Photoaging Elevated the Genotoxicity of Polystyrene Microplastics to Marine Mussel Mytilus trossulus (Gould, 1850)
Abstract
1. Introduction
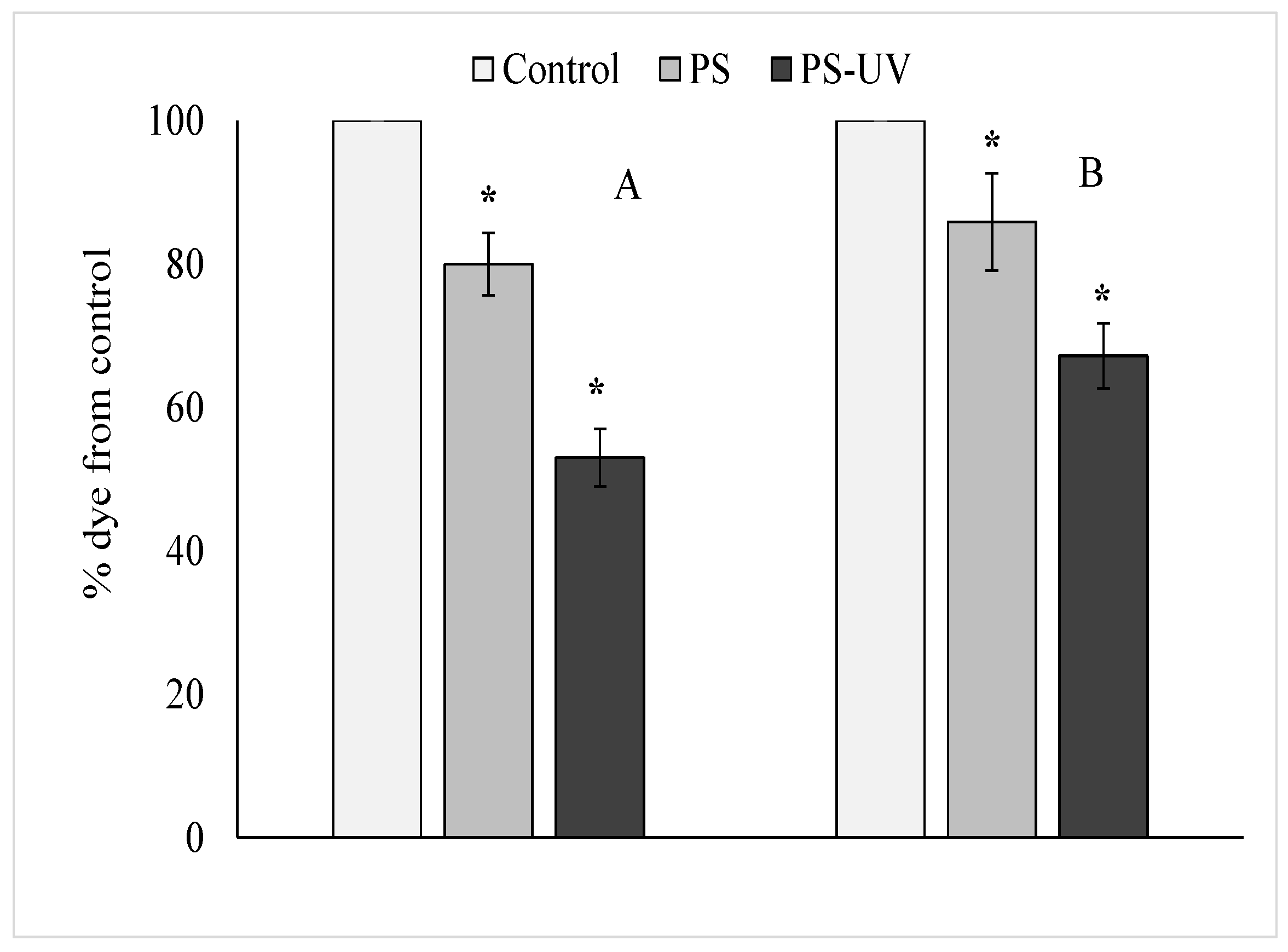
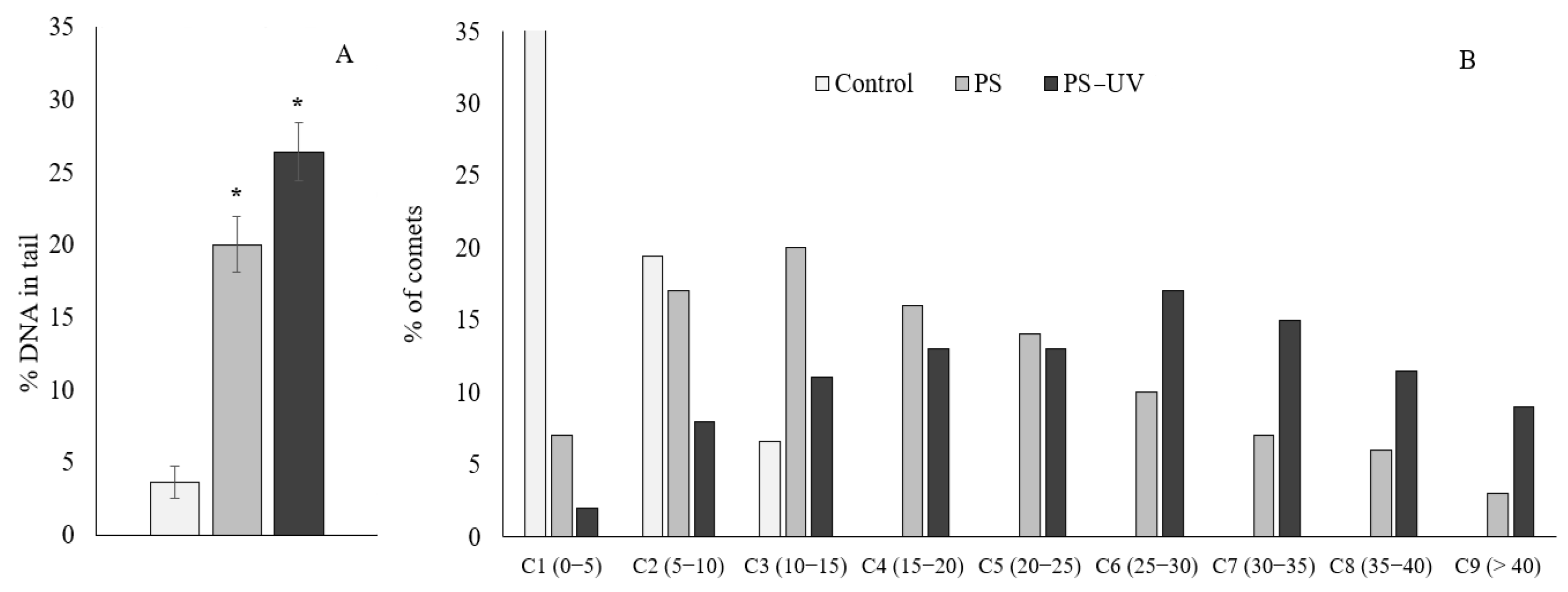
2. Results
3. Discussion
4. Materials and Methods
4.1. Description of the Experiment
4.2. Determination of Cytotoxicity
4.3. Comet Assay
4.4. Fourier-Transform Infrared (FTIR) Spectroscopy
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, M.N. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int. 2006, 32, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science 2015, 13, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.; Cowie, P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef]
- Desforges, J.P.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Galloway, T.S. Ingestion of nanoplastics and microplastics by pacific oyster larvae. Environ. Sci. Technol. 2015, 49, 14625–14632. [Google Scholar] [CrossRef]
- Vivekanand, A.C.; Mohapatra, S.; Tyagi, V.K. Microplastics in aquatic environment: Challenges and perspectives. Chemosphere 2021, 282, 131151. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Gorokhova, E. Screening for microplastic particles in plankton samples: How to integrate marine litter assessment into existing monitoring programs? Mar. Pollut. Bull. 2015, 99, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, J.; Tan, Z.; Gao, Y.; Zhan, Z.; Chen, Q.; Cai, L. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total. Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadi, A.; Papaefthimiou, C.; Genizegkini, E.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Bobori, D.C.; Kastrinaki, G.; Koumoundouros, G.; Lambropoulou, D.A.; et al. Adverse effects polystyrene microplastics exert on zebrafish heart—Molecular to individual level. J. Hazard. Mater. 2021, 416, 125969. [Google Scholar] [CrossRef] [PubMed]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Kukla, S.P.; Dovzhenko, N.V. Genotoxic Properties of Polystyrene (PS) Microspheres in the Filter-Feeder Mollusk Mytilus trossulus (Gould, 1850). J. Mar. Sci. Eng. 2022, 10, 273. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Slobodskova, V.V.; Kukla, S.P.; Mazur, A.A.; Dovzhenko, N.V.; Zhukovskaya, A.F.; Karpenko, A.A.; Karpenko, M.A.; Odintsov, V.S. Dietary Exposure to Particles of Polytetrafluoroethylene (PTFE) and Polymethylmethacrylate (PMMA) Induces Different Responses in Periwinkles Littorina brevicula. Int. J. Mol. Sci. 2023, 24, 8243. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, J.; Zhou, L.; Mao, Z.; Zhang, X.; Cai, J.; Huang, P. Enhanced hepatic metabolic perturbation of polystyrene nanoplastics by UV irradiation-induced hydroxyl radical generation. J. Environ. Sci. 2024, 142, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ding, P.; Li, X.; Huang, C.; Li, X.; Chen, X.; Zhang, L.; Qi, J. Environmentally persistent free radicals on photoaged microplastics from disposable plastic cups induce the oxidative stress associated toxicity. J. Hazard. Mater. 2024, 464, 132990. [Google Scholar] [CrossRef]
- Feldman, D. Polymer Weathering: Photo-Oxidation. J. Polymers Environ. 2002, 10, 4. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513. [Google Scholar] [CrossRef]
- Andrade, J.; Fernández-González, V.; López-Mahía, P.; Muniategui, S. A low-cost system to simulate environmental microplastic weathering. Mar. Pollut. Bull. 2019, 149, 110663. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.; Sandblom, O.; MacLeod, M. Identification of Chain Scission Products Released to Water by Plastic Exposed to Ultraviolet Light. Environ. Sci. Technol. Lett. 2018, 5, 272–276. [Google Scholar] [CrossRef]
- Shi, Y.; Qin, J.; Tao, Y.; Jie, G.; Wang, J. Natural weathering severity of typical coastal environment on polystyrene: Experiment and modeling. Polym. Test. 2019, 76, 138–145. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, J.; Zhou, T.; Zhao, Y.; Yu, F.; Ma, J. Laboratory simulation of microplastics weathering and its adsorption behaviors in an aqueous environment: A systematic review. Environ. Pollut. 2020, 265 Pt B, 114864. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Jiang, R.; You, J.; Ouyang, G. New insights into the photo-degraded polystyrene microplastic: Effect on the release of volatile organic compounds. J. Hazard. Mater. 2022, 431, 128523. [Google Scholar] [CrossRef]
- Zha, F.; Dai, J.; Han, Y.; Liu, P.; Wang, M.; Liu, H.; Guo, X. Release of millions of micro(nano)plastic fragments from photooxidation of disposable plastic boxes. Sci. Total Environ. 2023, 858 Pt 3, 160044. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Hüffer, T.; Weniger, A.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Rubin, A.E.; Zucker, I. Engineered Polystyrene-Based Microplastics of High Environmental Relevance. Environ. Sci. Technol. 2021, 55, 10491–10501. [Google Scholar] [CrossRef] [PubMed]
