Pharmacological Inhibition of MMP-12 Exerts Protective Effects on Angiotensin II-Induced Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice
Abstract
1. Introduction
2. Results
2.1. MMP-12 Is Elevated in Pro-Inflammatory Macrophages and Human AAAs, and Reduced MMP-12 Expression/Activity Retards Medial Fragmentation
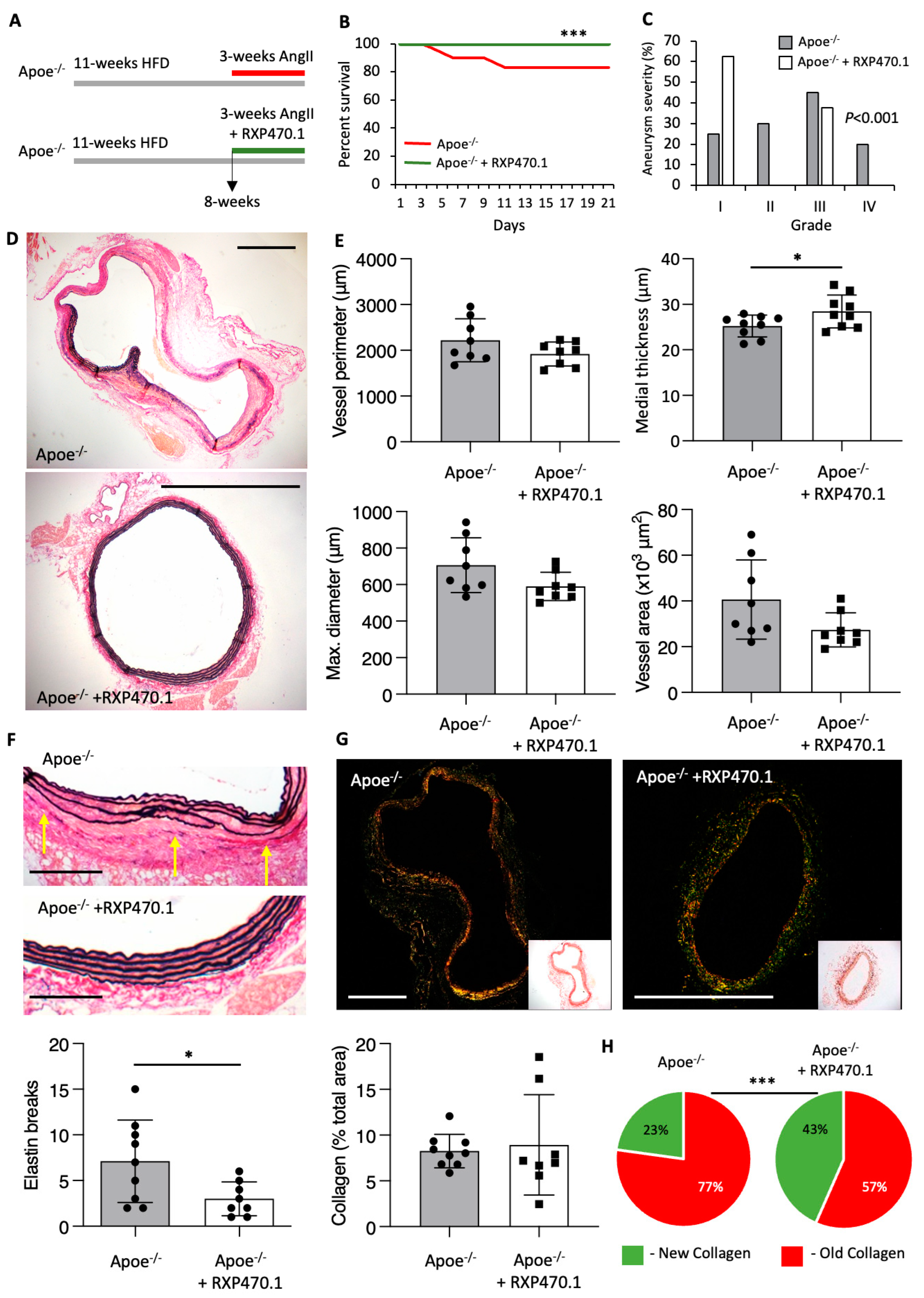
2.2. MMP-12 Inhibition Exerts Protective Effects on AAA Development
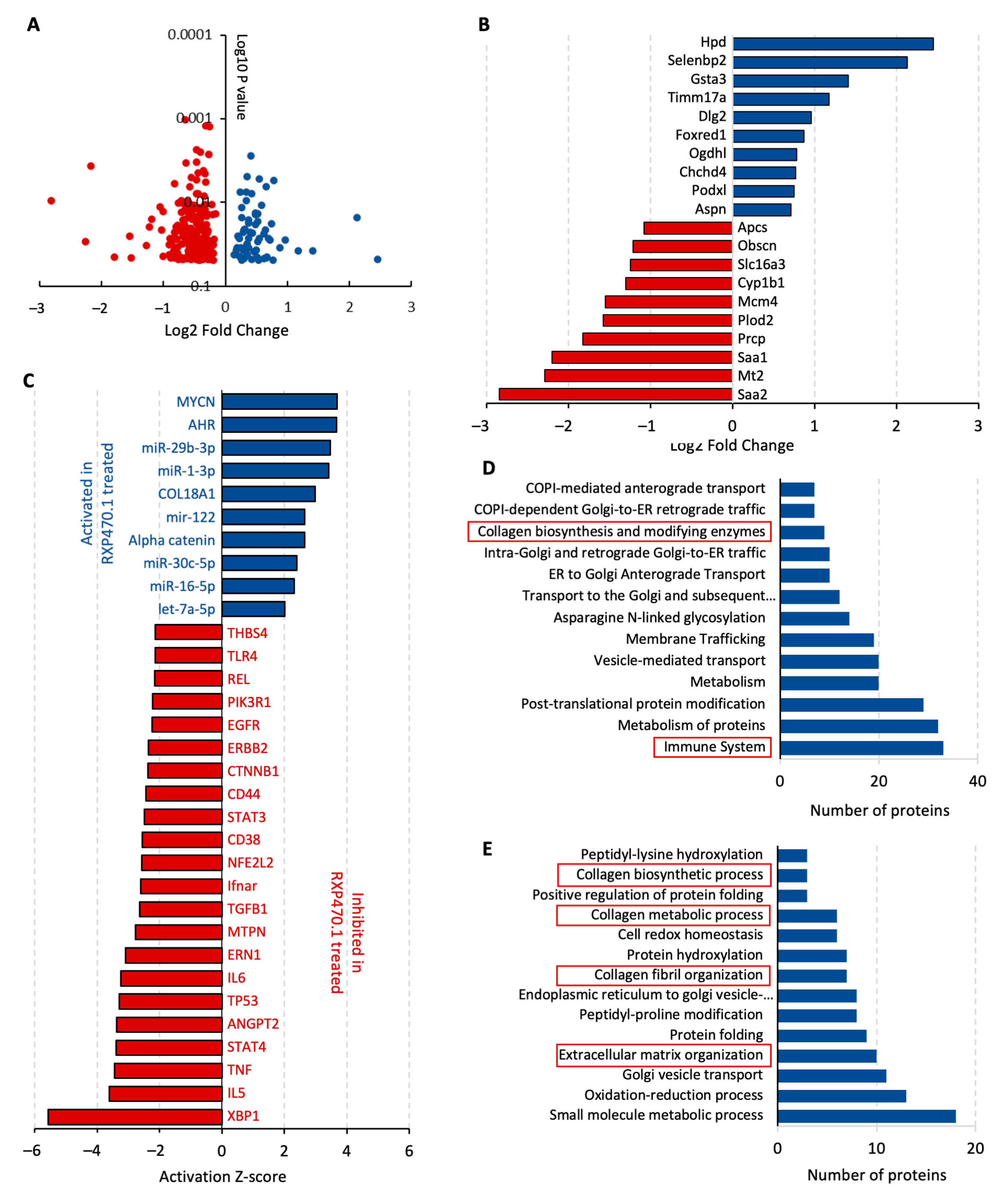
2.3. MMP-12 Inhibition Regulates ECM Remodeling Proteins and Inflammatory Pathways during Early AAA Formation
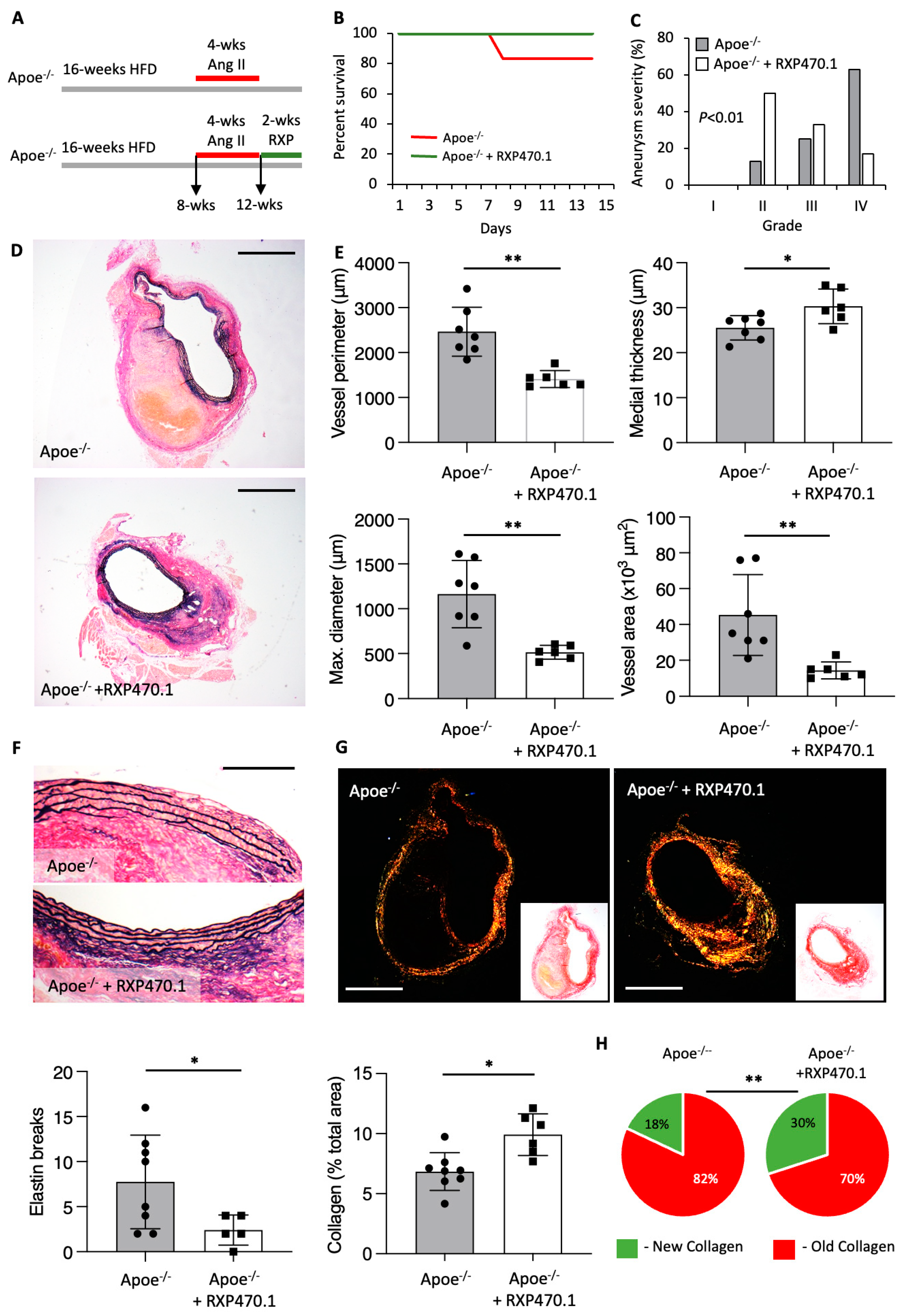
2.4. MMP-12 Inhibition Alleviates the Progression of Pre-Existing AAAs
3. Discussion
4. Materials and Methods
4.1. Human Aortic Tissue Samples and Cells
4.2. Gene Expression
4.3. Western Blotting
4.4. In Situ Zymography
4.5. Mice
4.6. Mouse Model of Ang II-Induced AAA
4.7. MMP-12 Inhibitor Administration
4.8. Termination
4.9. Histological Analyses
4.10. Proteomics
4.11. Statistical Analyses
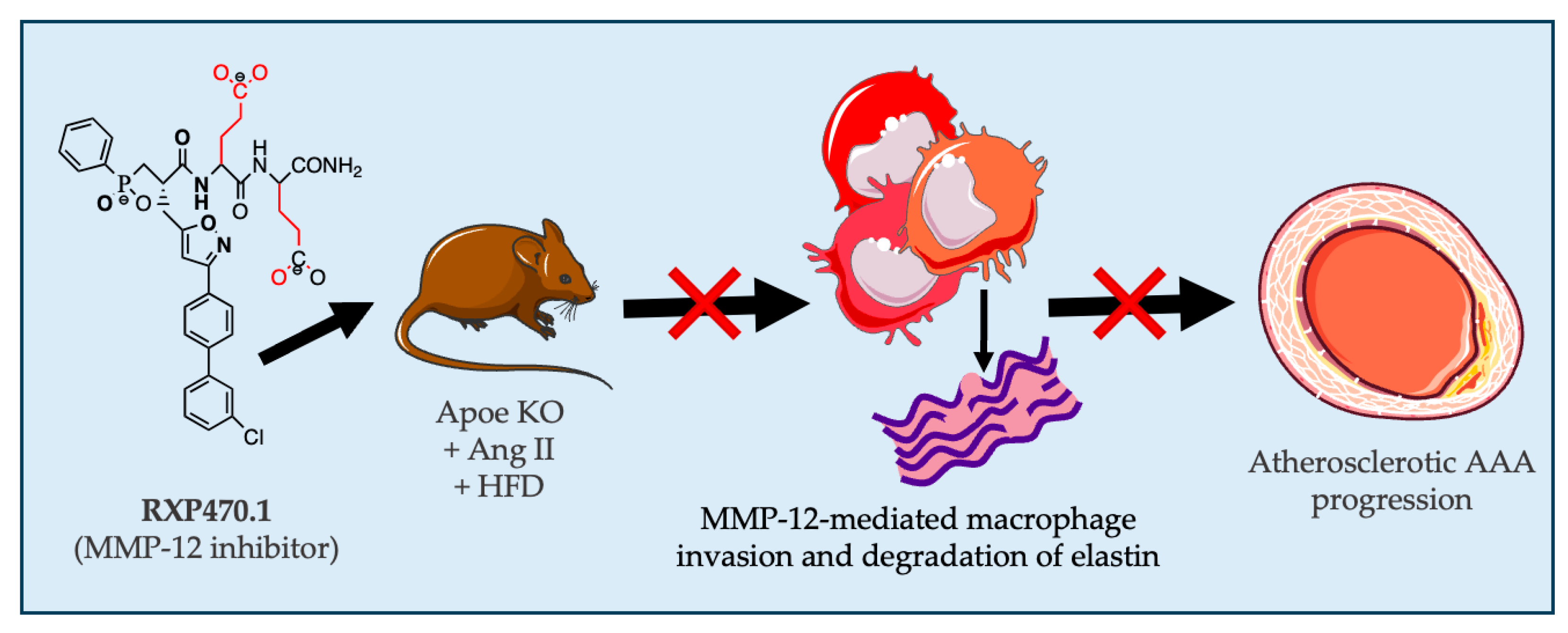
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golledge, J.; Norman, P.E. Atherosclerosis and abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Nordon, I.M.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011, 8, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Hellenthal, F.; Buurman, W.A.; Wodzig, W.; Schurink, G.W.H. Biomarkers of aaa progression. Part 1: Extracellular matrix degeneration. Nat. Rev. Cardiol. 2009, 6, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, G.; Moehle, C.W.; Pei, H.; Walton, S.P.; Yang, Z.; Kron, I.L.; Lau, C.L.; Owens, G.K. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2009, 138, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Hellenthal, F.; Buurman, W.A.; Wodzig, W.; Schurink, G.W.H. Biomarkers of abdominal aortic aneurysm progression. Part 2: Inflammation. Nat. Rev. Cardiol. 2009, 6, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.P.J.; Banerjee, A.; Fairhead, J.F.; Handa, A.; Silver, L.E.; Rothwell, P.M. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. Br. J. Surg. 2015, 102, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Tsao, P.S.; Dalman, R.L.; Norman, P.E. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation 2008, 118, 2382–2392. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Folsom, A.R.; Ballantyne, C.M.; Tang, W. Lipoprotein(a) and abdominal aortic aneurysm risk: The atherosclerosis risk in communities study. Atherosclerosis 2018, 268, 63–67. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.; Pertsemlidis, A.; Kavaslar, N.; Stewart, A.; Roberts, R.; Cox, D.R.; Hinds, D.A.; Pennacchio, L.A.; Tybjaerg-Hansen, A.; Folsom, A.R.; et al. A common allele on chromosome 9 associated with coronary heart disease. Science 2007, 316, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.R.; Golledge, J.; Cooper, J.A.; Hafez, H.; Norman, P.E.; Humphries, S.E. Sequence variant on 9p21 is associated with the presence of abdominal aortic aneurysm disease but does not have an impact on aneurysmal expansion. Eur. J. Hum. Genet. 2009, 17, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Aortic aneurysm formation. Lessons from human studies and experimental models. Circulation 1998, 98, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; LeMaire, S.A.; Webb, N.R.; Cassis, L.A.; Daugherty, A.; Lu, H.S. Aortic aneurysms and dissections series. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.-B.; Martin-Ventura, J.-L.; Egido, J.; Sakalihasan, N.; Treska, V.; Lindholt, J.; Allaire, E.; Thorsteinsdottir, U.; Cockerill, G.; Swedenborg, J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc. Res. 2011, 90, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Mitchell, R.N.; Libby, P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Bianco, R.; Di Gregoli, K.; Johnson, J.L. The contribution of matrix metalloproteinases and their inhibitors to the development, progression, and rupture of abdominal aortic aneurysms. Front. Cardiovasc. Med. 2023, 10, 1248561. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Matrix metalloproteinases and their inhibitors in cardiovascular pathologies: Current knowledge and clinical potential. Met. Med. 2014, 1, 21–36. [Google Scholar] [CrossRef][Green Version]
