Current Status of Cardiac Regenerative Therapy Using Induced Pluripotent Stem Cells
Abstract
1. Introduction
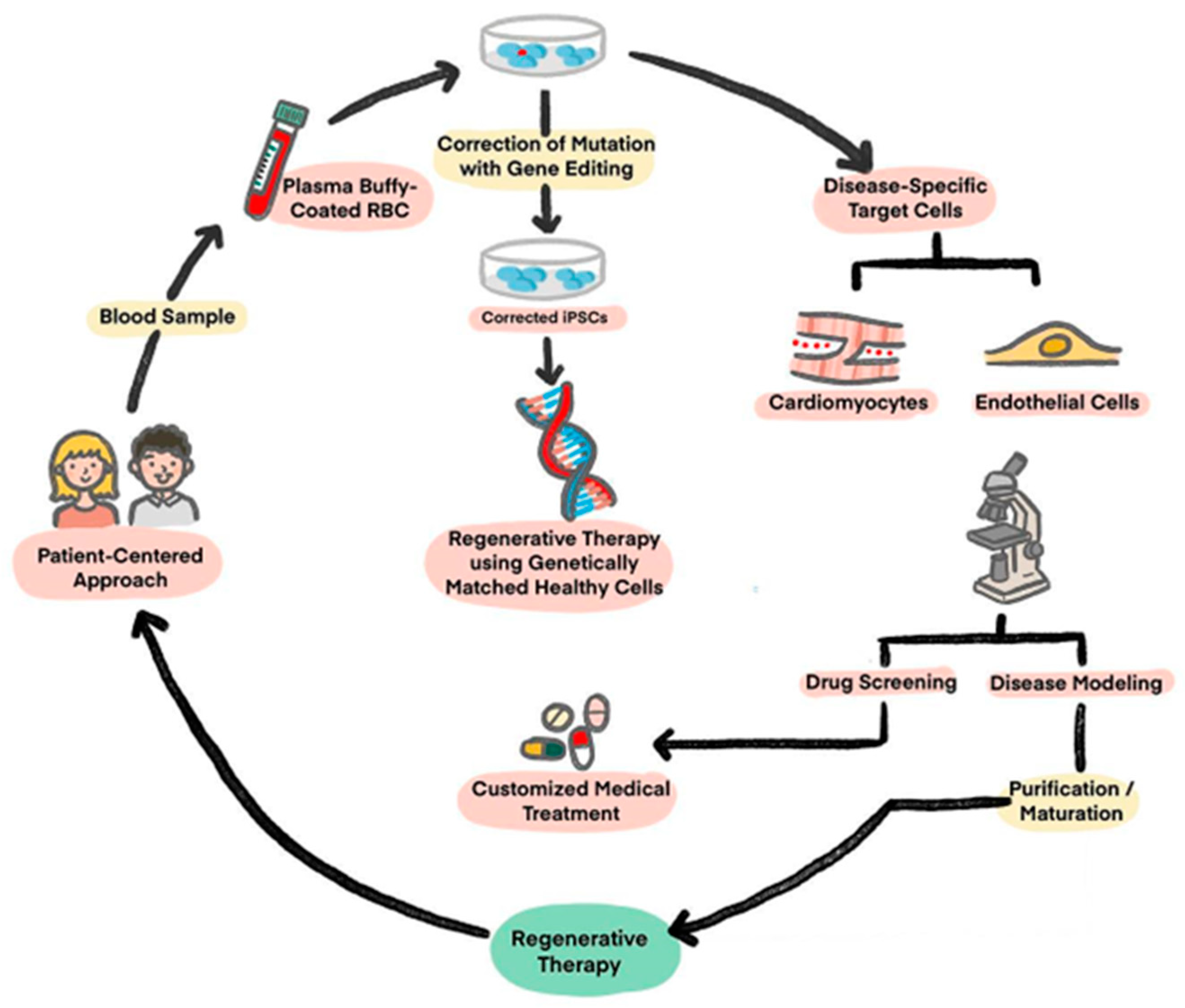
2. Induced Pluripotent Stem Cell (iPSC)
2.1. Disease Modeling
2.2. Drug Screening
2.3. Regenerative Therapies
3. Limitations and Solutions for Translation of iPSC-CMs Practically
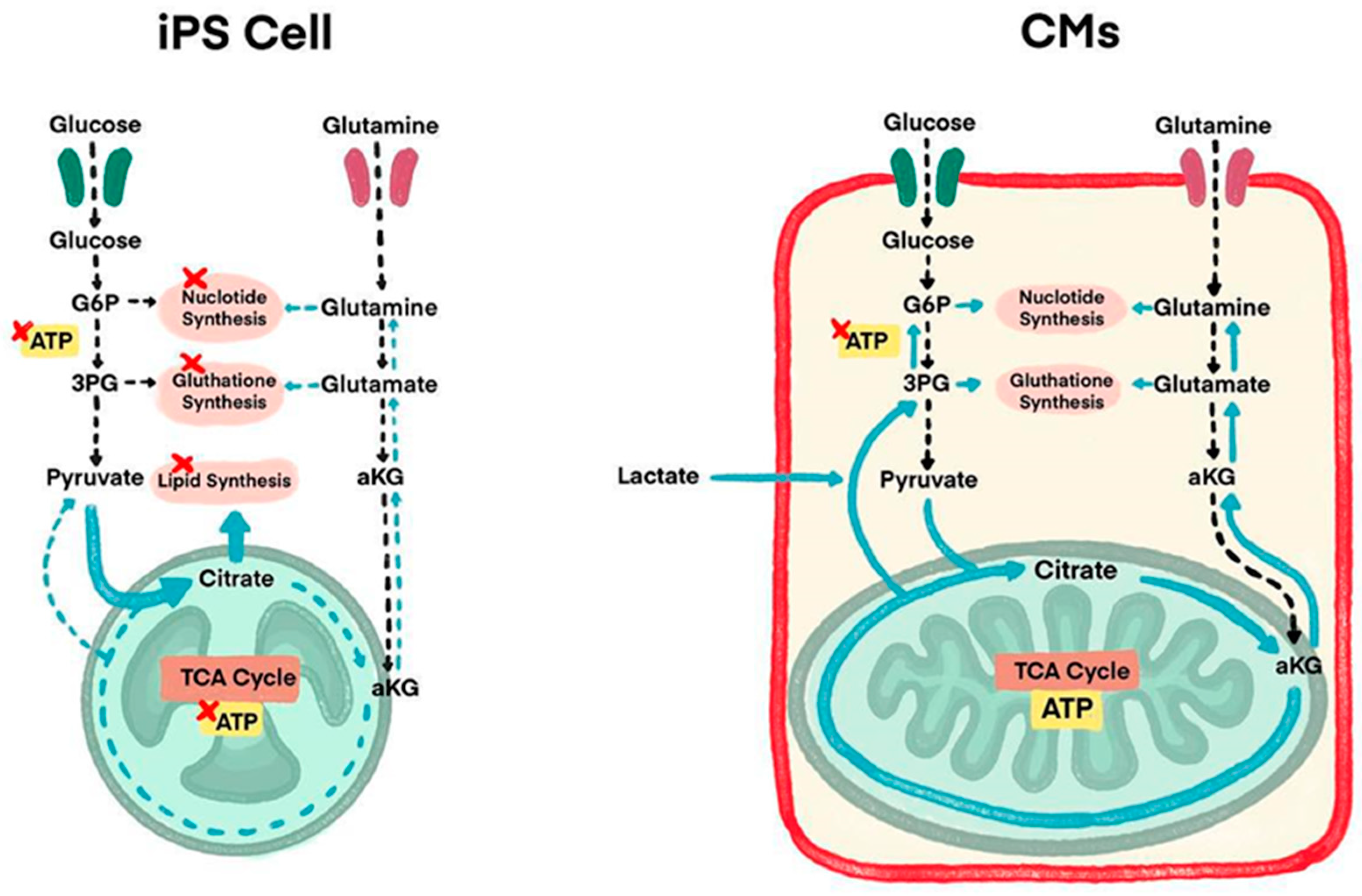
3.1. Overcoming iPSC-CMs Immaturity
3.2. Delivery Routes
3.3. Prevention of Immunogenicity
3.4. Solutions for Arrhythmogenesis
3.5. Solutions to Poor Engraftment
3.6. Prevention of Tumorigenesis
4. Current Therapeutic Clinical Trials
4.1. iPSC-Derived Cardiomyocyte Patch
4.2. Cardiac Spheroid
4.3. Biological Ventricular Assist Tissue (BioVAT)
4.4. Epicardial Injection of iPSC-CMs
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, A.M.; Mann, D.L. In search of new therapeutic targets and strategies for heart failure: Recent advances in basic science. Lancet 2011, 378, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Fonarow, G.C.; Opsha, Y.; Sandhu, A.T.; Sweitzer, N.K.; Warraich, H.J.; HFSA Scientific Statement Committee Members Chair. Economic issues in heat failure in the United States. J. Card. Fail. 2022, 28, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Dorsey, H.; Decleene, N.; Razo, C.; Stark, B.; Johnson, C. The global burden of heart failure: A systematic analysis for The Global Burden of Disease Study 2021. Eur. Heart J. 2023, 44 (Suppl. 2), ehad655.876. [Google Scholar] [CrossRef]
- Bristow, M.R. Management of heart failure. In Braunwald’s Heart Disease; Elsevier: Amsterdam, The Netherlands, 2005; pp. 603–624. [Google Scholar]
- Starling, R.C. Epidemiology of heart failure: Progression to pandemic? Heart Fail. Comb. Med. Surg. Approach 2007, 1–8. [Google Scholar] [CrossRef]
- Urbich, M.; Globe, G.; Pantiri, K.; Heisen, M.; Bennison, C.; Wirtz, H.S.; Di Tanna, G.L. A systematic review of medical costs associated with heart failure in USA (2014–2020). Pharmacoeconomics 2020, 38, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Reinecke, H.; Pabon, L.M. Regeneration gaps: Observations on stem cells and cardiac repair. J. Am. Coll. Cardiol. 2006, 47, 1777–1785. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Murry, C.E. Regenerating the heart. Nat. Biotechnol. 2005, 23, 845. [Google Scholar] [CrossRef]
- Hunt, S.A.; American College of Cardiology. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation 2005, 112, e154–e235. [Google Scholar] [PubMed]
- Azevedo, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.; Zornoff, L.A. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq. Bras. Cardiol. 2016, 106, 62–69. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S. Heart disease and stroke statistics—Update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar]