- Dailianis, S.; Rouni, M.; Ainali, N.M.; Vlastos, D.; Kyzas, G.Z.; Lambropoulou, D.A.; Bikiaris, D.N. New insights into the size-independent bioactive potential of pristine and UV-B aged polyethylene microplastics. Sci. Total Environ. 2024, 918, 170616. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, A.; Kuehnel, D.; Puntar, B.; Žgajnar Gotvajn, A.; Kalčikova, G. An exploratory ecotoxicity study of primary microplastics versus aged in natural waters and wastewaters. Environ. Pollut. 2019, 254 Pt A, 112980. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; MacLeod, M.; Breitholtz, M. Variability in Toxicity of Plastic Leachates as a Function of Weathering and Polymer Type: A Screening Study with the Copepod Nitocra spinipes. Biol. Bull. 2021, 240, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, Y.; Wang, C.; Hua, X.; Li, H.; Xie, D.; Xiang, M.; Yu, Y. Reproductive toxicity of UV-photodegraded polystyrene microplastics induced by DNA damage-dependent cell apoptosis in Caenorhabditis elegans. Sci. Total Environ. 2022, 811, 152350. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, G.; Purvaja, R.; Anandavelu, I.; Robin, R.S.; Ramesh, R. Accumulation and ecotoxicological risk of weathered polyethylene (wPE) microplastics on green mussel (Perna viridis). Ecotoxicol. Environ. Saf. 2021, 208, 111765. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, G.; Purvaja, R.; Anandavelu, I.; Robin, R.S.; Ramesh, R. Ingestion and toxic impacts of weathered polyethylene (wPE) microplastics and stress defensive responses in whiteleg shrimp (Penaeus vannamei). Chemosphere 2022, 300, 134487. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, C.; He, D.; Sun, J.; Li, J.; Pan, X. Effects of aging on environmental behavior of plastic additives: Migration, leaching, and ecotoxicity. Sci. Total Environ. 2022, 849, 157951. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Hof, D.; Dombrowski, A.; Schweyen, P.; Dierkes, G.; Ternes, T.; Schulte-Oehlmann, U.; Oehlmann, J. Enhanced in vitro toxicity of plastic leachates after UV irradiation. Water Res. 2021, 199, 117203. [Google Scholar] [CrossRef]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef]
