Differences in Metabolite Profiles of Dihydroberberine and Micellar Berberine in Caco-2 Cells and Humans—A Pilot Study
Abstract
1. Introduction
2. Results
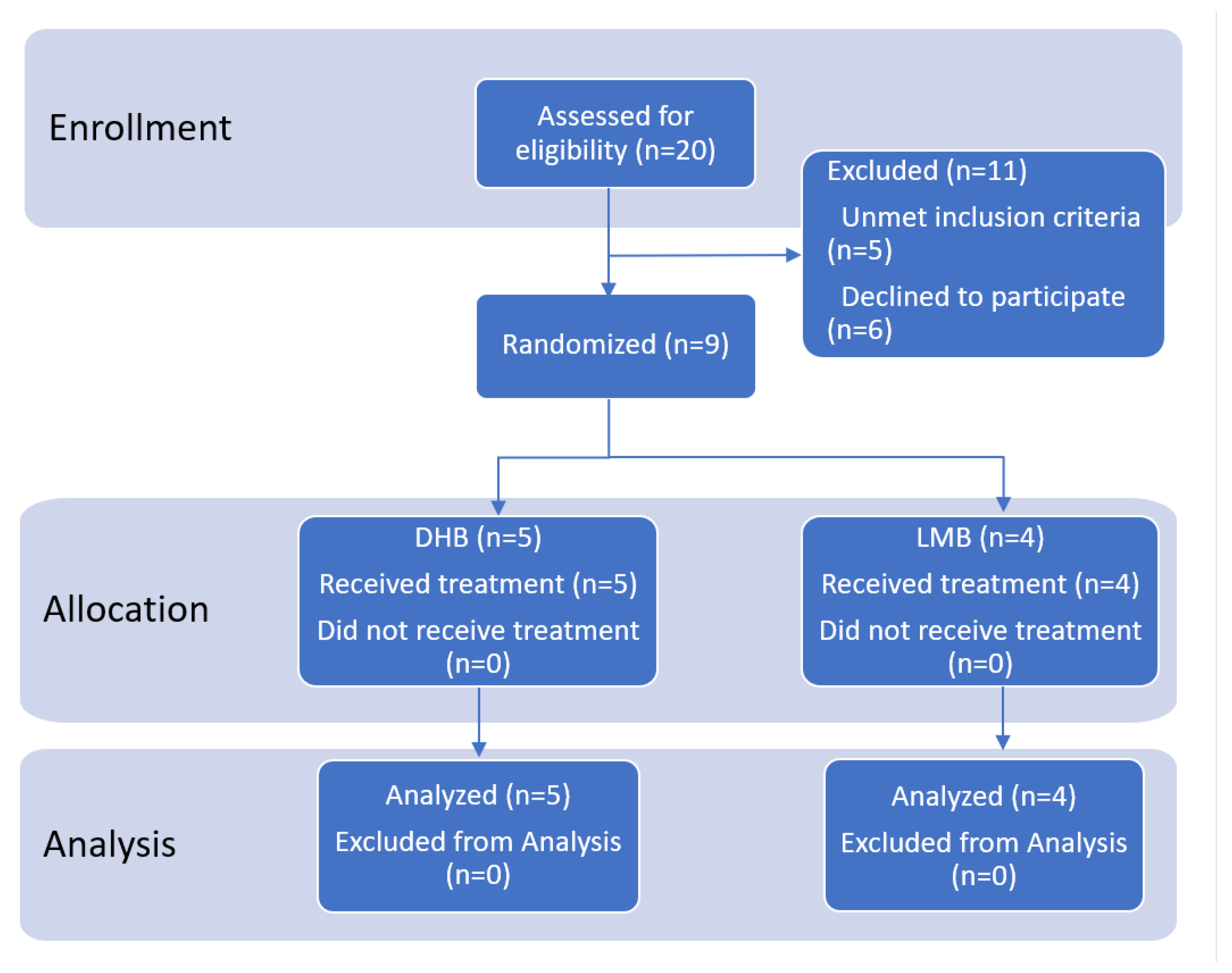
2.1. Participant Flowchart
2.2. Baseline Characteristics
2.3. Side Effects
2.4. Pharmacokinetics of Berberine and Metabolites in Human Blood
Pharmacokinetic Correlation of Metabolites
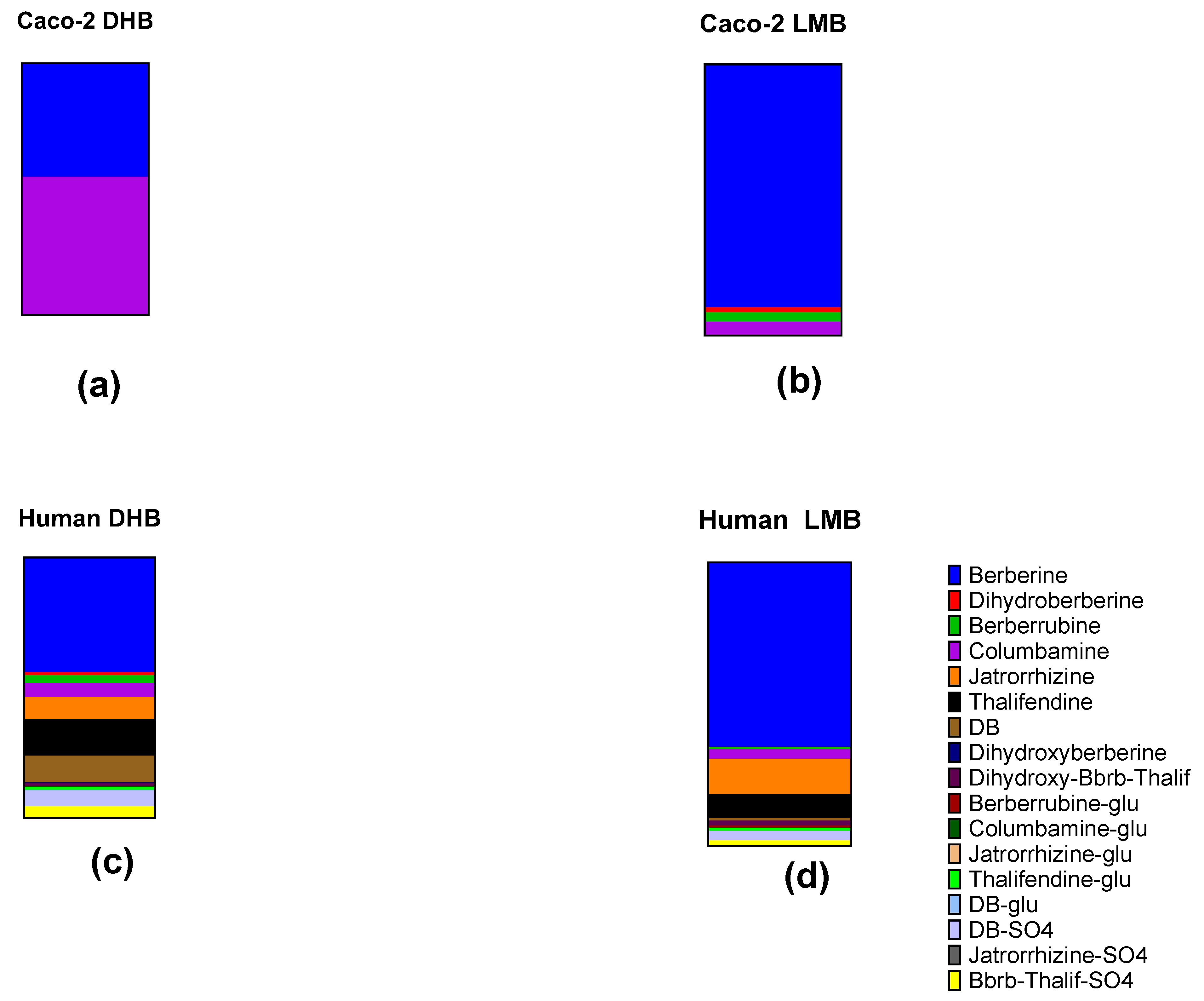
2.5. Metabolites from Caco-2 Cell Cultures
Comparison with Human Blood
3. Discussion
4. Materials and Methods
4.1. Study Treatments
4.2. Study Participants
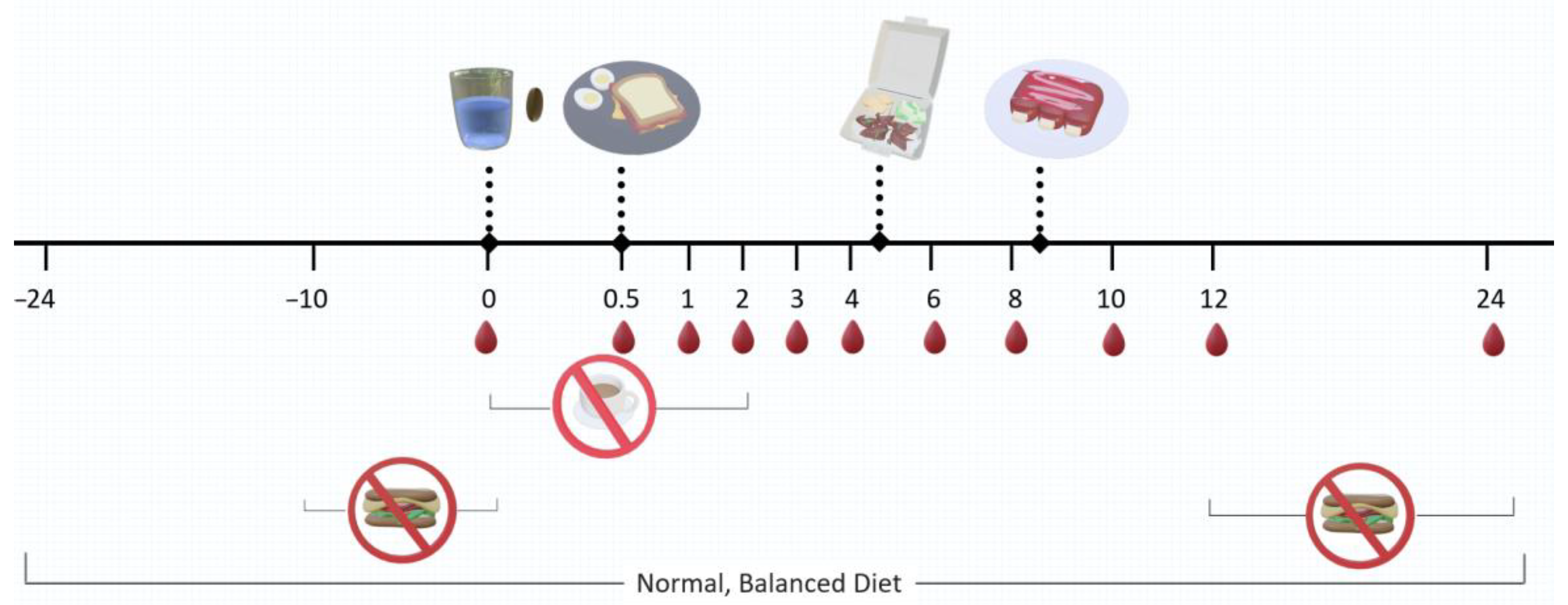
4.3. Study Design
4.4. Blood Collection and Sample Preparation
4.5. Cell Cultures
4.6. Analysis of Berberine and Metabolites
4.7. Data Analysis and Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current Knowledge and Pharmacological Profile of Berberine: An Update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
- Grycová, L.; Dostál, J.; Marek, R. Quaternary Protoberberine Alkaloids. Phytochemistry 2007, 68, 150–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.-D.; Zhao, W.; Wang, Z.-Z.; Wang, S.-K.; Zhou, Z.-X.; Song, D.-Q.; Wang, Y.-M.; et al. Berberine Lowers Blood Glucose in Type 2 Diabetes Mellitus Patients through Increasing Insulin Receptor Expression. Metabolism 2010, 59, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of Berberine on Blood Glucose in Patients with Type 2 Diabetes Mellitus: A Systematic Literature Review and a Meta-Analysis. Endocr. J. 2019, 66, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, X.; Wu, N.; Han, Y.; Wang, J.; Yu, Y.; Chen, Q. Efficacy and Safety of Berberine Alone for Several Metabolic Disorders: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2021, 12, 653887. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, M.; Zhao, Y.; Gong, J.; Su, H.; Yuan, F.; Fang, K.; Yuan, X.; Yu, X.; Dong, H.; et al. Berberine Protects against Diabetic Kidney Disease via Promoting PGC-1α-Regulated Mitochondrial Energy Homeostasis. Br. J. Pharmacol. 2020, 177, 3646–3661. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Šmejkal, K.; Malaník, M.; et al. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef]
- Sun, H.; Wang, H.; Zhang, A.; Yan, G.; Zhang, Y.; An, N.; Wang, X. Berberine Ameliorates Nonbacterial Prostatitis via Multi-Target Metabolic Network Regulation. OMICS 2015, 19, 186–195. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Yang, W. Role of Berberine in Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.-H.; Abdollahi, M.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Berberine and Neurodegeneration: A Review of Literature. Pharmacol. Rep. 2015, 67, 970–979. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Rahmani, A.H. Berberine: An Important Emphasis on Its Anticancer Effects through Modulation of Various Cell Signaling Pathways. Molecules 2022, 27, 5889. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef]