- Joseph, A.; Kaplan, S. After Criticism from Scientists, Congress Eases Its Pursuit of Faster Stem Cell Therapies. Stat News. 2016. Available online: https://www.statnews.com/2016/11/30/stem-cells-cures-act/ (accessed on 25 May 2024).
- Romito, A.; Cobellis, G. Pluripotent stem cells: Current understanding and future directions. Stem Cells Int. 2016, 2016, 9451492. [Google Scholar] [CrossRef] [PubMed]
- Rikhtegar, R.; Pezeshkian, M.; Dolati, S.; Safaie, N.; Afrasiabi Rad, A.; Mahdipour, M.; Nouri, M.; Jodati, A.R.; Yousefi, M. Stem cells as therapy for heart disease: iPSCs, ESCs, CSCs, and skeletal myoblasts. Biomed. Pharmacother. 2019, 109, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yuasa, S.; Fukuda, K. Cardiac regenerative medicine. Circ. J. 2008, 72, A49–A55. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.; Liu, C.; Wu, J.C. Translation of human-induced pluripotent stem cells: From clinical trial in a dish to precision medicine. J. Am. Coll. Cardiol. 2016, 67, 2161–2176. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.E.; Barrs, R.W.; Mei, Y. Transplantation of Human Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Regenerative Therapy. Front. Cardiovasc. Med. 2021, 8, 707890. [Google Scholar] [CrossRef] [PubMed]
- Shanak, S.; Helms, V. DNA methylation and the core pluripotency network. Dev. Biol. 2020, 464, 145–160. [Google Scholar] [CrossRef]
- Chin, M.H.; Mason, M.J.; Xie, W.; Volinia, S.; Singer, M.; Peterson, C.; Ambartsumyan, G.; Aimiuwu, O.; Richter, L.; Zhang, J.; et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 2009, 5, 111–123. [Google Scholar] [CrossRef]
- Newman, A.M.; Cooper, J.B. Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem Cell 2010, 7, 258–262. [Google Scholar] [CrossRef]
- De Korte, T.; Katili, P.A.; Mohd Yusof, N.A.N.; van Meer, B.J.; Saleem, U.; Burton, F.L.; Smith, G.L.; Clements, P.; Mummery, C.L.; Eschenhagen, T.; et al. Unlocking personalized biomedicine and drug discovery with human induced pluripotent stem cell-derived cardiomyocytes: Fit for purpose or forever elusive? Annu. Rev. Pharmacol. Toxicol. 2020, 6, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.T.; Macadangdang, J.; Leung, W.; Laflamme, M.A.; Kim, D.H. Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening. Biotechnol. Adv. 2017, 35, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.D.; Saito, Y.; Nakamura, K.; Iida, T.; Yuasa, S. Induced pluripotent stem cell-derived cardiomyocytes therapy for ischemic heart disease in animal model: A meta-analysis. Int. J. Mol. Sci. 2024, 25, 987. [Google Scholar] [CrossRef]