- Johnson, J.L.; George, S.J.; Newby, A.C.; Jackson, C.L. Divergent effects of matrix metalloproteinases −3, −7, −9 and −12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc. Natl. Acad. Sci. USA 2005, 102, 15575–15580. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Baker, A.H.; Oka, K.; Chan, L.; Newby, A.C.; Jackson, C.L.; George, S.J. Suppression of atherosclerotic plaque progression and instability by tissue inhibitor of metalloproteinase-2: Involvement of macrophage migration and apoptosis. Circulation 2006, 113, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Di Gregoli, K.; George, S.J.; Newby, A.C.; Johnson, J.L. Differential effects of tissue inhibitor of metalloproteinase (timp)-1 and timp-2 on atherosclerosis and monocyte/macrophage invasion. Cardiovasc. Res. 2016, 109, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Shipley, J.M.; Wesselschmidt, R.L.; Kobayashi, D.K.; Ley, T.J.; Shapiro, S.D. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 3942–3946. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Devel, L.; Czarny, B.; George, S.J.; Jackson, C.L.; Rogakos, V.; Beau, F.; Yiotakis, A.; Newby, A.C.; Dive, V. A selective matrix metalloproteinase-12 inhibitor retards atherosclerotic plaque development in apolipoprotein e-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 528–535. [Google Scholar] [CrossRef]
- Hinterseher, I.; Tromp, G.; Kuivaniemi, H. Genes and abdominal aortic aneurysm. Ann. Vasc. Surg. 2011, 25, 388–412. [Google Scholar] [CrossRef]
- Roychowdhury, T.; Klarin, D.; Levin, M.G.; Spin, J.M.; Rhee, Y.H.; Deng, A.; Headley, C.A.; Tsao, N.L.; Gellatly, C.; Zuber, V.; et al. Genome-wide association meta-analysis identifies risk loci for abdominal aortic aneurysm and highlights pcsk9 as a therapeutic target. Nat. Genet. 2023, 55, 1831–1842. [Google Scholar] [CrossRef]