- Och, A.; Podgórski, R.; Nowak, R. Biological Activity of Berberine—A Summary Update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ye, J.; Jia, W. Effects and Mechanisms of Berberine in Diabetes Treatment. Acta Pharm. Sin. B 2012, 2, 327–334. [Google Scholar] [CrossRef]
- Yang, F.; Gao, R.; Luo, X.; Liu, R.; Xiong, D. Berberine Influences Multiple Diseases by Modifying Gut Microbiota. Front. Nutr. 2023, 10, 1187718. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Interactions between Gut Microbiota and Berberine, a Necessary Procedure to Understand the Mechanisms of Berberine. J. Pharm. Anal. 2022, 12, 541–555. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, W.; Wu, S.; Zheng, H.-M.; Li, P.; Sheng, H.-F.; Chen, M.-X.; Chen, Z.-H.; Ji, G.-Y.; Zheng, Z.-D.-X.; et al. Linking Gut Microbiota, Metabolic Syndrome and Economic Status Based on a Population-Level Analysis. Microbiome 2018, 6, 172. [Google Scholar] [CrossRef]
- Cao, J.; Chen, M.; Xu, R.; Guo, M. Therapeutic Mechanisms of Berberine to Improve the Intestinal Barrier Function via Modulating Gut Microbiota, TLR4/NF-κ B/MTORC Pathway and Autophagy in Cats. Front. Microbiol. 2022, 13, 961885. [Google Scholar] [CrossRef]
- Gong, J.; Hu, M.; Huang, Z.; Fang, K.; Wang, D.; Chen, Q.; Li, J.; Yang, D.; Zou, X.; Xu, L.; et al. Berberine Attenuates Intestinal Mucosal Barrier Dysfunction in Type 2 Diabetic Rats. Front. Pharmacol. 2017, 8, 42. [Google Scholar] [CrossRef]
- Dehau, T.; Cherlet, M.; Croubels, S.; Van De Vliet, M.; Goossens, E.; Van Immerseel, F. Berberine-Microbiota Interplay: Orchestrating Gut Health through Modulation of the Gut Microbiota and Metabolic Transformation into Bioactive Metabolites. Front. Pharmacol. 2023, 14, 1281090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Y.; Yan, X.; Wang, W.; Tian, X.; Wang, L.; Zhu, W.; Gong, L.; Pan, G. Different Structures of Berberine and Five Other Protoberberine Alkaloids That Affect P-Glycoprotein-Mediated Efflux Capacity. Acta Pharmacol. Sin. 2019, 40, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The Metabolism of Berberine and Its Contribution to the Pharmacological Effects. Drug Metab. Rev. 2017, 49, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Chen, Y.; Chen, J.; Liao, H.; Li, Y.; Ma, Y. Jatrorrhizine: A Review of Sources, Pharmacology, Pharmacokinetics and Toxicity. Front. Pharmacol. 