Murine iPSC-Loaded Scaffold Grafts Improve Bone Regeneration in Critical-Size Bone Defects
Abstract
1. Introduction
2. Results
2.1. In Vitro Assessment of Osteogenic Differentiation Capacity of iPSCs
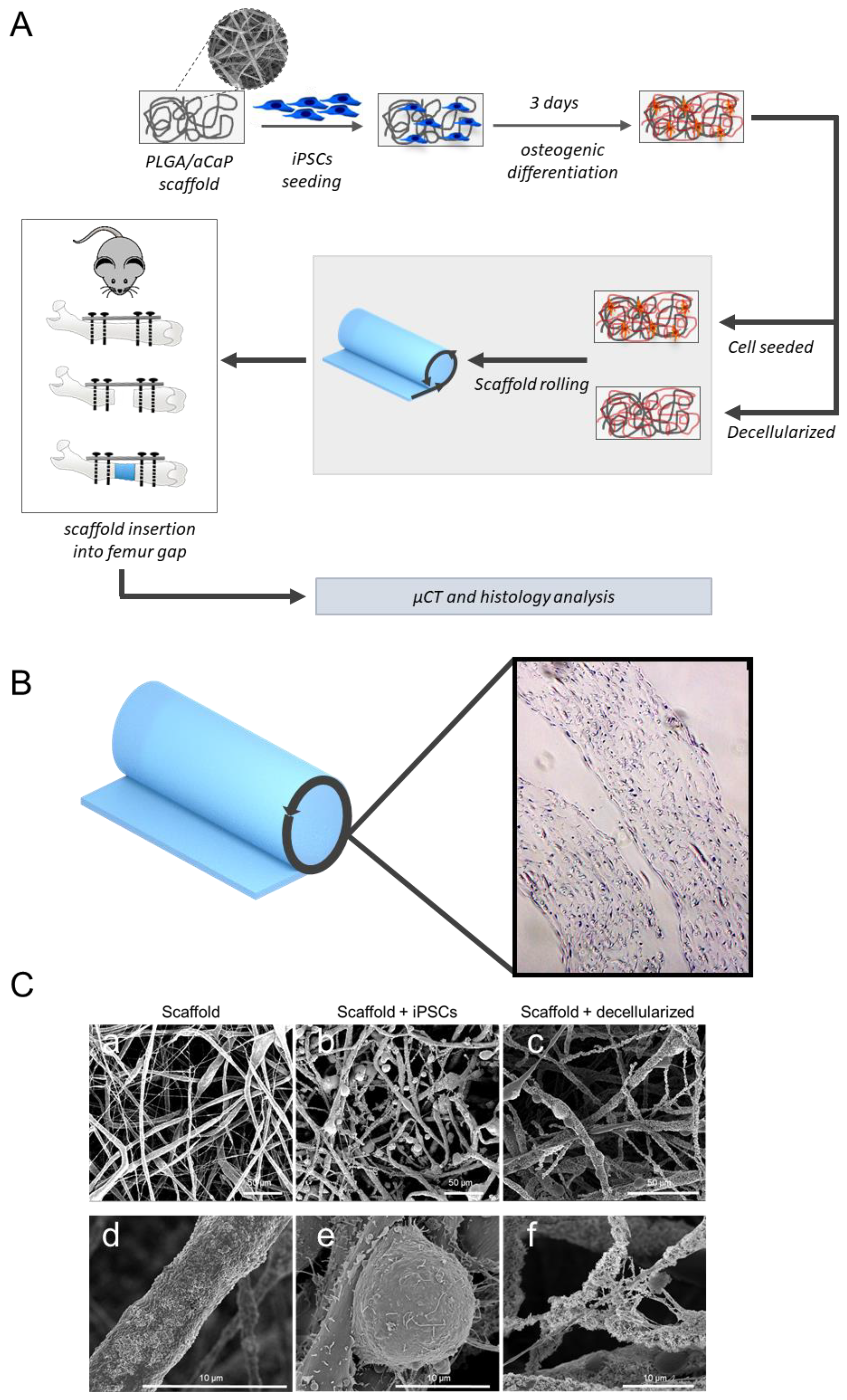
2.2. Scaffold Preparation
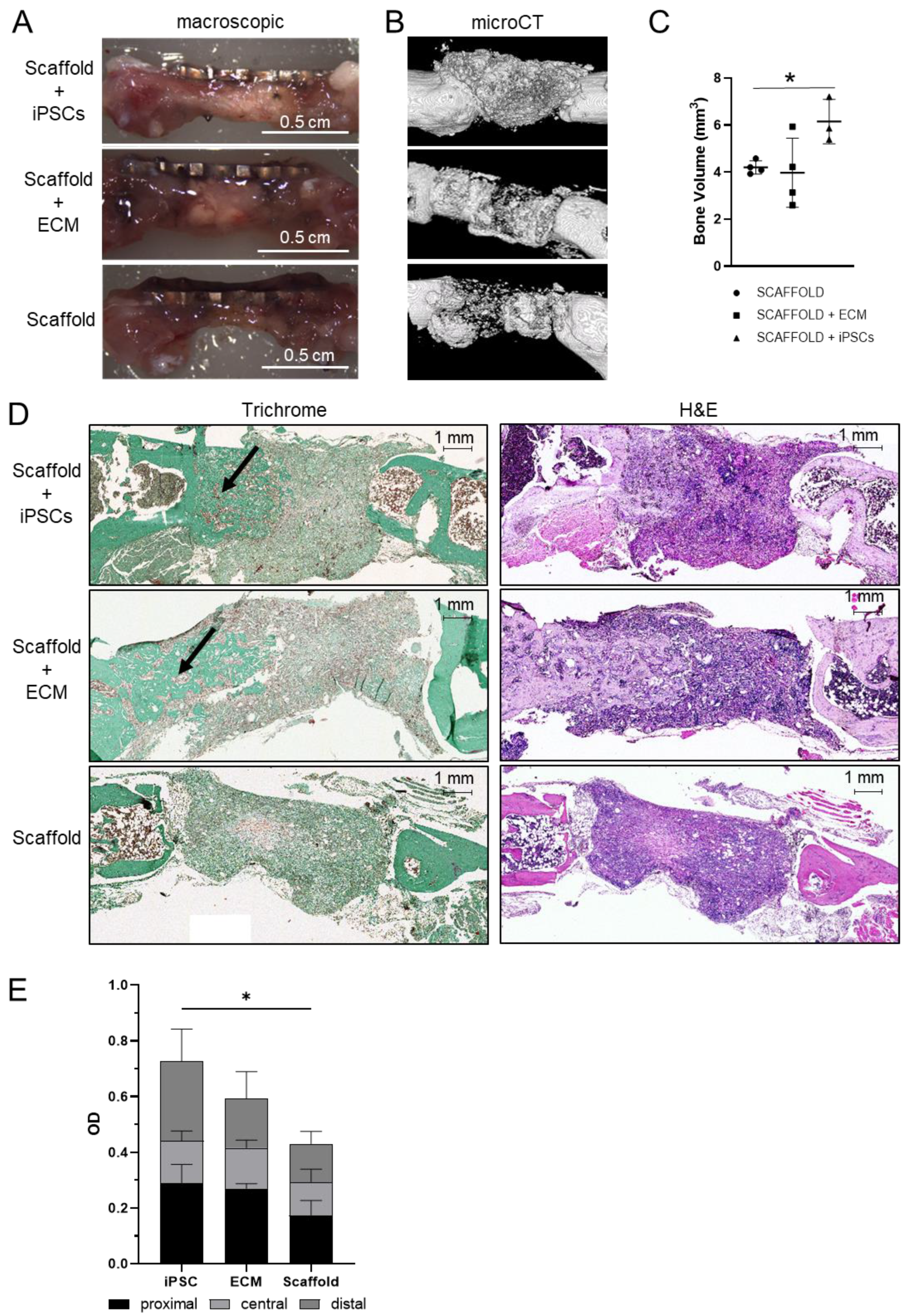
2.3. Mouse Model for Critical-Size Bone Defect
3. Discussion
4. Materials and Methods
4.1. Culture and Characterization of Mouse iPSCs
4.2. In Vitro Assessment of Osteogenic Differentiation Capacity
4.3. Alizarin Red Staining
4.4. Scaffolds
4.5. Scanning Electron Microscopy (SEM)
4.6. Animals and Surgical Procedures
4.7. MicroCT
4.8. Histological Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aCaP | amorphous calcium-phosphates nanoparticles |
| ALP | alkaline phosphatase |
| CD1 | cluster of differentiation 1 |
| c-Myc | MYC proto-oncogene |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s modified Eagle’s medium |
| e.g. | example given |
| ECM | extracellular matrix |
| FBS | fetal bovine serum |
| iPSC | induced pluripotent stem cell |
| Klf4 | KLF transcription factor 4 |
| LIF | leukemia inhibitory factor |
| MEF | mouse embryonic fibroblast |
| MSC | mesenchymal stromal cell |
| NEAA | non-essential amino acids |
| Oct4 | organic cation/carnitine transporter 4 |
| OD | optical density |
| PBS | phosphate-buffered saline |
| PLGA | poly (DL-lactic–glycolic acid) |
| RNA | ribonucleic acid |
| RTQ-PCR | real-time quantitative polymerase chaine reaction |
| SEM | scanning electron microscopy |
| SMA | smooth musce actin |
| Sox2 | SRY-box transcription factor 2 |
| SSEA-1 | stage specific embryonic antigen 1 |
| Tris | tris(hydroxymethyl)aminomethane |
| vol | volume |
References
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Kuehlfluck, P.; Moghaddam, A.; Helbig, L.; Child, C.; Wildemann, B.; Schmidmaier, G. RIA fractions contain mesenchymal stroma cells with high osteogenic potency. Injury 2015, 46 (Suppl. 8), S23–S32. [Google Scholar] [CrossRef]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M.L. Autogenous iliac crest bone graft. Complications and functional assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.D. Autograft and nonunions: Morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop. Clin. N. Am. 2010, 41, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38 (Suppl. 4), S3–S6. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.-I.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef]