- Curci, J.A.; Liao, S.; Huffman, M.D.; Shapiro, S.D.; Thompson, R.W. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J. Clin. Investig. 1998, 102, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Di Gregoli, K.; Somerville, M.; Bianco, R.; Thomas, A.C.; Frankow, A.; Newby, A.C.; George, S.J.; Jackson, C.L.; Johnson, J.L. Galectin-3 identifies a subset of macrophages with a potential beneficial role in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1491–1509. [Google Scholar] [CrossRef]
- Longo, G.M.; Buda, S.J.; Fiotta, N.; Xiong, W.; Griener, T.; Shapiro, S.; Baxter, B.T. Mmp-12 has a role in abdominal aortic aneurysms in mice. Surgery 2005, 137, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.L.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase b) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ait-Oufella, H.; Herbin, O.; Bonnin, P.; Ramkhelawon, B.; Taleb, S.; Huang, J.; Offenstadt, G.; Combadiare, C.; Renia, L.; et al. Tgf-b activity protects against inflammatory aortic aneurysm progression and complications in angiotensin ii-infused mice. J. Clin. Investig. 2010, 120, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Salarian, M.; Ghim, M.; Toczek, J.; Han, J.; Weiss, D.; Spronck, B.; Ramachandra, A.B.; Jung, J.-J.; Kukreja, G.; Zhang, J.; et al. Homeostatic, non-canonical role of macrophage elastase in vascular integrity. Circ. Res. 2023, 132, 432–448. [Google Scholar] [CrossRef]
- Johnson, J.L. Metalloproteinases in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Luttun, A.; Lutgens, E.; Manderveld, A.; Maris, K.; Collen, D.; Carmeliet, P.; Moons, L. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein e-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation 2004, 109, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F.; Kuivaniemi, H. Role of matrix metalloproteinase inhibitors in preventing abdominal aortic aneurysm. Ann. Vasc. Surg. 2007, 21, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Hautamaki, R.D.; Kobayashi, D.K.; Senior, R.M.; Shapiro, S.D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997, 277, 2002–2004. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Daugherty, A.; Lu, H.S. Divergent roles of matrix metalloproteinase 12 in abdominal aortic aneurysms. Circ. Res. 2023, 132, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Kitajima, S.; Quan, L.; Wang, Y.; Zhu, M.; Liu, E.; Lai, L.; Yan, H.; Fan, J. Macrophage elastase derived from adventitial macrophages modulates aortic remodeling. Front. Cell Dev. Biol. 2023, 10, 1097137. [Google Scholar] [CrossRef] [PubMed]
- Combadiere, C.; Potteaux, S.; Rodero, M.; Simon, T.; Pezard, A.; Esposito, B.; Merval, R.; Proudfoot, A.; Tedgui, A.; Mallat, Z. Combined Inhibition of CCL2, CX3CR1, and CCR5 Abrogates Ly6Chi and Ly6Clo Monocytosis and Almost Abolishes Atherosclerosis in Hypercholesterolemic Mice. Circulation 2008, 117, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Mellak, S.; Ait-Oufella, H.; Esposito, B.; Loyer, X.; Poirier, M.; Tedder, T.F.; Tedgui, A.; Mallat, Z.; Potteaux, S. Angiotensin ii mobilizes spleen monocytes to promote the development of abdominal aortic aneurysm in apoe−/− mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.G.; Martin-McNulty, B.; Sukovich, D.A.; Freay, A.; Halks-Miller, M.; Thinnes, T.; Loskutoff, D.J.; Carmeliet, P.; Dole, W.P.; Wang, Y.-X. Urokinase-type plasminogen activator plays a critical role in angiotensin ii–induced abdominal aortic aneurysm. Circ. Res. 2003, 92, 510–517. [Google Scholar] [PubMed]
- Michel, J.-B.; Jondeau, G.; Milewicz, D.M. From genetics to response to injury: Vascular smooth muscle cells in aneurysms and dissections of the ascending aorta. Cardiovasc. Res. 2018, 114, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Trachet, B.; Fraga-Silva, R.A.; Piersigilli, A.; Tedgui, A.; Sordet-Dessimoz, J.; Astolfo, A.; Van der