2022, 12, 783127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Zhang, M.N.; Wang, H.G.; Yang, X.Z.; Yu, C.G. Jatrorrhizine Alleviates Ulcerative Colitis via Regulating Gut Microbiota and NOS2 Expression. Gut Pathog. 2022, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, W.; Ouyang, T.; Shen, X.-Y.; Wang, F.; Qu, Y.; Zhang, M.; Luo, T.; Wang, H.-Q. Jatrorrhizine Balances the Gut Microbiota and Reverses Learning and Memory Deficits in APP/PS1 Transgenic Mice. Sci. Rep. 2019, 9, 19575. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, R.-Y.; Shi, M.-J.; Zhao, Y.-X.; Yan, Y.; Xu, X.-X.; Zhang, M.; Zhao, X.-T.; Zhang, Y.-B. Demethyleneberberine Alleviates Inflammatory Bowel Disease in Mice through Regulating NF-κB Signaling and T-Helper Cell Homeostasis. Inflamm. Res. 2017, 66, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Saklani, P.; Khan, H.; Singh, T.G.; Gupta, S.; Grewal, A.K. Demethyleneberberine, a Potential Therapeutic Agent in Neurodegenerative Disorders: A Proposed Mechanistic Insight. Mol. Biol. Rep. 2022, 49, 10101–10113. [Google Scholar] [CrossRef]
- Tan, X.-S.; Ma, J.-Y.; Feng, R.; Ma, C.; Chen, W.-J.; Sun, Y.-P.; Fu, J.; Huang, M.; He, C.-Y.; Shou, J.-W.; et al. Tissue Distribution of Berberine and Its Metabolites after Oral Administration in Rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef]
- Cao, S.; Xu, P.; Yan, J.; Liu, H.; Liu, L.; Cheng, L.; Qiu, F.; Kang, N. Berberrubine and Its Analog, Hydroxypropyl-berberrubine, Regulate LDLR and PCSK9 Expression via the ERK Signal Pathway to Exert Cholesterol-lowering Effects in Human Hepatoma HepG2 Cells. J. Cell. Biochem. 2018, 120. [Google Scholar] [CrossRef]
- Sun, R.; Kong, B.; Yang, N.; Cao, B.; Feng, D.; Yu, X.; Ge, C.; Feng, S.; Fei, F.; Huang, J.; et al. The Hypoglycemic Effect of Berberine and Berberrubine Involves Modulation of Intestinal Farnesoid X Receptor Signaling Pathway and Inhibition of Hepatic Gluconeogenesis. Drug Metab. Dispos. 2021, 49, 276–286. [Google Scholar] [CrossRef]
- Jeon, Y.W.; Jung, J.W.; Kang, M.; Chung, I.K.; Lee, W. NMR Studies on Antitumor Drug Candidates, Berberine and Berberrubine. Bull. Korean Chem. Soc. 2002, 23, 391–394. [Google Scholar] [CrossRef]
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A Systematic Review of the Anticancer Properties of Berberine, a Natural Product from Chinese Herbs. Anti-Cancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef]