- Canepa, D.D.; Casanova, E.A.; Arvaniti, E.; Tosevski, V.; Märsmann, S.; Eggerschwiler, B.; Cinelli, P. Identification of ALP+/CD73+ defining markers for enhanced osteogenic potential in human adipose-derived mesenchymal stromal cells by mass cytometry. Stem Cell Res. Ther. 2021, 12, 7. [Google Scholar] [CrossRef]
- Selich, A.; Daudert, J.; Hass, R.; Philipp, F.; von Kaisenberg, C.; Paul, G.; Cornils, K.; Fehse, B.; Rittinghausen, S.; Schambach, A.; et al. Massive Clonal Selection and Transiently Contributing Clones During Expansion of Mesenchymal Stem Cell Cultures Revealed by Lentiviral RGB-Barcode Technology. Stem Cells Transl. Med. 2016, 5, 591–601. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Egusa, H.; Kayashima, H.; Miura, J.; Uraguchi, S.; Wang, F.; Okawa, H.; Sasaki, J.-I.; Saeki, M.; Matsumoto, T.; Yatani, H. Comparative analysis of mouse-induced pluripotent stem cells and mesenchymal stem cells during osteogenic differentiation in vitro. Stem Cells Dev. 2014, 23, 2156–2169. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Inamura, M.; Kawabata, K.; Sakurai, F.; Yamanishi, K.; Hayakawa, T.; Mizuguchi, H. Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells 2009, 27, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chen, W.; Liu, J.; Weir, M.D.; Cheng, L.; Xu, H.H.K. Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng. Part A 2014, 20, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Lin, S.; Rizkalla, A.S.; Mequanint, K. Porous and biodegradable polycaprolactone-borophosphosilicate hybrid scaffolds for osteoblast infiltration and stem cell differentiation. J. Mech. Behav. Biomed. Mater. 2019, 92, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Mahboudi, H.; Soleimani, M.; Enderami, S.E.; Kehtari, M.; Ahvaz, H.H.; Ghanbarian, H.; Bandehpour, M.; Nojehdehi, S.; Mirzaei, S.; Kazemi, B. The effect of nanofibre-based polyethersulfone (PES) scaffold on the chondrogenesis of human induced pluripotent stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nie, H.; Xu, Z.; Niu, X.; Guo, S.; Yin, J.; Guo, F.; Li, G.; Wang, Y.; Zhang, C. The effect of 3D nanofibrous scaffolds on the chondrogenesis of induced pluripotent stem cells and their application in restoration of cartilage defects. PLoS ONE 2014, 9, e111566. [Google Scholar] [CrossRef] [PubMed]
- Tokita, R.; Nakajima, K.; Inoue, K.; Al-Wahabi, A.; Ser-Od, T.; Matsuzaka, K.; Inoue, T. Differentiation behavior of iPS cells cultured on PLGA with osteoinduction medium. Dent. Mater. J. 2017, 36, 103–110. [Google Scholar] [CrossRef][Green Version]
