CrossRef]
- Wagnieres, G.A.; Star, W.M.; Wilson, B.C. In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem. Photobiol. 1998, 68, 603–632. [Google Scholar] [CrossRef]
- Mahbub, S.B.; Ploschner, M.; Gosnell, M.E.; Anwer, A.G.; Goldys, E.M. Statistically strong label-free quantitative identification of native fluorophores in a biological sample. Sci. Rep. 2017, 7, 15792. [Google Scholar] [CrossRef]



| Pairing of Cell Groups | Information from Comparison |
|---|---|
| Young Control vs. Old Control | Identify changes in autofluorescence based on age and compare this to previous studies on the effects of ageing on MSC autofluorescence to ensure results are consistent. |
| Young Polypharmacy vs. Old Polypharmacy | Controlling for polypharmacy effects, identify changes in autofluorescence based on age. |
| Young Control vs. Young Polypharmacy | Identify differential changes in autofluorescence based on the effects of polypharmacy in young age. |
| Old Control vs. Old Polypharmacy | Identify differential changes in autofluorescence based on the effects of polypharmacy in old age. |
| Young Polypharmacy vs. Old Control | Compare the impacts of polypharmacy in young age and old age without polypharmacy. |
| Young Control vs. Old Polypharmacy | Compare the effects of young age without polypharmacy and polypharmacy in old age on MSCs. |
| Channel | Peak Excitation (nm) | Peak Emission (nm) | Fluorophore |
|---|---|---|---|
| 1 | 345 | 414 | NADH/Elastin |
| 2 | 345 | 451 | NADH |
| 3 | 345 | 575 | Flavin/Lipo-Pigments |
| 4 | 490 | 575 | Flavin |
| 5 | 505 | 575 | Flavin |
| 6 | 490 | 594 | Flavin |
| 7 | 505 | 594 | Flavin |
| 8 | 490 | 675 | Porphyrin |
| 9 | 505 | 675 | Porphyrin |
| 10 | 358 | 414 | NADH/Elastin |
| 11 | 371 | 414 | NADH/Elastin |
| 12 | 358 | 451 | NADH/Elastin |
| 13 | 377 | 451 | NADH/Elastin |
| 14 | 358 | 575 | Flavin |
| 15 | 381 | 575 | Flavin |
| 16 | 403 | 575 | Flavin |
| 17 | 430 | 575 | Flavin |
| 18 | 457 | 575 | Flavin |
| 19 | 358 | 594 | Flavin/Lipo-pigments |
| 20 | 377 | 594 | Flavin/Lipo-pigments |
| 21 | 381 | 594 | Flavin |
| 22 | 391 | 594 | Flavin |
| 23 | 400 | 594 | Flavin |
| 24 | 408 | 594 | Flavin |
| 25 | 437 | 594 | Flavin |
| 26 | 358 | 675 | Porphyrin |
| 27 | 381 | 675 | Porphyrin |
| 28 | 391 | 675 | Porphyrin |
| 29 | 437 | 675 | Porphyrin |
| 30 | 391 | 647 | Flavin/Porphyrin |
| 31 | 437 | 647 | Flavin/Porphyrin |
| 32 | 476 | 575 | Brightfield Image |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekara, C.M.N.; Gemikonakli, G.; Mach, J.; Sang, R.; Anwer, A.G.; Agha, A.; Goldys, E.M.; Hilmer, S.N.; Campbell, J.M. Ageing and Polypharmacy in Mesenchymal Stromal Cells: Metabolic Impact Assessed by Hyperspectral Imaging of Autofluorescence. Int. J. Mol. Sci. 2024, 25, 5830. https://doi.org/10.3390/ijms25115830
Chandrasekara CMN, Gemikonakli G, Mach J, Sang R, Anwer AG, Agha A, Goldys EM, Hilmer SN, Campbell JM. Ageing and Polypharmacy in Mesenchymal Stromal Cells: Metabolic Impact Assessed by Hyperspectral Imaging of Autofluorescence. International Journal of Molecular Sciences. 2024; 25(11):5830. https://doi.org/10.3390/ijms25115830
Chicago/Turabian StyleChandrasekara, Chandrasekara M. N., Gizem Gemikonakli, John Mach, Rui Sang, Ayad G. Anwer, Adnan Agha, Ewa M. Goldys, Sarah N. Hilmer, and Jared M. Campbell. 2024. "Ageing and Polypharmacy in Mesenchymal Stromal Cells: Metabolic Impact Assessed by Hyperspectral Imaging of Autofluorescence" International Journal of Molecular Sciences 25, no. 11: 5830. https://doi.org/10.3390/ijms25115830
APA StyleChandrasekara, C. M. N., Gemikonakli, G., Mach, J., Sang, R., Anwer, A. G., Agha, A., Goldys, E. M., Hilmer, S. N., & Campbell, J. M. (2024). Ageing and Polypharmacy in Mesenchymal Stromal Cells: Metabolic Impact Assessed by Hyperspectral Imaging of Autofluorescence. International Journal of Molecular Sciences, 25(11), 5830. https://doi.org/10.3390/ijms25115830






