Advances in Anti-Cancer Drug Development: Metformin as Anti-Angiogenic Supplemental Treatment for Glioblastoma
Abstract
1. Introduction
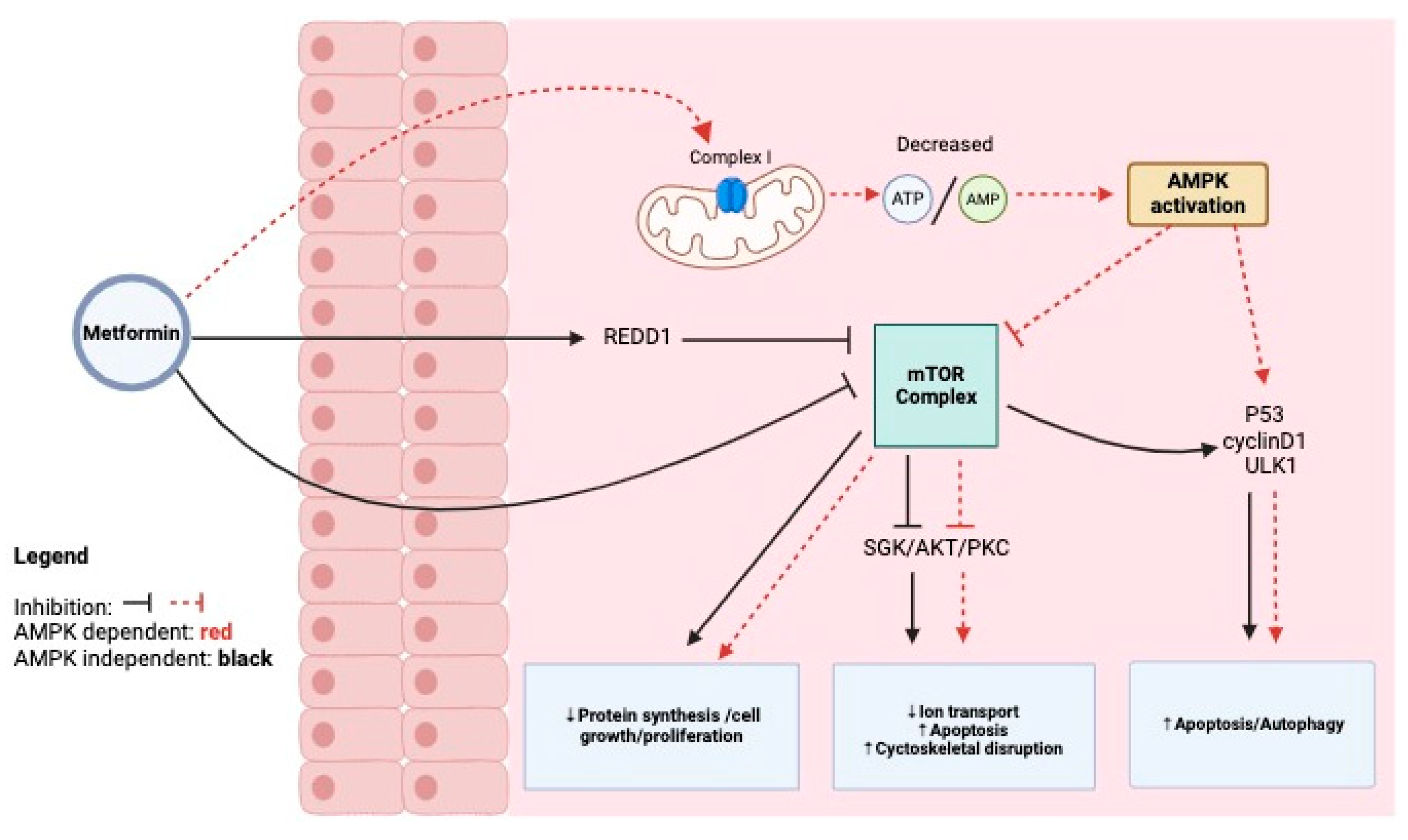
2. Mechanism of Action of Metformin on Cancer Cells, Antineoplastic Action, and Edema Reduction
3. Glioblastoma Stem Cell Target for Metformin
4. Metformin for High-Grade Glioblastoma+Trials
5. Adjuvant Therapy Metformin and Temozolomide for Newly Diagnosed Glioblastoma
6. Results
6.1. Metformin’s Mechanism of Action in Cancer Cells
6.2. Impact on Edema and Brain Tumor Environment
6.3. Adjuvant Therapy with Temozolomide
6.4. Effectiveness in High-Grade Glioblastoma
7. Discussion
8. Conclusions
9. The Limitations of This Study
Author Contributions
Funding
Conflicts of Interest
References
- Goodenberger, M.L.; Jenkins, R.B. Genetics of adult glioma. Cancer Genet. 2012, 205, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Shah, S. Novel Therapies in Glioblastoma Treatment: Review of Glioblastoma; Current Treatment Options; and Novel Oncolytic Viral Therapies. Med. Sci. 2023, 12, 1. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Song, K.; Wu, S.; Hameed, N.F.; Kudulaiti, N.; Xu, H.; Qin, Z.Y.; Wu, J.S. The prognosis of glioblastoma: A large, multifactorial study. Br. J. Neurosurg. 2021, 35, 555–561. [Google Scholar] [CrossRef]
- Oraiopoulou, M.E.; Tzamali, E.; Papamatheakis, J.; Sakkalis, V. Phenocopying Glioblastoma: A Review. IEEE Rev. Biomed. Eng. 2023, 16, 456–471. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- Ahmad, E.; Sargeant, J.A.; Zaccardi, F.; Khunti, K.; Webb, D.R.; Davies, M.J. Where does metformin stand in modern day management of type 2 diabetes? Pharmaceuticals 2020, 13, 427. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin—Mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Yousef, M.; Tsiani, E. Metformin in lung cancer: Review of in vitro and in vivo animal studies. Cancers 2017, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xue, Y.; Xi, Y.R.; Xie, K. Progress in the application and mechanism of metformin in treating non-small cell lung cancer. Oncol. Lett. 2017, 13, 2873–2880. [Google Scholar] [CrossRef]
- Farmer, R.E.; Ford, D.; Forbes, H.J.; Chaturvedi, N.; Kaplan, R.; Smeeth, L.; Bhaskaran, K. Metformin and cancer in type 2 diabetes: A systematic review and comprehensive bias evaluation. Int. J. Epidemiol. 2017, 46, 745. [Google Scholar] [CrossRef] [PubMed]
- Seliger, C.; Luber, C.; Gerken, M.; Schaertl, J.; Proescholdt, M.; Riemenschneider, M.J.; Meier, C.R.; Bogdahn, U.; Leitzmann, M.F.; Klinkhammer-Schalke, M.; et al. Use of metformin and survival of patients with high-grade glioma. Int. J. Cancer 2019, 144, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Schiff, D.; Wen, P.Y.; van den Bent, M.J. Neurological adverse effects caused by cytotoxic and targeted therapies. Nat. Rev. Clin. Oncol. 2009, 6, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Iannantuoni, F.; Gruevska, A.; Muntane, J.; Rocha, M.; Victor, V.M. Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020, 34, 101517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dutta, S.; Shah, R.B.; Singhal, S.; Dutta, S.B.; Bansal, S.; Sinha, S.; Haque, M. Metformin: A Review of Potential Mechanism and Therapeutic Utility Beyond Diabetes. Drug Des. Dev. Ther. 2023, 17, 1907–1932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; McGirt, M.J.; Woodworth, G.F.; Datoo, G.; Tamargo, R.J.; Weingart, J.; Olivi, A.; Brem, H.; Quinones-Hinojosa, A. Persistent outpatient hyperglycemia is independently associated with survival, recurrence and malignant degeneration following surgery for hemispheric low grade gliomas. Neurol. Res. 2010, 32, 442–448. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and its benefits for various diseases. Front. Endocrinol. 2020, 11, 490991. [Google Scholar] [CrossRef]
- Jiralerspong, S.; Palla, S.L.; Giordano, S.H.; Meric-Bernstam, F.; Liedtke, C.; Barnett, C.M.; Hsu, L.; Hung, M.C.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Gritti, M.; Würth, R.; Angelini, M.; Barbieri, F.; Peretti, M.; Pizzi, E.; Pattarozzi, A.; Carra, E.; Sirito, R.; Daga, A.; et al. Metformin repositioning as antitumoral agent: Selective antiproliferative effects in human glioblastoma stem cells, via inhibition of CLIC1-mediated ion current. Oncotarget 2014, 5, 11252–11268. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Sahra, I.B.; Le Marchand-Brustel, Y.; Tanti, J.F. Metformin and cancer therapy. Curr. Opin. Oncol. 2012, 24, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.Z.; Dias, M.M.; Ropelle, E.R.; Osório-Costa, F.; Rossato, F.A.; Vercesi, A.E.; Saad, M.J.; Carvalheira, J.B. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 3993–4005. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Sunayama, J.; Okada, M.; Watanabe, E.; Seino, S.; Shibuya, K.; Suzuki, K.; Narita, Y.; Shibui, S.; Kayama, T.; et al. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl. Med. 2012, 1, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Karpel-Massler, G.; Halatsch, M.E. Can the therapeutic effects of temozolomide be potentiated by stimulating AMP-activated protein kinase with olanzepine and metformin? Br. J. Pharmacol. 2011, 164, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Würth, R.; Pattarozzi, A.; Gatti, M.; Bajetto, A.; Corsaro, A.; Parodi, A.; Sirito, R.; Massollo, M.; Marini, C.; Zona, G.; et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: A role for metformin-induced inhibition of Akt. Cell Cycle 2013, 12, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Vincent, E.E.; Poffenberger, M.C.; Jones, R.G. The AMP-activated protein kinase (AMPK) and cancer: Many faces of a metabolic regulator. Cancer Lett. 2015, 356, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in cancer: A molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef]
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef]
- Joyce, C.; Rayi, A.; Kasi, A. Tumor-Suppressor Genes. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Zhang, Y.; Dube, C.; Gibert, M., Jr.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The p53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef]