Donckt, C.; Modregger, P.; Stampanoni, M.F.M.; Segers, P.; et al. Dissecting abdominal aortic aneurysm in ang ii-infused mice: Suprarenal branch ruptures and apparent luminal dilatation. Cardiovasc. Res. 2015, 105, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Trachet, B.; Piersigilli, A.; Fraga-Silva, R.A.; Aslanidou, L.; Sordet-Dessimoz, J.; Astolfo, A.; Stampanoni, M.F.M.; Segers, P.; Stergiopulos, N. Ascending aortic aneurysm in angiotensin ii–infused mice: Formation, progression, and the role of focal dissections. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Saraff, K.; Babamusta, F.; Cassis, L.A.; Daugherty, A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin ii-infused, apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, M.K.; Vijungco, J.D.; Flores, A.; Borensztajn, J.; Shively, V.; Pearce, W.H. Enhanced abdominal aortic aneurysm in timp-1-deficient mice. J. Surg. Res. 2005, 123, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Allaire, E.; Forough, R.; Clowes, M.; Starcher, B.; Clowes, A.W. Local overexpression of timp-1 prevents aortic aneurysm degeneration and rupture in a rat model. J. Clin. Investig. 1998, 102, 1413–1420. [Google Scholar] [CrossRef]
- Xiong, W.F.; Knispel, R.; Mactaggart, J.; Baxter, B.T. Effects of tissue inhibitor of metalloproteinase 2 deficiency on aneurysm formation. J. Vasc. Surg. 2006, 44, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Di Gregoli, K.; Mohamad Anuar, N.N.; Bianco, R.; White, S.J.; Newby, A.C.; George, S.J.; Johnson, J.L. Microrna-181b controls atherosclerosis and aneurysms through regulation of timp-3 and elastin. Circ. Res. 2017, 120, 49–65. [Google Scholar] [CrossRef]
- Basu, R.; Fan, D.; Kandalam, V.; Lee, J.; Das, S.K.; Wang, X.; Baldwin, T.A.; Oudit, G.Y.; Kassiri, Z. Loss of timp3 gene leads to abdominal aortic aneurysm formation in response to angiotensin II. J. Biol. Chem. 2012, 287, 44083–44096. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, I.; Bengtsson, E.; Colhoun, H.M.; Shore, A.C.; Palombo, C.; Natali, A.; Edsfeldt, A.; Dunér, P.; Fredrikson, G.N.; Björkbacka, H.; et al. Elevated plasma levels of mmp-12 are associated with atherosclerotic burden and symptomatic cardiovascular disease in subjects with type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.M.; Lynch, A.I.; Brophy, V.H.; Eckfeldt, J.H.; Davis, B.R.; Ford, C.E.; Boerwinkle, E.; Arnett, D.K. Pharmacogenetic associations of mmp9 and mmp12 variants with cardiovascular disease in patients with hypertension. PLoS ONE 2011, 6, e23609. [Google Scholar] [CrossRef] [PubMed]
- Jguirim-Souissi, I.; Jelassi, A.; Addad, F.; Hassine, M.; Najah, M.; Ben Hamda, K.; Maatouk, F.; Ben Farhat, M.; Bouslema, A.; Rouis, M.; et al. Plasma metalloproteinase-12 and tissue inhibitor of metalloproteinase-1 levels and presence, severity, and outcome of coronary artery disease. Am. J. Cardiol. 2007, 100, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Shiraya, S.; Miyake, T.; Aoki, M.; Yoshikazu, F.; Ohgi, S.; Nishimura, M.; Ogihara, T.; Morishita, R. Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis 2009, 202, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme oxygenase-1: An anti-inflammatory effector in cardiovascular, lung, and related metabolic disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Skrzypek, K.; Tertil, M.; Golda, S.; Ciesla, M.; Weglarczyk, K.; Collet, G.; Guichard, A.; Kozakowska, M.; Boczkowski, J.; Was, H.; et al. Interplay between heme oxygenase-1 and mir-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid. Redox Signal 2013, 19, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-C.; Wu, M.-L.; Gung, P.-Y.; Chen, C.-H.; Kuo, C.-C.; Yet, S.-F. Heme oxygenase-1 deficiency exacerbates angiotensin ii-induced aortic aneurysm in mice. Oncotarget 2016, 7, 67760–67776. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, A.; Klóska, D.; Werner, E.; Hajduk, K.; Grochot-Przęczek, A.; Józkowicz, A.; Piechota-Polańczyk, A. A dual role of heme oxygenase-1 in angiotensin ii-induced abdominal aortic aneurysm in the normolipidemic mice. Cells 2021, 10, 163. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Rateri, D.L.; Charo, I.F.; Owens, A.P.; Howatt, D.A.; Cassis, L.A. Angiotensin ii infusion promotes ascending aortic aneurysms: Attenuation by CCR2 deficiency in apoe−/− mice. Clin. Sci. 2010, 118, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.R.; Iacobazzi, D.; Abdul-Ghani, S.; Ghorbel, M.T.; Heesom, K.J.; George, S.J.; Caputo, M.; Suleiman, M.S.; Tulloh, R.M. The cardiac proteome in patients with congenital ventricular septal defect: A comparative study between right atria and right ventricles. J. Proteom. 2019, 191, 107–113. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gregoli, K.; Atkinson, G.; Williams, H.; George, S.J.; Johnson, J.L. Pharmacological Inhibition of MMP-12 Exerts Protective Effects on Angiotensin II-Induced Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2024, 25, 5809. https://doi.org/10.3390/ijms25115809
Di Gregoli K, Atkinson G, Williams H, George SJ, Johnson JL. Pharmacological Inhibition of MMP-12 Exerts Protective Effects on Angiotensin II-Induced Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice. International Journal of Molecular Sciences. 2024; 25(11):5809. https://doi.org/10.3390/ijms25115809
Chicago/Turabian StyleDi Gregoli, Karina, Georgia Atkinson, Helen Williams, Sarah J. George, and Jason L. Johnson. 2024. "Pharmacological Inhibition of MMP-12 Exerts Protective Effects on Angiotensin II-Induced Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice" International Journal of Molecular Sciences 25, no. 11: 5809. https://doi.org/10.3390/ijms25115809
APA StyleDi Gregoli, K., Atkinson, G., Williams, H., George, S. J., & Johnson, J. L. (2024). Pharmacological Inhibition of MMP-12 Exerts Protective Effects on Angiotensin II-Induced Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice. International Journal of Molecular Sciences, 25(11), 5809. https://doi.org/10.3390/ijms25115809