- Pandi, P.; Madhuvandhi, J.; Priya, K.K.; Thiagarajan, R.; Gopalakrishnan, S.; Elumalai, S.; Thilagam, H. Weathered polyethylene microplastics exposure leads to modulations in glutathione-S-transferase activity in fish. Front. Mar. Sci. 2022, 9, 990351. [Google Scholar] [CrossRef]
- Faggio, C.; Tsarpali, V.; Dailianis, S. Mussel digestive gland as a model tissue for assessing xenobiotics: An overview. Sci. Total Environ. 2018, 636, 220–229. [Google Scholar] [CrossRef]
- Ward, J.E.; Zhao, S.Y.; Holohan, B.A.; Mladinich, K.M.; Griffin, T.W.; Wozniak, J.; Shumway, S.E. Selective ingestion and egestion of plastic particles by the blue mussel (Mytilus edulis) and Eastern Oyster (Crassostrea virginica): Implications for using bivalves as bioindicators of microplastic pollution. Environ. Sci. Technol. 2019, 53, 8776–8784. [Google Scholar] [CrossRef]
- Völkl, M.; Jérôme, V.; Weig, A.; Jasinski, J.; Meides, N.; Strohriegl, P.; Scheibel, T.; Freitag, R. Pristine and artificially-aged polystyrene microplastic particles differ in regard to cellular response. J. Hazard. Mater. 2022, 435, 128955. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, J.; Zhang, G.; Liu, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Interactive effects of microplastics and selected pharmaceuticals on red tilapia: Role of microplastic aging. Sci. Total Environ. 2021, 752, 142256. [Google Scholar] [CrossRef]
- Rummel, C.D.; Escher, B.I.; Sandblom, O.; Plassmann, M.M.; Arp, H.P.H.; MacLeod, M.; Jahnke, A. Effects of Leachates from UV-Weathered Microplastic in Cell-Based Bioassays. Environ. Sci. Technol. 2019, 53, 9214–9223. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lavogina, D.; Lust, H.; Tahk, M.-J.; Laasfeld, T.; Vellama, H.; Nasirova, N.; Vardja, M.; Eskla, K.-L.; Salumets, A.; Rinken, A.; et al. Revisiting the Resazurin-Based Sensing of Cellular Viability: Widening the Application Horizon. Biosensors 2022, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Mahaye, N.; Thwala, M.; Cowan, D.A.; Musee, N. Genotoxicity of metal based engineered nanoparticles in aquatic organisms: A review. Mutat. Res. 2017, 773, 134–160. [Google Scholar] [CrossRef]
- Gómez-Mendikute, A.; Exteberria, A.; Olabarrieta, I.; Cajaraville, M.P. Oxygen radicals production and actin filament disruption in bivalve haemocytes treated with benzo(a)pyrene. Mar. Environ. Res. 2002, 54, 431–436. [Google Scholar] [CrossRef]
- Bandow, N.; Will, V.; Wachtendorf, V.; Simon, F.G. Contaminant release from aged microplastic. Environ. Chem. 2017, 14, 394–405. [Google Scholar] [CrossRef]