- Cerezo, M.; Tichet, M.; Abbe, P.; Ohanna, M.; Lehraiki, A.; Rouaud, F.; Allegra, M.; Giacchero, D.; Bahadoran, P.; Bertolotto, C.; et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol. Cancer Ther. 2013, 12, 1605–1615. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Skuli, S.J.; Alomari, S.; Gaitsch, H.; Bakayoko, A.; Skuli, N.; Tyler, B.M. Metformin and cancer, an ambiguanidous relationship. Pharmaceuticals 2022, 15, 626. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Hawley, S.A.; Scott, J.W. AMP-activated protein kinase—development of the energy sensor concept. J. Physiol. 2006, 574 Pt 1, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ben Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef]
- Pistollato, F.; Rampazzo, E.; Abbadi, S.; Della Puppa, A.; Scienza, R.; D’Avella, D.; Denaro, L.; Te Kronnie, G.; Panchision, D.M.; Basso, G. Molecular mechanisms of HIF-1alpha modulation induced by oxygen tension and BMP2 in glioblastoma derived cells. PLoS ONE 2009, 4, e6206. [Google Scholar] [CrossRef] [PubMed]
- Sesen, J.; Dahan, P.; Scotland, S.J.; Saland, E.; Dang, V.T.; Lemarié, A.; Tyler, B.M.; Brem, H.; Toulas, C.; Cohen-Jonathan Moyal, E.; et al. Metformin inhibits growth of human glioblastoma cells and enhances therapeutic response. PLoS ONE 2015, 10, e0123721. [Google Scholar] [CrossRef]
- Dowling, R.J.; Zakikhani, M.; Fantus, I.G.; Pollak, M.; Sonenberg, N. Metformin inhibits mammalian target of ra-pamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007, 67, 10804–10812. [Google Scholar] [CrossRef]
- Guarnaccia, L.; Navone, S.E.; Masseroli, M.M.; Balsamo, M.; Caroli, M.; Valtorta, S.; Moresco, R.M.; Campanella, R.; Schisano, L.; Fiore, G.; et al. Effects of Metformin as Add-On Therapy against Glioblastoma: An Old Medicine for Novel Oncology Therapeutics. Cancers 2022, 14, 1412. [Google Scholar] [CrossRef]
- Mazurek, M.; Litak, J.; Kamieniak, P.; Kulesza, B.; Jonak, K.; Baj, J.; Grochowski, C. Metformin as Potential Therapy for High-Grade Glioma. Cancers 2020, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chen, Q.; Li, X.; Zeng, Z.; Xiong, W.; Li, G.; Li, X.; Yang, J.; Xiang, B.; Yi, M. Pyroptosis: A new paradigm of cell death for fighting against cancer. J. Exp. Clin. Cancer Res. 2021, 40, 153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Bian, Y.; Zhang, Y.; Ren, G.; Li, G. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle 2020, 19, 1089–1104. [Google Scholar] [CrossRef]
- Stummer, W. Mechanisms of tumor-related brain edema. Neurosurg. Focus 2007, 22, E8. [Google Scholar] [CrossRef]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Takata, F.; Dohgu, S.; Matsumoto, J.; Machida, T.; Kaneshima, S.; Matsuo, M.; Sakaguchi, S.; Takeshige, Y.; Yamauchi, A.; Kataoka, Y. Metformin induces up-regulation of blood-brain barrier functions by activating AMP-activated protein kinase in rat brain microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2013, 433, 586–590. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Kreso, A.; Jamieson, C.H. Cancer stem cells and self-renewal. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 3113–3120. [Google Scholar] [CrossRef]