- Yang, N.; Sun, R.; Chen, X.; Zhen, L.; Ge, C.; Zhao, Y.; He, J.; Geng, J.; Guo, J.; Yu, X.; et al. In Vitro Assessment of the Glucose-Lowering Effects of Berberrubine-9-O-β-D-Glucuronide, an Active Metabolite of Berberrubine. Acta Pharmacol. Sin. 2017, 38, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, G.; Wang, Y.-X.; Kong, W.-J.; Yang, P.; Wang, Y.-M.; Li, Y.-H.; Yi, H.; Li, Z.-R.; Song, D.-Q.; et al. Bioactivities of Berberine Metabolites after Transformation through CYP450 Isoenzymes. J. Transl. Med. 2011, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, Y.; Ai, G.; Luo, C.; Chen, H.; Li, C.; Zeng, H.; Xie, J.; Chen, J.; Su, Z. Dihydroberberine, a Hydrogenated Derivative of Berberine Firstly Identified in Phellodendri Chinese Cortex, Exerts Anti-Inflammatory Effect via Dual Modulation of NF-κB and MAPK Signaling Pathways. Int. Immunopharmacol. 2019, 75, 105802. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Shou, J.-W.; Zhao, Z.-X.; He, C.-Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.-S.; Li, X.-Y.; Wen, B.-Y.; et al. Transforming Berberine into Its Intestine-Absorbable Form by the Gut Microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef]
- Buchanan, B.; Meng, Q.; Poulin, M.-M.; Zuccolo, J.; Azike, C.G.; Gabriele, J.; Baranowski, D.C. Comparative Pharmacokinetics and Safety Assessment of Transdermal Berberine and Dihydroberberine. PLoS ONE 2018, 13, e0194979. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.M.; Ratliff, K.M.; Hagele, A.M.; Stecker, R.A.; Mumford, P.W.; Kerksick, C.M. Absorption Kinetics of Berberine and Dihydroberberine and Their Impact on Glycemia: A Randomized, Controlled, Crossover Pilot Trial. Nutrients 2021, 14, 124. [Google Scholar] [CrossRef]
- Solnier, J.; Zhang, Y.; Kuo, Y.C.; Du, M.; Roh, K.; Gahler, R.; Wood, S.; Chang, C. Characterization and Pharmacokinetic Assessment of a New Berberine Formulation with Enhanced Absorption In Vitro and in Human Volunteers. Pharmaceutics 2023, 15, 2567. [Google Scholar] [CrossRef]
- Wang, Y.; Zidichouski, J.A. Update on the Benefits and Mechanisms of Action of the Bioactive Vegetal Alkaloid Berberine on Lipid Metabolism and Homeostasis. Cholesterol 2018, 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Yu, P.; Peng, L.; Luo, L.; Liu, J.; Li, S.; Lai, X.; Luan, F.; Meng, X. Berberine: A Review of Its Pharmacokinetics Properties and Therapeutic Potentials in Diverse Vascular Diseases. Front. Pharmacol. 2021, 12, 762654. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Tenori, L.; Cascante, M.; De Atauri Carulla, P.R.; Martins Dos Santos, V.A.P.; Saccenti, E. From Correlation to Causation: Analysis of Metabolomics Data Using Systems Biology Approaches. Metabolomics 2018, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Jahagirdar, S.; Saccenti, E. On the Use of Correlation and MI as a Measure of Metabolite—Metabolite Association for Network Differential Connectivity Analysis. Metabolites 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Whomsley, R.; Poggesi, I.; Cawello, W.; Mathy, F.