- Vučković, S.; Dinani, R.; Nollet, E.E.; Kuster, D.W.; Buikema, J.W.; Houtkooper, R.H.; Nabben, M.; van der Velden, J.; Goversen, B. Characterization of cardiac metabolism in iPSC-derived cardiomyocytes: Lessons from maturation and disease modeling. Stem Cell Res. Ther. 2022, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Pourrier, M.; Fedida, D. The emergence of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) as a platform to model arrhythmogenic diseases. Int. J. Mol. Sci. 2020, 21, 657. [Google Scholar] [CrossRef] [PubMed]
- McDermott-Roe, C.; Lv, W.; Maximova, T.; Wada, S.; Bukowy, J.; Marquez, M.; Lai, S.; Shehu, A.; Benjamin, I.; Geurts, A.; et al. Investigation of a dilated cardiomyopathy–associated variant in BAG3 using genome-edited iPSC-derived cardiomyocytes. JCI Insight 2019, 4, e128799. [Google Scholar] [CrossRef] [PubMed]
- Ovics, P.; Regev, D.; Baskin, P.; Davidor, M.; Shemer, Y.; Neeman, S.; Ben-Haim, Y.; Binah, O. Drug development and the use of induced pluripotent stem cell-derived cardiomyocytes for disease modeling and drug toxicity screening. Int. J. Mol. Sci. 2020, 21, 7320. [Google Scholar] [CrossRef] [PubMed]
- Hnatiuk, A.P.; Briganti, F.; Staudt, D.W.; Mercola, M. Human iPSC modeling of heart disease for drug development. Cell Chem. Biol. 2021, 28, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Pence, L.; Woodling, K.; Bagam, P.; Beger, R.; Gamboa da Costa, G.; Pang, L. Effects of serum and compound preparation methods on delayed repolarization evaluation with human iPSC-CMs. Toxicol. Sci. 2022, 188, 48–61. [Google Scholar] [CrossRef]
- Cashman, J.R. Reengineering Mexiletine by chemical synthesis to decrease toxicity and improve pharmacological properties with patient-derived iPSC cardiomyocytes. Arch. Clin. Toxicol. 2022, 4, 5–10. [Google Scholar]
- Huang, C.Y.; Nicholson, M.W.; Wang, J.Y.; Ting, C.Y.; Tsai, M.H.; Cheng, Y.C.; Liu, C.L.; Chan, D.Z.H.; Lee, Y.C.; Hsu, C.C.; et al. Population-based high-throughput toxicity screen of human iPSC-derived cardiomyocytes and neurons. Cell Rep. 2022, 39, 110643. [Google Scholar] [CrossRef]
- Denning, C.; Borgdorff, V.; Crutchley, J.; Firth, K.S.; George, V.; Kalra, S.; Kondrashov, A.; Hoang, M.D.; Mosqueira, D.; Patel, A.; et al. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta 2016, 1863, 1728–1748. [Google Scholar] [CrossRef]
- Yang, X.L.; Pabon, L.; Murry, C.E. Engineering adolescence: Maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014, 114, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Deng, G.; Sai, X.; Guo, H.; Huang, H.; Zhu, P. Maturation strategies and limitations of induced pluripotent stem cell-derived cardiomyocytes. Biosci. Rep. 2021, 41, BSR20200833. [Google Scholar] [CrossRef]