- Galli, R.; Binda, E.; Orfanelli, U.; Cipelletti, B.; Gritti, A.; De Vitis, S.; Fiocco, R.; Foroni, C.; Dimeco, F.; Vescovi, A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004, 64, 7011–7021. [Google Scholar] [CrossRef]
- Sunayama, J.; Sato, A.; Matsuda, K.; Tachibana, K.; Watanabe, E.; Seino, S.; Suzuki, K.; Narita, Y.; Shibui, S.; Sakurada, K.; et al. FoxO3a functions as a key integrator of cellular signals that control glioblastoma stem-like cell differentiation and tumorigenicity. Stem Cells 2011, 29, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Setti, M.; Savalli, N.; Osti, D.; Richichi, C.; Angelini, M.; Brescia, P.; Fornasari, L.; Carro, M.S.; Mazzanti, M.; Pelicci, G. Functional role of CLIC1 ion channel in glioblastoma-derived stem/progenitor cells. J. Natl. Cancer Inst. 2013, 105, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Bosio, A.G.; Pattarozzi, A.; Tonelli, M.; Bajetto, A.; Verduci, I.; Cianci, F.; Cannavale, G.; Palloni, L.M.G.; Francesconi, V.; et al. Chloride intracellular channel 1 activity is not required for glioblastoma development but its inhibition dictates glioma stem cell responsivity to novel biguanide derivatives. J. Exp. Clin. Cancer Res. 2022, 41, 53. [Google Scholar] [CrossRef] [PubMed]
- Ugwueze, C.V.; Ogamba, O.J.; Young, E.E.; Onyenekwe, B.M.; Ezeokpo, B.C. Metformin: A Possible Option in Cancer Chemotherapy. Anal. Cell. Pathol. 2020, 2020, 7180923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al Hassan, M.; Fakhoury, I.; El Masri, Z.; Ghazale, N.; Dennaoui, R.; El Atat, O.; Kanaan, A.; El-Sibai, M. Metformin Treatment Inhibits Motility and Invasion of Glioblastoma Cancer Cells. Anal. Cell. Pathol. 2018, 2018, 5917470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, E.H.; Lee, J.H.; Oh, Y.; Koh, I.; Shim, J.K.; Park, J.; Choi, J.; Yun, M.; Jeon, J.Y.; Huh, Y.M.; et al. Inhibition of glioblastoma tumorspheres by combined treatment with 2-deoxyglucose and metformin. Neuro Oncol. 2017, 19, 197–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Z.; Zhao, G.; Li, P.; Li, Y.; Zhou, G.; Chen, Y.; Xie, G. Temozolomide in combination with metformin act synergistically to inhibit proliferation and expansion of glioma stem-like cells. Oncol. Lett. 2016, 11, 2792–2800. [Google Scholar] [CrossRef]
- Adeberg, S.; Bernhardt, D.; Ben Harrabi, S.; Bostel, T.; Mohr, A.; Koelsche, C.; Diehl, C.; Rieken, S.; Debus, J. Metformin influences progression in diabetic glioblastoma patients. Strahlenther. Onkol. 2015, 191, 928–935. [Google Scholar] [CrossRef]
- Elmaci, İ.; Altinoz, M.A. A Metabolic Inhibitory Cocktail for Grave Cancers: Metformin, Pioglitazone and Lithium Combination in Treatment of Pancreatic Cancer and Glioblastoma Multiforme. Biochem. Genet. 2016, 54, 573–618. [Google Scholar] [CrossRef] [PubMed]
- Soritau, O.; Tomuleasa, C.; Aldea, M.; Petrushev, B.; Susman, S.; Gheban, D.; Ioani, H.; Cosis, A.; Brie, I.; Irimie, A.; et al. Metformin plus temozolomide-based chemotherapy as adjuvant treatment for WHO grade III and IV malignant gliomas. J. Buon 2011, 16, 282–289. [Google Scholar] [PubMed]