-X.; Delporte, M.-L.; Papeleu, P.; Watelet, J.-B. Drug Metabolism and Pharmacokinetics. Drug Metab. Rev. 2009, 41, 344–390. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, Q.; Yang, M.; Liu, D.; Hou, X.; Tang, L.; Wang, X.; Lyu, Y.; Chen, X.; Liu, K.; et al. Current Trends in Drug Metabolism and Pharmacokinetics. Acta Pharm. Sin. B 2019, 9, 1113–1144. [Google Scholar] [CrossRef]
- Yang, S.; Cao, S.; Li, C.; Zhang, J.; Liu, C.; Qiu, F.; Kang, N. Berberrubine, a Main Metabolite of Berberine, Alleviates Non-Alcoholic Fatty Liver Disease via Modulating Glucose and Lipid Metabolism and Restoring Gut Microbiota. Front. Pharmacol. 2022, 13, 913378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, P.; Luan, H.; Jiang, H.; Xu, Y.; Zhang, Y.; Zhang, Y.; Li, R. Demethyleneberberine Alleviated the Inflammatory Response by Targeting MD-2 to Inhibit the TLR4 Signaling. Front. Immunol. 2023, 14, 1130404. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, K.; Hong, Y.; Gong, Y.; Ni, J.; Yang, N.; Ding, W. A New Perspective on the Antimicrobial Mechanism of Berberine Hydrochloride Against Staphylococcus Aureus Revealed by Untargeted Metabolomic Studies. Front. Microbiol. 2022, 13, 917414. [Google Scholar] [CrossRef]
- Fang, X.; Wu, H.; Wang, X.; Lian, F.; Li, M.; Miao, R.; Wei, J.; Tian, J. Modulation of Gut Microbiota and Metabolites by Berberine in Treating Mice With Disturbances in Glucose and Lipid Metabolism. Front. Pharmacol. 2022, 13, 870407. [Google Scholar] [CrossRef]
- Cui, H.-M.; Zhang, Q.-Y.; Wang, J.-L.; Chen, J.-L.; Zhang, Y.-L.; Tong, X.-L. In Vitro Studies of Berberine Metabolism and Its Effect of Enzyme Induction on HepG2 Cells. J. Ethnopharmacol. 2014, 158, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lin, H.; Huang, W. Modulating Gut Microbiota as an Anti-Diabetic Mechanism of Berberine. Med. Sci. Monit. 2011, 17, RA164–RA167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Zheng, M.; Yang, Y.; Ren, H.; Kong, Y.; Wang, S.; Wang, J.; Jiang, Y.; Yang, J.; et al. Berberine Slows the Progression of Prediabetes to Diabetes in Zucker Diabetic Fatty Rats by Enhancing Intestinal Secretion of Glucagon-Like Peptide-2 and Improving the Gut Microbiota. Front. Endocrinol. 2021, 12, 609134. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Yang, J.; Kong, Y.; Wang, X.; Zheng, M.; Xu, Y.-G.; Wang, Y.; Ren, H.; Chang, B.-C.; Chen, L. Alteration of the Intestinal Barrier and GLP2 Secretion in Berberine Treated Type 2 Diabetic Rats. J. Endocrinol. 2013, 218, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yu, J.; Yang, Y.; Zhang, F.; Su, W.; Fan, Q.; Wu, C.; Wu, S. Berberine, a Potential Prebiotic to Indirectly Promote Akkermansia Growth through Stimulating Gut Mucin Secretion. Biomed. Pharmacother. 