Abstract
Colorectal cancer (CRC) is one of the most prevalent cancers worldwide, ranking as the third most malignant. The incidence of CRC has been increasing with time, and it is reported that Westernized diet and lifestyle play a significant role in its higher incidence and rapid progression. The intake of high amounts of omega-6 (n − 6) PUFAs and low levels of omega-3 (n − 3) PUFAs has an important role in chronic inflammation and cancer progression, which could be associated with the increase in CRC prevalence. Oxylipins generated from PUFAs are bioactive lipid mediators and have various functions, especially in inflammation and proliferation. Carcinogenesis is often a consequence of chronic inflammation, and evidence has shown the particular involvement of n − 6 PUFA arachidonic acid-derived oxylipins in CRC, which is further described in this review. A deeper understanding of the role and metabolism of PUFAs by their modifying enzymes, their pathways, and the corresponding oxylipins may allow us to identify new approaches to employ oxylipin-associated immunomodulation to enhance immunotherapy in cancer. This paper summarizes oxylipins identified in the context of the initiation, development, and metastasis of CRC. We further explore CRC chemo-prevention strategies that involve oxylipins as potential therapeutics.
1. Introduction
Colorectal cancer (CRC) is one of the most prevalent cancers, ranking as the third most common malignancy globally in 2018 and second in mortality rankings [1]. There have been more than 1.9 million new cases of CRC and 935,000 deaths in 2020. The increase in the incidence of CRC is considered a sign of economic and social development [2].
Common risk factors for CRC include smoking, drinking alcohol, low dietary fiber/calcium intake, increased red meat consumption, excess body weight, and physical inactivity [3]. Therefore, the change in lifestyle and dietary habits in most parts of the world toward Westernized diets and lifestyles probably contributes to the increased incidence of CRC. The majority of CRC entities are adenocarcinomas resulting from sporadic pathological epithelial damage and follow the adenoma–carcinoma sequence [4]. One of the well-known risk factors for the occurrence and development of CRC is a chronic inflammatory environment [5].
Eating habits around the world have changed dramatically in the Western world with increasing calorie and fat intake but also a predominance of the essential omega-6 polyunsaturated fatty acids (n − 6 PUFAs) in many diets, as compared with lower levels of the other essential fatty acid class, omega-3 (n − 3) PUFAs. These lifestyle changes could be an important reason for the increase in CRC prevalence. It is widely believed that for most of human history, our diet was based on an equal ratio of n − 6 to n − 3 PUFA uptake, while the present ratio is assumed to be approximately 15:1. This difference indicates a fundamental change [6].
2. PUFAs and Enzymatically Formed Oxylipins
Oxylipins are produced when omega-3 (n − 3) or omega-6 (n − 6) PUFAs are oxygenated by COX (cyclooxygenase), LOX (lipoxygenase), and CYP (cytochrome P450 monooxygenase) enzymes [7]. The process is mostly initiated by rising intercellular calcium levels that induce the cPLA2 (cytosolic phospholipase A2)-regulated release of PUFAs from the sn2-position of phospholipids in the cell membrane [8]. COXs are heme-containing enzymes with both oxygenase and peroxidase activities; they are able to convert free PUFAs into thromboxanes and 1-, 2-, 3-, and dihomo-2-series prostanoids, such as prostaglandin-D2 (PGD2) and prostaglandin-E2 (PGE2) [9]. PGE2 especially plays a key role in the context of CRC, is discussed extensively in Section 5, and is displayed both in Figure 1 and Figure 2.