- Song, J.A.; Choi, C.Y.; Park, H.S. Exposure of bay scallop Argopecten irradians to micro-polystyrene: Bioaccumulation and toxicity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 236, 108801. [Google Scholar] [CrossRef] [PubMed]
- Rouillon, C.; Bussiere, P.O.; Desnoux, E.; Collin, S.; Vial, C.; Therias, S.; Gardette, J.L. Is carbonyl index a quantitative probe to monitor polypropylene photodegradation? Polymer Degrad. Stabil. 2016, 128, 200–208. [Google Scholar] [CrossRef]
- De Bomfim, A.S.C.; Maciel, M.M.D.; Voorwald, H.J.C.; Benini, K.C.C.; de Oliveira, D.M.; Cioffi, M.O.H. Effect of different degradation types on properties of plastic waste obtained from espresso coffee capsules. Waste Manag. 2019, 83, 123–130. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Massarelli, C.; Uricchio, V.F. Fourier transform infrared spectroscopy to assess the degree of alteration of artificially aged and environmentally weathered microplastics. Polymers 2023, 15, 911. [Google Scholar] [CrossRef]
- Duan, J.; Li, Y.; Gao, J.; Cao, R.; Shang, E.; Zhang, W. ROS-mediated photoaging pathways of nano- and micro-plastic particles under UV irradiation. Water Res. 2022, 216, 118320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, R. Solar radiation stimulates release of semi-labile dissolved organic matter from microplastics. Front. Mar. Sci. 2023, 10, 1284280. [Google Scholar] [CrossRef]
- Schwarz, W.; Wegener, S.; Schertzinger, G.; Pannekens, H.; Schweyen, P.; Dierkes, G.; Klein, K.; Ternes, T.A.; Oehlmann, J.; Dopp, E. Chemical and toxicological assessment of leachates from UV-degraded plastic materials using in-vitro bioassays. PeerJ 2023, 11, 15192. [Google Scholar] [CrossRef]
- Liu, S.; Huang, W.; Yang, J.; Xiong, Y.; Huang, Z.; Wang, J.; Cai, T.; Dang, Z.; Yang, C. Formation of environmentally persistent free radicals on microplastics under UV irradiations. J. Hazard. Mater. 2023, 453, 131277. [Google Scholar] [CrossRef]
- Du, H.; Ma, H.; Xing, B. Identification of naturally weathering microplastics and their interactions with ion dyes in aquatic environments. Mar. Pollut. Bull. 2022, 174, 113186. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Zhao, S.; Xia, T.; Guo, X.; Wang, T.; Zhu, L. Formation of Environmentally Persistent Free Radicals on Microplastics under Light Irradiation. Environ. Sci. Technol. 2019, 53, 8177–8186. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yu, D.; Zhang, Y.; Zhu, C.; Yin, Q.; Hu, Y.; Zhang, X.; Yue, R.; Xiong, X. Polystyrene microplastics-triggered mitophagy and oxidative burst via activation of PERK pathway. Sci. Total Environ. 2021, 781, 146753. [Google Scholar] [CrossRef]