- Seliger, C.; Rauer, L.; Wüster, A.L.; Moeckel, S.; Leidgens, V.; Jachnik, B.; Ammer, L.M.; Heckscher, S.; Dettmer, K.; Riemenschneider, M.J.; et al. Heterogeneity of Amino Acid Profiles of Proneural and Mesenchymal Brain-Tumor Initiating Cells. Int. J. Mol. Sci. 2023, 24, 3199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mayer, A.; Vaupel, P.; Struss, H.G.; Giese, A.; Stockinger, M.; Schmidberger, H. Strong adverse prognostic impact of hyperglycemic episodes during adjuvant chemoradiotherapy of glioblastoma multiforme. Strahlenther. Onkol. 2014, 190, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Bowker, S.L.; Yasui, Y.; Veugelers, P.; Johnson, J.A. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: Assessing effects of time-varying exposure. Diabetologia 2010, 53, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.; Than, K.; Ruiz, A.J.; Olivi, A.; Quiñones-Hinojosa, A. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery 2008, 63, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Chambless, L.B.; Parker, S.L.; Hassam-Malani, L.; McGirt, M.J.; Thompson, R.C. Type 2 diabetes mellitus and obesity are independent risk factors for poor outcome in patients with high-grade glioma. J. Neuro Oncol. 2012, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.R.; Grommes, C. Retrospective analysis of the effects of steroid therapy and antidiabetic medication on survival in diabetic glioblastoma patients. CNS Oncol. 2013, 2, 237–246. [Google Scholar] [CrossRef]
- Mohammad, A.H.; Jatana, S.; Ruiz-Barerra, M.A.; Khalaf, R.; Al-Saadi, T.; Diaz, R.J. Metformin use is associated with longer survival in glioblastoma patients with MGMT gene silencing. J. Neurooncol. 2023, 165, 209–218. [Google Scholar] [CrossRef]
- Grimaldi, C.; Chiarini, F.; Tabellini, G.; Ricci, F.; Tazzari, P.L.; Battistelli, M.; Martelli, A.M. AMP-dependent kinase/mammalian target of rapamycin complex 1 signaling in T-cell acute lymphoblastic leukemia: Therapeutic implications. Leukemia 2012, 26, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Gerthofer, V.; Kreutz, M.; Renner, K.; Jachnik, B.; Dettmer, K.; Oefner, P.; Riemenschneider, M.J.; Proescholdt, M.; Vollmann-Zwerenz, A.; Hau, P.; et al. Combined Modulation of Tumor Metabolism by Metformin and Diclofenac in Glioma. Int. J. Mol. Sci. 2018, 19, 2586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Z.; Zhao, G.; Xie, G.; Zhao, L.; Chen, Y.; Yu, H.; Zhang, Z.; Li, C.; Li, Y. Metformin and temozolomide act synergistically to inhibit growth of glioma cells and glioma stem cells in vitro and in vivo. Oncotarget 2015, 6, 32930–32943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Title | Country | Population (n) | Outcomes/Objectives | Trial No. |
|---|---|---|---|---|
| Temozolomide, Memantine Hydrochloride, Mefloquine, and Metformin Hydrochloride in Treating Patients With Glioblastoma Multiforme After Radiation Therapy | USA | 144 | The purpose of this study was to determine the safety and tolerability of temozolomide (TMZ) in combination with metformin (metformin hydrochloride) (MFRMN), mefloquine (MFLOQ), and/or memantine (memantine hydrochloride) (MEMTN) in patients receiving adjuvant therapy after completing external beam radiotherapy (XRT) in combination with chemotherapy for newly diagnosed glioblastoma multiforme. | NCI-2011-03038 |