2021, 139, 111595. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia Muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ni, Y.; Qian, L.; Fang, Q.; Zheng, T.; Zhang, M.; Gao, Q.; Zhang, Y.; Ni, J.; Hou, X.; et al. Decreased Abundance of Akkermansia Muciniphila Leads to the Impairment of Insulin Secretion and Glucose Homeostasis in Lean Type 2 Diabetes. Adv. Sci. 2021, 8, 2100536. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chang, L.; Zhang, X.; Ning, Z.; Mayne, J.; Ye, Y.; Stintzi, A.; Liu, J.; Figeys, D. Berberine and Its Structural Analogs Have Differing Effects on Functional Profiles of Individual Gut Microbiomes. Gut Microbes 2020, 11, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Hao, H.-P.; Xie, H.-G.; Lai, L.; Wang, Q.; Liu, C.-X.; Wang, G.-J. Extensive Intestinal First-Pass Elimination and Predominant Hepatic Distribution of Berberine Explain Its Low Plasma Levels in Rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Gao, Z.; Zhang, Q.; Gu, C. The Mechanism of Berberine Alleviating Metabolic Disorder Based on Gut Microbiome. Front. Cell. Infect. Microbiol. 2022, 12, 854885. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Gao, Q.-Y.; Zou, T.-H.; Wang, B.-M.; Liu, S.-D.; Sheng, J.-Q.; Ren, J.-L.; Zou, X.-P.; Liu, Z.-J.; Song, Y.-Y.; et al. Berberine versus Placebo for the Prevention of Recurrence of Colorectal Adenoma: A Multicentre, Double-Blinded, Randomised Controlled Study. Lancet Gastroenterol. Hepatol. 2020, 5, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Fang, C.; Yang, G.; Li, J.; Liu, Y.; Zhang, L.; Yang, P.; Fang, J.; Gu, Y.; Zhang, Y.; et al. Identification of FtfL as a Novel Target of Berberine in Intestinal Bacteria. BMC Biol. 2023, 21, 280. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass Spectrometry-Based Metabolomics: A Guide for Annotation, Quantification and Best Reporting Practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.T.; Lim, H.B. Determination of Isoquinoline Alkaloids by UPLC-ESI-Q-TOF MS: Application to Chelidonium majus L. Anal. Sci. Technol. 2017, 30, 379–389. [Google Scholar] [CrossRef]
- Xu, P.; Xu, C.; Li, X.; Li, D.; Li, Y.; Jiang, J.; Yang, P.; Duan, G. Rapid Identification of Berberine Metabolites in Rat Plasma by UHPLC-Q-TOF-MS. Molecules 2019, 24, 1994. [Google Scholar] [CrossRef] [PubMed]
- Basera, I.A.; Girme, A.; Bhatt, V.P.; Saste, G.; Pawar, S.; Hingorani, L.; Shah, M.B. Development of Validated UHPLC–PDA with ESI–MS-MS Method for Concurrent Estimation of Magnoflorine, Berbamine, Columbamine, Jatrorrhizine, Palmatine and Berberine in Berberis Aristata. Acta Chromatogr. 2021, 1, 412–421. [Google Scholar] [CrossRef]