- Zuang, V. The neutral red release assay: A review. Altern Lab Anim. 2001, 29, 575–599. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, Z.; Miao, J.; Tian, Y.; Pan, L. Effects of benzo[a]pyrene exposure on oxidative stress and apoptosis of gill cells of Chlamys farreri in vitro. Environ. Toxicol. Pharmacol. 2022, 93, 103867. [Google Scholar] [CrossRef]
- Knapp, J.L.A.; González-Pinzón, R.; Haggerty, R. The resazurin-resorufin system: Insights from a decade of “smart” tracer development for hydrologic applications. Water Res. 2018, 54, 6877–6889. [Google Scholar] [CrossRef]
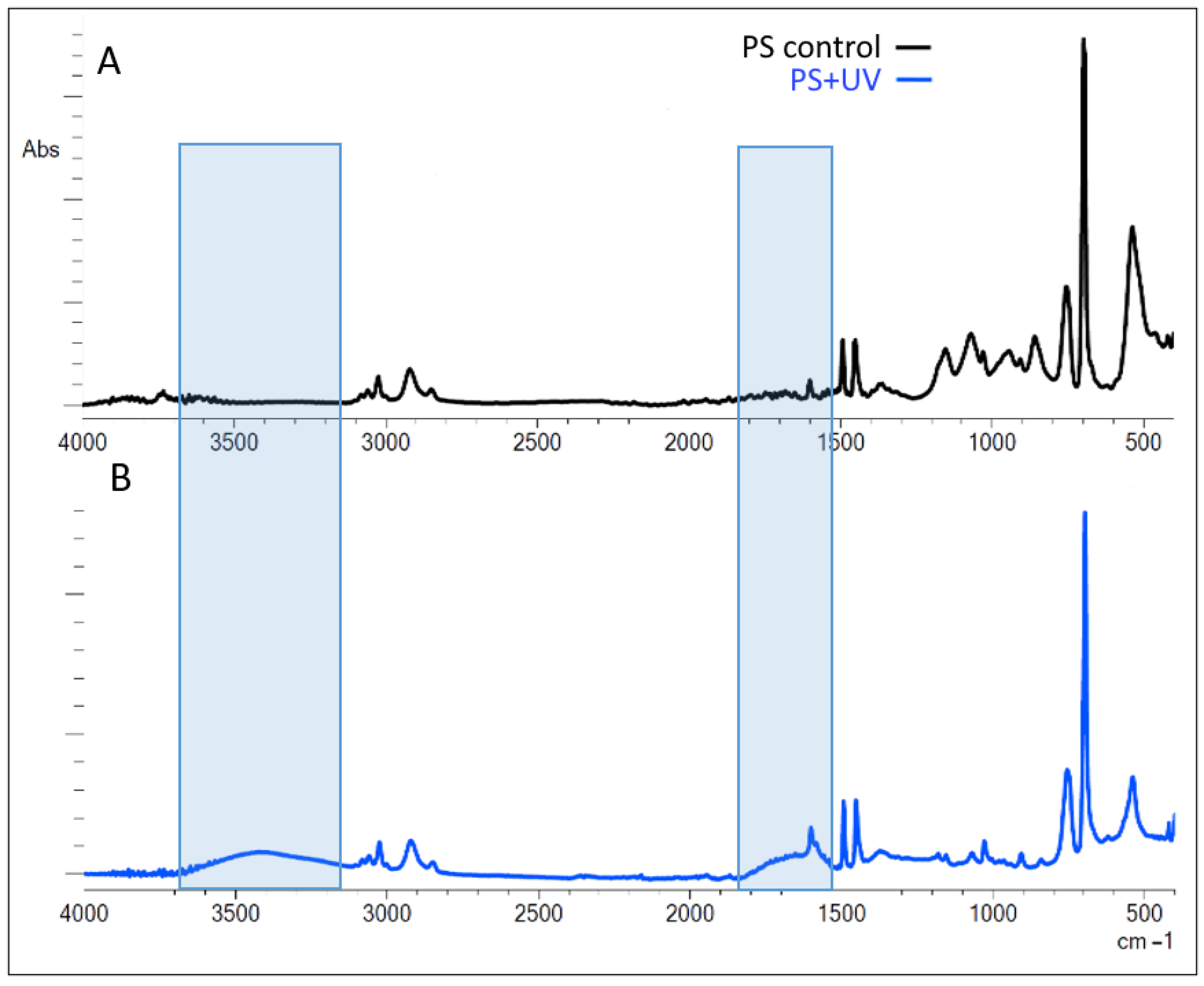
| Index | Formula | PS Control | PS UV Irradiation |
|---|---|---|---|
| CI | (1635–1650)/1452 | 0.39 ± 0.07 | 1.01 ± 0.12 * |
| HI | (3420–3550)/1452 | 0.03 ± 0.01 | 0.39 ± 0.04 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelomin, V.P.; Slobodskova, V.V.; Dovzhenko, N.V.; Mazur, A.A.; Kukla, S.P. Photoaging Elevated the Genotoxicity of Polystyrene Microplastics to Marine Mussel Mytilus trossulus (Gould, 1850). Int. J. Mol. Sci. 2024, 25, 5740. https://doi.org/10.3390/ijms25115740
Chelomin VP, Slobodskova VV, Dovzhenko NV, Mazur AA, Kukla SP. Photoaging Elevated the Genotoxicity of Polystyrene Microplastics to Marine Mussel Mytilus trossulus (Gould, 1850). International Journal of Molecular Sciences. 2024; 25(11):5740. https://doi.org/10.3390/ijms25115740
Chicago/Turabian StyleChelomin, Victor Pavlovich, Valentina Vladimirovna Slobodskova, Nadezhda Vladimirovna Dovzhenko, Andrey Alexandrovich Mazur, and Sergey Petrovich Kukla. 2024. "Photoaging Elevated the Genotoxicity of Polystyrene Microplastics to Marine Mussel Mytilus trossulus (Gould, 1850)" International Journal of Molecular Sciences 25, no. 11: 5740. https://doi.org/10.3390/ijms25115740
APA StyleChelomin, V. P., Slobodskova, V. V., Dovzhenko, N. V., Mazur, A. A., & Kukla, S. P. (2024). Photoaging Elevated the Genotoxicity of Polystyrene Microplastics to Marine Mussel Mytilus trossulus (Gould, 1850). International Journal of Molecular Sciences, 25(11), 5740. https://doi.org/10.3390/ijms25115740