| Metformin, Neo-adjuvant Temozolomide, and Hypo- hypo-accelerated radiotherapy Followed by Adjuvant TMZ in Patients With GBM | Canada | 50 | It is expected that the proposed study treatment will improve the median survival from current values of 20 months (current MUHC neoadjuvant Phase 2 data) to 25 months. This means an improved outcome of 25%. Using one-tailed statistics, with a power of 0.8 and an alpha of 0.05, the sample size for this Phase II trial will be 50 patients. | NCT02780024 |
| Bioavailability of Disulfiram and Metformin in Glioblastomas | Sweden | 3 | Does the drug get there? Does the drug perform what it is intended to perform? To improve the chances of clinical success, there is a need for the rational and intelligent selection of potential drugs in future trials. This is an initiative for analyzing the tumor concentration of preoperatively administered repurposed drugs. | NCT03151772 |
| A Pilot Study of Ketogenic Diet and Metformin in Glioblastoma: Feasibility and Metabolic Imaging | USA | 36 | Not published. | NCT04691960 |
| Study on Low Dose Temozolomide Plus Metformin or Placebo in Patient With Recurrent or Refractory Glioblastoma | South Korea | 81 | A comparison of progression-free survival obtained from the progression-free survival curve. | NCT03243851 |
| Phase 2, Open-label, Single-arm Study on the Use of Metformin as Adjunctive Therapy in High-grade Glioma | Italy | 25 | The clinical trial will be single-arm to evaluate the biological activity and effects of metformin in combination with TMZ in patients with GBM. The efficacy at the recommended dose (RD) of metformin in patients with GBM. | NCT05929495 |
| Oxidative Phosphorylation Targeting In Malignant Glioma Using Metformin Plus Radiotherapy Temozolomide | France | 640 | Progression-free survival (PFS) estimated by the RANO (Response Assessment in Neuro-Oncology) criteria. | NCT04945148 |
| Treatment of Recurrent Brain Tumors: Metabolic Manipulation Combined With Radiotherapy | Israel | 18 | The short-term implementation of the metabolic intervention (i.e., combined diet and metformin therapy) before, during, and after hypofractionated (2 weeks) radiation therapy is expected to increase tolerability, increase compliance, and avoid the chronic metabolic complications associated with extreme carbohydrate-restriction diets. | NCT02149459 |
| Country | Study ID | Population (n) | Objectives/Outcomes | Treatment |
|---|---|---|---|---|
| France and Italy | NCT04945148 | 640 | The investigators have observed in vivo (a reduction of >50% of tumor growth) and hypothesize that metformin could be specifically efficient to treat up-front patients affected by OXPHOS+ GBM, in association with the standard first-line treatment with radiotherapy and temozolomide (RT-TMZ). | Metformin (1500–3000 mg daily) plus radiation and temozolomide |
| Israel | NCT02149459 | 18 | To enhance the radiosensitivity of recurrent brain tumors through metabolic reprogramming induced by a low-carbohydrate diet and metformin therapy. | Metformin, radiation, and low carbohydrate diet |