- Jeon, O.H.; Panicker, L.M.; Lu, Q.; Chae, J.J.; Feldman, R.A.; Elisseeff, J.H. Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Sci. Rep. 2016, 6, 26761. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Nguyen, B.-N.B.; Moriarty, R.A.; Kamalitdinov, T.; Etheridge, J.M.; Fisher, J.P. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J. Biomed. Mater. Res. A 2017, 105, 1123–1131. [Google Scholar] [CrossRef]
- Santos, M.I.; Reis, R.L. Vascularization in bone tissue engineering: Physiology, current strategies, major hurdles and future challenges. Macromol. Biosci. 2010, 10, 12–27. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Zhu, Y.; Wang, L.; Tomsia, A.P.; Mao, C. Phage nanofibers induce vascularized osteogenesis in 3D printed bone scaffolds. Adv. Mater. 2014, 26, 4961–4966. [Google Scholar] [CrossRef]
- Pele, K.G.; Amaveda, H.; Mora, M.; Marcuello, C.; Lostao, A.; Alamán-Díez, P.; Pérez-Huertas, S.; Pérez, M.; García-Aznar, J.M.; García-Gareta, E. Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering. Gels 2023, 9, 505. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef]
- Gröninger, O.; Hess, S.; Mohn, D.; Schneider, E.; Stark, W.; Märsmann, S.; Buschmann, J. Directing Stem Cell Commitment by Amorphous Calcium Phosphate Nanoparticles Incorporated in PLGA: Relevance of the Free Calcium Ion Concentration. Int. J. Mol. Sci. 2020, 21, 2627. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.; Stark, W.; Mohn, D.; Cohrs, N.; Märsmann, S.; Calcagni, M.; Cinelli, P.; Buschmann, J. Gene expression in human adipose-derived stem cells: Comparison of 2D films, 3D electrospun meshes or co-cultured scaffolds with two-way paracrine effects. Eur. Cell Mater. 2017, 34, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.M.; Yuan, Y.; Li, X.; Jashashvili, T.; Jamieson, M.; Urata, M.; Chen, Y.; Chai, Y. Mesenchymal stem cells and three-dimensional-osteoconductive scaffold regenerate calvarial bone in critical size defects in swine. Stem Cells Transl. Med. 2021, 10, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Eslaminejad, M.B.; Nazarian, H. Mesenchymal stem cells enhance bone regeneration in rat calvarial critical size defects more than platelete-rich plasma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 356–362; discussion 363. [Google Scholar] [CrossRef] [PubMed]
- Casanova, E.A.; Rodriguez-Palomo, A.; Stähli, L.; Arnke, K.; Gröninger, O.; Generali, M.; Liebi, M. SAXS imaging reveals optimized osseointegration properties of bioengineered oriented 3D-PLGA/aCaP scaffolds in a critical size bone defect model. Biomaterials 2023, 294, 121989. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.A.; Bartolomei, G.; Hottiger, M.O.; Cinelli, P. Artd1/Parp1 regulates reprogramming by transcriptional regulation of Fgf4 Via Sox2 ADP-Ribosylation. Stem Cells 2013, 31, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Legan, L.; Retko, K.; Ropret, P. Vibrational spectroscopic study on degradation of alizarin carmine. Microchem. J. 2016, 127, 36–45. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red S stains for calcium. J. Histochem. Cytochem. Off J. Histochem. Soc. 1969, 17, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, W.; Otto, L.; Hess, S.C.; Stark, W.J.; Märsmann, S.; Bürgisser, G.M.; Buschmann, J. Cartilage/bone interface fabricated under perfusion: Spatially organized commitment of adipose-derived stem cells without medium supplementation. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1833–1843. [Google Scholar] [CrossRef]
- Baumgartner, W.; Schneider, I.; Hess, S.C.; Stark, W.J.; Marsmann, S.; Brunelli, M.; Calcagni, M.; Cinelli, P.; Buschmann, J. Cyclic uniaxial compression of human stem cells seeded on a bone biomimetic nanocomposite decreases anti-osteogenic commitment evoked by shear stress. J. Mech. Behav. Biomed. Mater. 2018, 83, 84–93. [Google Scholar] [CrossRef]
- Buschmann, J.; Balli, E.; Hess, S.C.; Stark, W.J.; Cinelli, P.; Märsmann, S.; Welti, M.; Weder, W.; Jungraithmayr, W. Effects of seeding adipose-derived stem cells on electrospun nanocomposite used as chest wall graft in a murine model. Injury 2017, 48, 2080–2088. [Google Scholar] [CrossRef]
- Manassero, M.; Viateau, V.; Matthys, R.; Deschepper, M.; Vallefuoco, R.; Bensidhoum, M.; Petite, H. A novel murine femoral segmental critical-sized defect model stabilized by plate osteosynthesis for bone tissue engineering purposes. Tissue Eng. Part C Methods 2013, 19, 271–280. [Google Scholar] [CrossRef]
- König, M.A.; Canepa, D.D.; Cadosch, D.; Casanova, E.; Heinzelmann, M.; Rittirsch, D.; Plecko, M.; Hemmi, S.; Simmen, H.-P.; Cinelli, P.; et al. Direct transplantation of native pericytes from adipose tissue: A new perspective to stimulate healing in critical size bone defects. Cytotherapy 2016, 18, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Lou, X. Induced Pluripotent Stem Cells as a new Strategy for Osteogenesis and Bone Regeneration. Stem Cell Rev. Rep. 2015, 11, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Sabareeswaran, A.; Basu, B.; Shenoy, S.J.; Jaffer, Z.; Saha, N.; Stamboulis, A. Early osseointegration of a strontium containing glass ceramic in a rabbit model. Biomaterials 2013, 34, 9278–9286. [Google Scholar] [CrossRef] [PubMed]