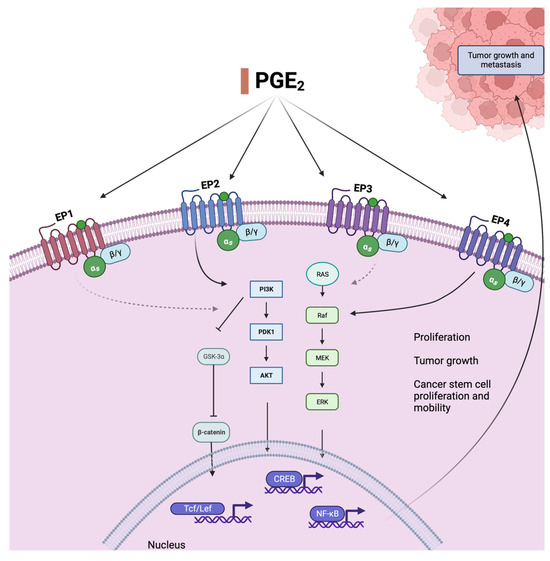
Figure 1.
Prostaglandin E2, an oxylipin derived from arachidonic acid AA (20:4, n − 6), activates relevant cancer-promoting pathways. Binding to mostly two of its receptors (EP2 and EP4), it leads to the activation of Ras and Raf, upregulation of the PI3K/AKT pathway, and subsequent activation of pro-proliferative transcription factors. The receptors and pathways that are most relevant and best understood in this context are shown as full black arrows. The PI3K/AKT pathway is involved in cell survival, differentiation, and proliferation and additionally increases CRC-stem cell count and mobility, leading to metastasis and drug resistance, through activation of NF-kB. This effect is also caused by MEK-associated ERK activation, which is mostly triggered by binding to EP4. PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase, PDK1 = pyruvate dehydrogenase kinase 1, AKT = protein-kinase B, MEK = mitogen-activated protein kinase, ERK = extracellular-signal-regulated kinase, NF-kB = nuclear factor kappa-B, Tcf/Lef = T cell factor/lymphoid enhancer factor family, GSKa = glycogen synthase kinase 3.
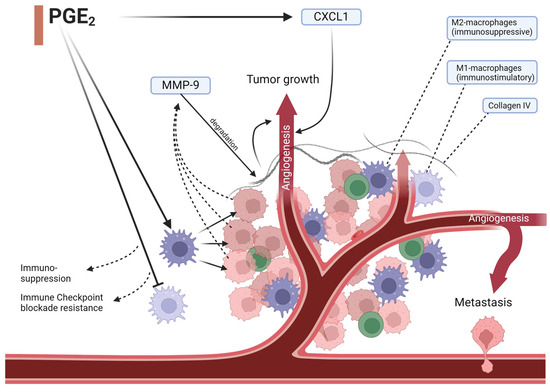
Figure 2.
PGE2 impacts angiogenesis in multiple ways. It induces angiopoietin-2 expression, an important vascular growth factor, and impacts immune cell composition. Immunosuppressive macrophages get overexpressed and cause tumor cells to release increased amounts of MMP-9, which degrades collagen IV in the base membrane and the extracellular matrix enabling cancer-cell angiogenesis and metastasis. Angiogenesis allows for further cancer cell mobility and growth. Lower expression of immunostimulatory macrophages additionally hinders an appropriate immune response and restricts checkpoint inhibition therapy. MMP-9 = matrix metalloprotease-9, CXCL1 = CXC motif chemokine ligand 1.
Another pathway is carried out by LOX enzymes, which starts with the production of hydroxy FAs (e.g., 5-HETE) and then subsequent modification into keto (e.g., oxo-ETE) or dihydroxy derivates (e.g., 5,15-diHETE). Activated LOX-5 catalyzes the formation of leukotrienes, and together with multiple consecutive LOX enzyme chain modifications, di- and tri-hydroxy FAs are created [10,11]. They include lipoxins, resolvins, protectins, and maresins. LOX-derived lipoxins like leukotrien-B4 (LTB4), lipoxin-A4 (LXA4), and 12-hydroxyeicosatetraenoic acid (12-HETE) are also featured in this review later on and are depicted in Figure 3.