| Parameter | DHB | LMB | p-Value |
|---|---|---|---|
| n | 5 | 4 | |
| Males|Females | 2|3 | 3|1 | |
| Age (years) | 38.0 ± 5.4 | 36.0 ± 4.2 | 0.9995 |
| Weight (kg) | 67.0 ± 3.9 | 64.0 ± 2.9 | 0.9890 |
| BMI (kg/m2) | 23.7 ± 0.4 | 21.7 ± 0.9 | 0.9207 |
| Fasting Blood Glucose (mmol/L) | 5.39 ± 0.22 | 5.64 ± 0.34 | 0.9995 |
| Total Cholesterol (mmol/L) | 4.49 ± 0.58 | 5.17 ± 0.41 | >0.9999 |
| Triglyceride (mmol/L) | 0.87 ± 0.15 | 1.82 ± 0.72 | >0.9999 |
| High Density Lipoprotein (mmol/L) | 1.60 ± 0.22 | 1.50 ± 0.14 | >0.9999 |
| Low Density Lipoprotein (mmol/L) | 2.50 ± 0.42 | 2.84 ± 0.32 | >0.9999 |
| Metabolites | AUC0–24 (ng·h/mL) | Cmax (ng/mL) | Tmax (h) | |||
|---|---|---|---|---|---|---|
| DHB | LMB | DHB | LMB | DHB | LMB | |
| Berberine | 41.1 ± 7.0 | 26.0 ± 14.2 | 11.2 ± 3.0 | 3.3 ± 1.7 | 1.2 ± 0.5 | 10.5 ± 5.0 |
| Dihydroberberine | 1.2 ± 0.5 | 0.05 ± 0.03 | 0.85 ± 0.45 | 0.01 ± 0.01 | 1.6 ± 0.4 | 2.5 ± 1.3 |
| Berberrubine | 2.9 ± 1.0 * | 0.33 ± 0.11 * | 1.3 ± 0.4 | 0.09 ± 0.03 | 1.6 ± 0.2 | 5.3 ± 2.2 |
| Columbamine | 4.8 ± 2.8 | 1.3 ± 0.5 | 1.4 ± 0.8 | 0.32 ± 0.13 | 2.4 ± 0.9 | 3.5 ± 0.9 |
| Jatrorrhizine | 7.9 ± 0.8 | 5.0 ± 1.0 | 1.1 ± 0.3 | 0.58 ± 0.16 | 10.4 ± 3.7 | 7.8 ± 2.0 |
| Thalifendine | 13.2 ± 4.4 | 3.4 ± 0.8 | 3.6 ± 1.4 | 0.73 ± 0.12 | 2.4 ± 0.9 | 5.5 ± 1.7 |
| DB | 9.6 ± 2.1 * | 0.33 ± 0.15 * | 7.9 ± 2.7 * | 0.12 ± 0.05 * | 1.6 ± 0.4 | 2.8 ± 1.1 |
| Dihydroxyberberine | 0.34 ± 0.11 | 0.003 ± 0.002 | 0.16 ± 0.06 | 0.002 ± 0.001 | 2.4 ± 0.9 | 2.3 ± 1.9 |
| Dihydroxy-Bbrb/Thalif | 0.95 ± 0.46 | 0.75 ± 0.35 | 0.25 ± 0.10 | 0.25 ± 0.10 | 6.3 ± 4.5 | 4.3 ± 2.3 |
| Berberrubine-glu | ND * | 0.27 ± 0.04 * | ND * | 0.06 ± 0.01 * | ND * | 2 ± 0 * |
| Columbamine-glu | 0.27 ± 0.15 | 0.02 ± 0.01 | 0.17 ± 0.14 | 0.007 ± 0.003 | 2.0 ± 0.4 | 3.3 ± 1.3 |
| Jatrorrhizine-glu | 0.18 ± 0.13 | 0.06 ± 0.02 | 0.14 ± 0.14 | 0.02 ± 0.01 | 1.4 ± 0.7 | 1.5 ± 0.3 |
| Thalifendine-glu | 1.1 ± 0.4 | 0.40 ± 0.09 | 0.54 ± 0.45 | 0.06 ± 0.01 | 3.4 ± 0.7 | 4.8 ± 0.8 |
| DB-glu | 0.21 ± 0.11 | 0.03 ± 0.01 | 0.17 ± 0.12 | 0.010 ± 0.004 | 1.6 ± 0.4 | 2 ± 0 |
| DB-SO4 | 5.7 ± 1.9 | 1.30 ± 0.05 | 3.9 ± 2.3 * | 0.35 ± 0.05 * | 1.6 ± 0.4 | 2 ± 0 |
| Jatrorrhizine-SO4 | 0.066 ± 0.035 | 0.002 ± 0.001 | 0.045 ± 0.025 | 0.001 ± 0.001 | 1.8 ± 0.5 | 1.0 ± 0.6 |
| Bbrb/Thalif-SO4 | 3.8 ± 2.0 | 0.70 ± 0.11 | 2.9 ± 2.2 | 0.17 ± 0.04 | 1.6 ± 0.4 | 2.3 ± 0.3 |
| Metabolites | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Berberine | |||||||||||||||||
| 2. Dihydroberberine | 0.08 | ||||||||||||||||
| 3. Berberrubine | 0.27 | 0.74 * | |||||||||||||||
| 4. Columbamine | 0.35 | 0.75 * | 0.62 * | ||||||||||||||