| Canada | NCT02780024 | 50 | This trial seeks to validate the adjunctive use of metformin with established GBM therapies, hypothesizing that its metabolic effects will synergistically enhance the efficacy of standard treatment protocols, thereby significantly improving patient survival outcomes. | Metformin and neoadjuvant temozolomide followed by combined radiation and temozolomide |
| USA | NCT04691960 | 36 | The purpose of the study is to evaluate the tolerability of a ketogenic diet in conjunction with metformin and whether maintaining and the diet with metformin will have any effect on participants. Participants will prepare their own meals with the help of a nutritionist. Participants will continue on treatment as long as they are responding to therapy and not experiencing unacceptable side effects. | Metformin (ramp up to 850 mg three times daily) and ketogenic diet |
| USA | NCT05183204 | 33 | The purpose of this study is to assess the safety of Paxalisib while maintaining a ketogenic diet (a high-fat, low-carbohydrate diet) and metformin (a drug approved by the Food and Drug Administration to treat type 2 diabetes) and to see what effects it has on glioblastoma. | Metformin (ramp up to 850 mg three times daily as tolerated), and ketogenic diet |
| USA | NCT01430351 | 144 | From the study entry, the median survival was 21 months, and the 2-year survival rate was 43%. Memantine, mefloquine, and metformin can be combined safely with TMZ in patients with newly diagnosed glioblastoma. | Metformin (dose not specified), mefloquine, memantine, hydrochloride, hydrochloride, and temozolomide |
| South Korea | NCT03243851 | 81 | Although the metformin plus temozolomide regimen was well tolerated, it did not confer a clinical benefit in patients with recurrent or refractory GBM. | Metformin (ramp up to 2000 mg daily) and low-dose temozolomide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.; Mansour, H.M.; Aguilar, T.M.; Lucke-Wold, B. Advances in Anti-Cancer Drug Development: Metformin as Anti-Angiogenic Supplemental Treatment for Glioblastoma. Int. J. Mol. Sci. 2024, 25, 5694. https://doi.org/10.3390/ijms25115694
Shah S, Mansour HM, Aguilar TM, Lucke-Wold B. Advances in Anti-Cancer Drug Development: Metformin as Anti-Angiogenic Supplemental Treatment for Glioblastoma. International Journal of Molecular Sciences. 2024; 25(11):5694. https://doi.org/10.3390/ijms25115694
Chicago/Turabian StyleShah, Siddharth, Hadeel M. Mansour, Tania M. Aguilar, and Brandon Lucke-Wold. 2024. "Advances in Anti-Cancer Drug Development: Metformin as Anti-Angiogenic Supplemental Treatment for Glioblastoma" International Journal of Molecular Sciences 25, no. 11: 5694. https://doi.org/10.3390/ijms25115694
APA StyleShah, S., Mansour, H. M., Aguilar, T. M., & Lucke-Wold, B. (2024). Advances in Anti-Cancer Drug Development: Metformin as Anti-Angiogenic Supplemental Treatment for Glioblastoma. International Journal of Molecular Sciences, 25(11), 5694. https://doi.org/10.3390/ijms25115694