- Nawawi, N.; Alqap, A.S.F.; Sopyan, I. Recent Progress on Hydroxyapatite-Based Dense Biomaterials for Load Bearing Bone Substitutes. Recent Pat. Mater. Sci. 2011, 4, 63–80. [Google Scholar] [CrossRef]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- O’Keefe, R.J.; Mao, J. Bone tissue engineering and regeneration: From discovery to the clinic—An overview. Tissue Eng. Part B Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. Organ engineering--combining stem cells, biomaterials, and bioreactors to produce bioengineered organs for transplantation. Bioessays 2013, 35, 163–172. [Google Scholar] [CrossRef]
- Liu, H.; Peng, H.; Wu, Y.; Zhang, C.; Cai, Y.; Xu, G.; Li, Q.; Chen, X.; Ji, J.; Zhang, Y.; et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 2013, 34, 4404–4417. [Google Scholar] [CrossRef]
- Peng, H.; Yin, Z.; Liu, H.; Chen, X.; Feng, B.; Yuan, H.; Su, B.; Ouyang, H.; Zhang, Y. Electrospun biomimetic scaffold of hydroxyapatite/chitosan supports enhanced osteogenic differentiation of mMSCs. Nanotechnology 2012, 23, 485102. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-H.; Xu, Y.-J.; Gao, J.; Yan, S.-G.; Zhao, J.; Tu, Q.; Zhang, J.; Duan, X.-J.; Sommer, C.A.; Mostoslavsky, G.; et al. Critical-size calvarial bone defects healing in a mouse model with silk scaffolds and SATB2-modified iPSCs. Biomaterials 2011, 32, 5065–5076. [Google Scholar] [CrossRef]
- Tam, W.L.; Mendes, L.F.; Chen, X.; Lesage, R.; Van Hoven, I.; Leysen, E.; Kerckhofs, G.; Bosmans, K.; Chai, Y.C.; Yamashita, A.; et al. Human pluripotent stem cell-derived cartilaginous organoids promote scaffold-free healing of critical size long bone defects. Stem Cell Res. Ther. 2021, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Watanabe, K.; Saito, A.; Onodera, S.; Azuma, T.; Takano, M. Bone regeneration of induced pluripotent stem cells derived from peripheral blood cells in collagen sponge scaffolds. J. Appl. Oral Sci. 2022, 30, e20210491. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Yang, C.; Sun, G. Recent advances in immunomodulatory hydrogels biomaterials for bone tissue regeneration. Mol. Immunol. 2023, 163, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Deng, Y.; Zhou, P.; Luo, Z.; Li, Q.; Xie, B.; Zhang, X.; Chen, T.; Pei, D.; Tang, Z.; et al. In vitro culture and directed osteogenic differentiation of human pluripotent stem cells on peptides-decorated two-dimensional microenvironment. ACS Appl. Mater. Interfaces 2015, 7, 4560–4572. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, B.; Hu, K.; Cao, C.; Man, Y.; Wang, P. Deriving Osteogenic Cells from Induced Pluripotent Stem Cells for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2017, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hanetseder, D.; Levstek, T.; Teuschl-Woller, A.H.; Frank, J.K.; Schaedl, B.; Redl, H.; Marolt Presen, D. Engineering of extracellular matrix from human iPSC-mesenchymal progenitors to enhance osteogenic capacity of human bone marrow stromal cells independent of their age. Front. Bioeng. Biotechnol. 