- Lundy, S.; Zhu, W.; Regnier, M.; Laflamme, M.A. Structural and functional maturation of cardiomyocytes derived from stem cells. Stem Cells Dev. 2013, 22, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Mosqueira, M.; Hecker, M.; Ullrich, N.D. Substrate stiffness influences structural and functional remodeling in induced pluripotent stem cell-derived cardiomyocytes. Front. Physiol. 2021, 12, 710619. [Google Scholar] [CrossRef] [PubMed]
- Crestani, T.; Steichen, C.; Neri, E.; Rodrigues, M.; Fonseca-Alaniz, M.H.; Ormrod, B.; Holt, M.R.; Pandey, P.; Harding, S.; Ehler, E.; et al. Electrical stimulation applied during differentiation drives the hiPSC-CMs towards a mature cardiac conduction-like cells. Biochem. Biophys. Res. Commun. 2020, 533, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Lyra-Leite, D.M.; Gutiérrez-Gutiérrez, Ó.; Wang, M.; Zhou, Y.; Cyganek, L.; Burridge, P.W. A review of protocols for human iPSC culture, cardiac differentiation, subtype-specification, maturation, and direct reprogramming. STAR Protoc. 2022, 3, 101560. [Google Scholar] [CrossRef]
- Dou, W.; Wang, L.; Malhi, M.; Liu, H.; Zhao, Q.; Plakhotnik, J.; Xu, Z.; Huang, Z.; Simmons, C.A.; Maynes, J.T.; et al. A microdevice platform for characterizing the effect of mechanical strain magnitudes on the maturation of iPSC-Cardiomyocytes. Biosens. Bioelectron. 2021, 175, 112875. [Google Scholar] [CrossRef]
- Huang, C.Y.; Maia-Joca, R.P.; Ong, C.S.; Wilson, I.; DiSilvestre, D.; Tomaselli, G.F.; Reich, D.H. Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. J. Mol. Cell. Cardiol. 2020, 138, 1–11. [Google Scholar] [CrossRef]
- Ziang, Y.; Miller, K.; Guan, J.; Kiratitanaporn, W.; Tang, M.; Chen, S. 3d Bioprinting of complex tissues in vitro state-of-the-art and future perspectives. Arch. Toxicol. 2022, 96, 691–710. [Google Scholar]
- Fukuda, K. Establishment and Industrialization of a New Treatment Method Using Regenerative Cardiomyocyte Transplantation for Refractory Severe Heart Failure-Secondary Publication. JMA J. 2023, 6, 388–392. [Google Scholar] [PubMed]
- Kishino, Y.; Fukuda, K. Unlocking the Pragmatic Potential of Regenerative Therapies in Heart Failure with Next-Generation Treatments. Biomedicines 2023, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Tadevosyan, K.; Igesias-Garcia, O.; Mazo, M.M.; Prósper, F.; Raya, A. Engineering and assessing cardiac tissue complexity. Int. J. Mol. Sci. 2021, 22, 1479. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.H.; Wang, S.P.; Chang, H.Y.; Yang, P.J.; Liu, P.Y.; Liu, Y.W. Immunogenicity in stem cell therapy for cardiac regeneration. Acta Cardiol. Sin. 2020, 36, 588. [Google Scholar] [PubMed]