| 5. Jatrorrhizine | 0.28 | 0.58 | 0.55 | 0.61 * | |||||||||||||
| 6. Thalifendine | 0.73 * | 0.48 | 0.54 | 0.76 ** | 0.54 | ||||||||||||
| 7. DB | 0.53 | 0.54 | 0.61 * | 0.51 | 0.23 | 0.67 * | |||||||||||
| 8. Dihydroxyberberine | −0.12 | 0.53 | 0.39 | 0.37 | 0.57 | 0.25 | 0.09 | ||||||||||
| 9. Dihydroxy-Bbrb/Thalif | −0.10 | 0.42 | 0.23 | 0.44 | 0.60 * | 0.30 | −0.11 | 0.73 * | |||||||||
| 10. Berberrubine-glu | 0.01 | 0.25 | 0.46 | 0.28 | 0.24 | 0.14 | −0.12 | 0.17 | 0.28 | ||||||||
| 11. Columbamine-glu | −0.22 | 0.77 ** | 0.67 * | 0.46 | 0.58 * | 0.16 | 0.06 | 0.75 ** | 0.60 * | 0.48 | |||||||
| 12. Jatrorrhizine-glu | −0.28 | 0.45 | 0.60 * | 0.28 | 0.28 | 0.03 | 0.00 | 0.51 | 0.19 | 0.48 | 0.76 ** | ||||||
| 13. Thalifendine-glu | −0.19 | 0.62 * | 0.75 ** | 0.57 | 0.65 * | 0.26 | 0.16 | 0.63 * | 0.55 | 0.53 | 0.81 ** | 0.71 * | |||||
| 14. DB-glu | −0.34 | 0.62 * | 0.65 * | 0.47 | 0.58 | 0.11 | 0.06 | 0.69 * | 0.62 * | 0.56 | 0.86 ** | 0.72 * | 0.94 *** | ||||
| 15. DB-SO4 | 0.51 | 0.38 | 0.63 * | 0.51 | 0.42 | 0.65 * | 0.65 * | 0.11 | 0.05 | −0.04 | 0.19 | 0.15 | 0.37 | 0.21 | |||
| 16. Jatrorrhizine-SO4 | −0.41 | 0.51 | 0.28 | 0.36 | 0.52 | 0.08 | −0.11 | 0.78 ** | 0.76 ** | 0.18 | 0.77 ** | 0.55 | 0.64 * | 0.79 ** | 0.05 | ||
| 17. Bbrb/Thalif-SO4 | 0.30 | 0.49 | 0.76 ** | 0.54 | 0.48 | 0.51 | 0.52 | 0.26 | 0.15 | 0.15 | 0.45 | 0.40 | 0.63 * | 0.49 | 0.92 *** | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.; Roh, Y.S.; Du, M.; Kuo, Y.C.; Zhang, Y.; Hardy, M.; Gahler, R.; Solnier, J. Differences in Metabolite Profiles of Dihydroberberine and Micellar Berberine in Caco-2 Cells and Humans—A Pilot Study. Int. J. Mol. Sci. 2024, 25, 5625. https://doi.org/10.3390/ijms25115625
Chang C, Roh YS, Du M, Kuo YC, Zhang Y, Hardy M, Gahler R, Solnier J. Differences in Metabolite Profiles of Dihydroberberine and Micellar Berberine in Caco-2 Cells and Humans—A Pilot Study. International Journal of Molecular Sciences. 2024; 25(11):5625. https://doi.org/10.3390/ijms25115625
Chicago/Turabian StyleChang, Chuck, Yoon Seok Roh, Min Du, Yun Chai Kuo, Yiming Zhang, Mary Hardy, Roland Gahler, and Julia Solnier. 2024. "Differences in Metabolite Profiles of Dihydroberberine and Micellar Berberine in Caco-2 Cells and Humans—A Pilot Study" International Journal of Molecular Sciences 25, no. 11: 5625. https://doi.org/10.3390/ijms25115625
APA StyleChang, C., Roh, Y. S., Du, M., Kuo, Y. C., Zhang, Y., Hardy, M., Gahler, R., & Solnier, J. (2024). Differences in Metabolite Profiles of Dihydroberberine and Micellar Berberine in Caco-2 Cells and Humans—A Pilot Study. International Journal of Molecular Sciences, 25(11), 5625. https://doi.org/10.3390/ijms25115625