2023, 11, 1214019. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kishida, T.; Nakai, K.; Sato, Y.; Kotani, S.-I.; Nishizawa, Y.; Yamamoto, T.; Kanamura, N.; Mazda, O. Direct phenotypic conversion of human fibroblasts into functional osteoblasts triggered by a blockade of the transforming growth factor-β signal. Sci. Rep. 2018, 8, 8463. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kishida, T.; Sato, Y.; Nishioka, K.; Ejima, A.; Fujiwara, H.; Kubo, T.; Yamamoto, T.; Kanamura, N.; Mazda, O. Direct conversion of human fibroblasts into functional osteoblasts by defined factors. Proc. Natl. Acad. Sci. USA 2015, 112, 6152–6157. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sato, Y.; Honjo, K.; Ichioka, H.; Oseko, F.; Sowa, Y.; Yamamoto, T.; Kanamura, N.; Kishida, T.; Mazda, O. Generation of Directly Converted Human Osteoblasts That Are Free of Exogenous Gene and Xenogenic Protein. J. Cell Biochem. 2016, 117, 2538–2545. [Google Scholar] [CrossRef]
- Takahashi, K.; Okita, K.; Nakagawa, M.; Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007, 2, 3081–3089. [Google Scholar] [CrossRef] [PubMed]
- Eggerschwiler, B.; Canepa, D.D.; Pape, H.-C.; Casanova, E.A.; Cinelli, P. Automated digital image quantification of histological staining for the analysis of the trilineage differentiation potential of mesenchymal stem cells. Stem. Cell Res. Ther. 2019, 10, 69. [Google Scholar] [CrossRef]
- Loher, S.; Stark, W.J.; Maciejewski, M.; Baiker, A.; Pratsinis, S.E.; Reichardt, D.; Maspero, F.; Krumeich, F.; Günther, D. Fluoro-apatite and Calcium Phosphate Nanoparticles by Flame Synthesis. Chem. Mater. 2005, 17, 36–42. [Google Scholar] [CrossRef]
- Schneider, O.D.; Loher, S.; Brunner, T.J.; Uebersax, L.; Simonet, M.; Grass, R.N.; Merkle, H.P.; Stark, W.J. Cotton wool-like nanocomposite biomaterials prepared by electrospinning: In vitro bioactivity and osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Baumgartner, W.; Groeninger, O.; Stark, W.J.; Maersmann, S.; Calcagni, M.; Cinelli, P.; Wolint, P.; Buschmann, J. 3D microtissue-derived human stem cells seeded on electrospun nanocomposites under shear stress: Modulation of gene expression. J. Mech. Behav. Biomed. Mater. 2020, 102, 103481. [Google Scholar] [CrossRef]
- 2010/63/Eu; Directive-2010/63-EN-EUR-Lex (europa.eu). European Union: Maastricht, The Netherlands, 2010.