- Mattapally, S.; Pawlik, K.M.; Fast, V.G.; Zumaquero, E.; Lund, F.E.; Randall, T.D.; Townes, T.M.; Zhang, J. Human leukocyte antigen class I and II knockout human induced pluripotent stem cell0derived cells: Universal donor for cell therapy. J. Am. Heart Assoc. 2018, 7, e010239. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef]
- Ogasawara, T.; Okano, S.; Ichimura, H.; Kadota, S.; Tanaka, Y.; Minami, I.; Uesugi, M.; Wada, Y.; Saito, N.; Okada, K. Impact of extracellular matrix on engraftment and maturation of pluripotent stem cell-derived cardiomyocytes in a rat myocardial infarct model. Sci. Rep. 2017, 7, 8630. [Google Scholar] [CrossRef]
- Stevens, K.R.; Murry, C.E. Human pluripotent stem cell-derived engineered tissues: Clinical considerations. Cell Stem Cell 2018, 22, 294–297. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, Q.; Wang, X.; Mansoor, A.; Lee, J.; Feygin, J.; Zhang, G.; Suntharalingam, P.; Boozer, S.; Mhashilkar, A. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation 2017, 115, 1866–1875. [Google Scholar] [CrossRef]
- Robey, T.E.; Saiget, M.K.; Reinecke, H.; Murry, C.E. Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell Cardiol. 2008, 45, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Nakada, Y.; Wei, Y.; Bian, W.; Chu, Y.; Borovjagin, A.V.; Xie, M.; Zhu, W.; Nguyen, T.; Zhou, Y.; et al. Cyclin D2 overexpression enhances the efficacy of human induced pluripotent stem cell-derived cardiomyocytes for myocardial repair in a swine model of myocardial infarction. Circulation 2021, 144, 210–228. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Zhao, M.; Fan, C.; Fast, V.G.; Valarmathi, M.T.; Zhu, W.; Zhang, J. N-cadherin overexpression enhances the reparative potency of human induced pluripotent stem cell-derived cardiomyocytes in infarcted mouse hearts. Cardiovasc. Res. 2020, 116, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, J.; Qiang, B.; Romagnuolo, R.; Gagliardi, M.; Keller, G.; Laflamme, M.A.; Li, R.K.; Nunes, S.S. Transplanted microvessels improve pluripotent stem cell-derived cardiomyocyte engraftment and cardiac function after infarction in rats. Sci. Transl. Med. 2020, 12, eaax2992. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sato, Y.; Yasuda, S.; Shikamura, M.; Tamura, T.; Takenaka, C.; Takasu, N.; Nomura, M.; Dohi, H.; Takahashi, M.; et al. Correlation between genetic abnormalities in induced pluripotent stem cell-derivatives and abnormal tissue formation in tumorigencity tests. Stem Cells Transl. Med. 2022, 11, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Prieto Gonzalez, E.A. A multilevel approach to the causes of genetic instability in stem cells. In Handbook of Stem Cell Therapy; Springer: Singapore, 2022; pp. 1–55. [Google Scholar]
- Kim, J.Y.; Nam, Y.; Rim, Y.A.; Ju, J.H. Review of the Current Trends in Clinical Trials Involving Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2022, 18, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012, 126, S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Miki, K.; Funakoshi, S.; Yoshida, Y.; Yamanaka, S.; Shimizu, T.; Okano, T.; et al. Enhanced therapeutic effects of human iPS cell derived cardiomyocyte by combined cell-sheets with omental flap technique in porcine ischemic cardiomyopathy model. Sci. Rep. 2017, 7, 8824. [Google Scholar] [CrossRef]
- Ishida, M.; Miyagawa, S.; Saito, A.; Fukushima, S.; Harada, A.; Ito, E.; Ohashi, F.; Watabe, T.; Hatazawa, J.; Matsuura, K.; et al. Transplantation of human-induced pluripotent stem cell derived cardiomyocytes is superior to somatic stem cell therapy for restoring cardiac function and oxygen consumption in a porcine model of myocardial infarction. Transplantation 2019, 103, 291–298. [Google Scholar] [CrossRef]
- Kawamura, T.; Ito, Y.; Ito, E.; Takeda, M.; Mikami, T.; Taguchi, T.; Mochizuki-Oda, N.; Sasai, M.; Shimamoto, T.; Nitta, Y.; et al. Safety confirmation of induced pluripotent stem cell-derived cardiomyocyte patch transplantation for ischemic cardiomyopathy: First three case reports. Front. Cardiovasc. Med. 2023, 10, 1182209. [Google Scholar] [CrossRef] [PubMed]
- Oikonomopoulos, A.; Kitani, T.; Wu, J.C. Pluripotent Stem Cell-Derived Cardiomyocytes as a Platform for Cell Therapy Applications: Progress and Hurdles for Clinical Translation. Mol. Ther. 2018, 26, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Erratum: Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 899. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, S.; Fujita, B.; Aboud, A.; Paitazoglou, C.; Eitel, I.; Jurczyk, D.; Mezger, M.; Yebran, F.; Kutschka, I.; Tiburcy, M.; et al. Safety and efficacy of induced pluripotent stem cell-derived engineered human myocardium as biological ventricular assist tissue in terminal heart failure (BioVAT-DZHK20): Trial design and experience at UKSH Lubeck. Thorac. Cardiovasc. Surg. 2024, 72, S1–S68. [Google Scholar]
- Mallapaty, S. Revealed: Two men in China were first to receive pioneering stem-cell treatment for heart disease. Nature 2020, 581, 249–250. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; Pan, T.; Zhu, X.; Chong, H.; Xu, C.; Fan, F.; Cao, H.; Zhang, B.; Pan, J.; et al. Epicardial injection of allogeneic human-induced-pluripotent stem cell-derived cardiomyocytes in patients with advanced heart failure: Protocol for a phase I/IIa dose-escalation clinical trial. BMJ Open 2022, 12, e056264. [Google Scholar] [CrossRef]