- Arnke, K.; Pfister, P.; Reid, G.; Vasella, M.; Ruhl, T.; Seitz, A.-K.; Lindenblatt, N.; Cinelli, P.; Kim, B.-S. Impact of a High-Fat Diet at a Young Age on Wound Healing in Mice. Int. J. Mol. Sci. 2023, 24, 17299. [Google Scholar] [CrossRef]

| Genes | Primer Forward (5′ → 3′) | Primer Reverse (5′ → 3′) |
|---|---|---|
| Nanog | ACA AGG GTC TGC TAC TGA GAT GC | GGA GAC TTC TTG CAT CTG CTG G |
| Oct4 | GGC GTT CGC TTT GGA AAG GTG TTC | CTC GAA CCA CAT CCT TCT CT |
| Sox2 | TAG AGC TAG ACT CCG GGC GAT GA | TTG CCT TAA ACA AGA CCA CGA AA |
| Alp | CGC CAG AGT ACG CTC CCG CC | TGT ACC CTG AGA TTC GT |
| Ocn | CAG CCC CTC AGC AGA CTG AA | GTT GTC AGC CAC CAC CTC CT |
| Actab | CAT CCA GGC TGT GCT GTC CCT GTA TGC | GAT CTT CAT GGT GCT AGG AGC CAG AGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessler, F.; Arnke, K.; Eggerschwiler, B.; Neldner, Y.; Märsmann, S.; Gröninger, O.; Casanova, E.A.; Weber, F.A.; König, M.A.; Stark, W.J.; et al. Murine iPSC-Loaded Scaffold Grafts Improve Bone Regeneration in Critical-Size Bone Defects. Int. J. Mol. Sci. 2024, 25, 5555. https://doi.org/10.3390/ijms25105555
Kessler F, Arnke K, Eggerschwiler B, Neldner Y, Märsmann S, Gröninger O, Casanova EA, Weber FA, König MA, Stark WJ, et al. Murine iPSC-Loaded Scaffold Grafts Improve Bone Regeneration in Critical-Size Bone Defects. International Journal of Molecular Sciences. 2024; 25(10):5555. https://doi.org/10.3390/ijms25105555
Chicago/Turabian StyleKessler, Franziska, Kevin Arnke, Benjamin Eggerschwiler, Yvonne Neldner, Sonja Märsmann, Olivier Gröninger, Elisa A. Casanova, Fabienne A. Weber, Matthias A. König, Wendelin J. Stark, and et al. 2024. "Murine iPSC-Loaded Scaffold Grafts Improve Bone Regeneration in Critical-Size Bone Defects" International Journal of Molecular Sciences 25, no. 10: 5555. https://doi.org/10.3390/ijms25105555
APA StyleKessler, F., Arnke, K., Eggerschwiler, B., Neldner, Y., Märsmann, S., Gröninger, O., Casanova, E. A., Weber, F. A., König, M. A., Stark, W. J., Pape, H.-C., Cinelli, P., & Tiziani, S. (2024). Murine iPSC-Loaded Scaffold Grafts Improve Bone Regeneration in Critical-Size Bone Defects. International Journal of Molecular Sciences, 25(10), 5555. https://doi.org/10.3390/ijms25105555