| Clinical Trial | Sponsor | Study Title | Inclusion | Intervention | Sample Size | Start Date | Country |
|---|---|---|---|---|---|---|---|
| NCT 03763136 Single-Blind RCT Phase I/IIa | Help Therapeutics, Nanjing University Medical School | Epicardial injection of allogeneic human pluripotent stem cell- derived cardiomyocytes to treat severe chronic heart failure | Chronic LV dysfunction LVEF 20–45% 35–75 years old NYHA Class III-IV | Intramyocardial injection of allogeneic iPSC-CMs at time of CABG surgery | 20 | May 2019 | China |
| NCT 04696328 None-Masking Single-Arm Trial | Cuorips Inc., Osaka University Hospital | Clinical trial of human (allogeneic) iPS cell-derived cardiomyocytes sheet for ischemic cardiomyopathy | Ischemic cardiomyopathy LVEF ≤ 35% ≥20 years old NYHA Class III-IV | Transplantation of human (allogeneic) iPSC-CM Sheet | 8 | December 2019 | Japan |
| NCT 04396899 None-Masking Single-Arm Trial Phase I/II | Repairon, University Medical Center Goettingen | Safety and efficacy of induced pluripotent stem cell-derived engineered human myocardium as biological ventricular assist tissue in terminal heart failure | HFrEF LVEF < 35% 18–80 years old NYHA Class III-IV | Implantation of Engineered Human Myocardium (EHM) on dysfunctional left or right ventricular myocardium | 53 | February 2020 | Germany |
| jRCTa 032200189 None-Masking Single-Arm Trial Phase I/II | Heartseed Inc., Keio University School of Medicine | Safety study of induced pluripotent stem cell-derived cardiac spheres transplantation (IPSCS study) | HFrEF LVEF 15–40% 20–75 years old NYHA Class III-IV | Intramyocardial injection of iPSC-CMs spheroids at time of CABG surgery | 10 | November 2020 | Japan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiura, T.; Shahannaz, D.C.; Ferrell, B.E. Current Status of Cardiac Regenerative Therapy Using Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2024, 25, 5772. https://doi.org/10.3390/ijms25115772
Sugiura T, Shahannaz DC, Ferrell BE. Current Status of Cardiac Regenerative Therapy Using Induced Pluripotent Stem Cells. International Journal of Molecular Sciences. 2024; 25(11):5772. https://doi.org/10.3390/ijms25115772
Chicago/Turabian StyleSugiura, Tadahisa, Dhienda C. Shahannaz, and Brandon E. Ferrell. 2024. "Current Status of Cardiac Regenerative Therapy Using Induced Pluripotent Stem Cells" International Journal of Molecular Sciences 25, no. 11: 5772. https://doi.org/10.3390/ijms25115772
APA StyleSugiura, T., Shahannaz, D. C., & Ferrell, B. E. (2024). Current Status of Cardiac Regenerative Therapy Using Induced Pluripotent Stem Cells. International Journal of Molecular Sciences, 25(11), 5772. https://doi.org/10.3390/ijms25115772