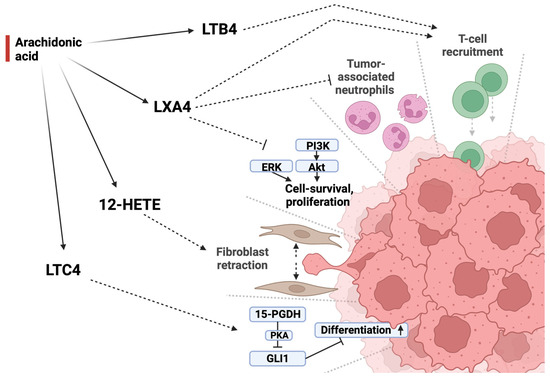
Figure 3.
Different LOX-enzymes produce a variety of oxylipins from AA (20:4 n − 6) which exhibit different effects on tumor development. Lipoxin A4 acts in an antitumorigenic manner by downregulating both tumor-associated neutrophils as well as tumor-promoting pathways while promoting T-cell recruitment. This immunostimulatory effect is further supported by leukotriene B4. Leukotriene C4 acts in an antiproliferative manner by promoting cell differentiation through PKA-mediated inhibition of GLI1. 12-HETE, in contrast, promotes tumor development by causing fibroblasts in the tumor-adjacent stroma to retract, opening space for metastasis and tumor growth. LXB4 = lipoxin A4, LTB4 = leukotriene B4, 12-HETE = 12-hydroxyeicosatetraenoic acid, LTC4 = leukotriene C4, PI3K = phosphoinositid-3-Kinase, Akt = protein kinase B, ERK = extracellular-signal-regulated kinases, 15-PGDH = 15-prostaglandin dehydrogenase, PKA = protein kinase A, GLI1 = glioma-associated oncogene.
Finally, CYP enzymes convert PUFAs in two main ways. Firstly, via epoxygenase, e.g., into epoxy-eicosatetraenoic acid (EpETE), epoxy-eicosatrienoic acid (EpETrE), and epoxy-docosapentaenoic acid (EpDPE), which are then remodeled by soluble epoxide hydrolase to become dihydroxy FAs such as dihydroxy-eicosatrienoic acid (DiHETrE). Secondly, via ω-hydroxylase, which creates, for instance, 20-hete, 19-hete, 20-hydroxyl leukotriene B4 (20-OH-LTB4), hydroxy-eicosapentaenoic acid (HEPE), HDoHE, and multiple ω-hydroxilated prostaglandins [11].
3. Increased Dietary Omega-3 PUFAs Might Lower Colorectal Cancer Risk
Given that the n − 6 PUFAs arachidonic acid (AA, 20:4 n − 6) is the precursor of the powerful often pro-inflammatory prostaglandin lipid mediators [12], the idea to change this disbalance by adding some n − 3 PUFAs is a straightforward concept in order to promote a less inflammatory and probably less CRC-prone nutrition environment.
Indeed, during the last three decades, data from both human and experimental studies have delivered evidence supporting the preventive use of n − 3 PUFA supplements in the context of CRC. A study published in 1993 investigated the effect of n − 3 PUFA oral supplementation with fish oil containing n − 3 PUFAs in a small group of twelve healthy people for 4 weeks and demonstrated reduced cell proliferation (used as a biomarker of decreased cancer risk) and decreased levels of PGE2 in rectal mucosa biopsy [13]. Another study, performed as a double-blind, placebo-controlled clinical study, was conducted in patients with sporadic adenomatous colorectal polyps treated with fish oil including eicosapentaenoic acid (EPA 20:5 n − 3, 4.1 g/day) and docosahexaenoic acid (DHA 22:6 n − 3, 3.6 g/day) for 12 weeks. The study also showed reduced proliferation in the upper part of colonic crypts [14].
Evidence from epidemiological studies supports these findings. The Physician’s Health Study recorded 500 male CRC patients over the course of 22 years and found an inverse correlation between fish and shellfish intake, or n − 3 PUFAs from other sources, and CRC risk [15]. Another large follow-up study collected data from 141,143 patients who were included in the Nurses’ Health Study 1, the Nurses’ Health Study 2, and the Health Professionals Follow-up Study, taking into consideration diet (assessing n − 3 PUFA intake through a validated food questionnaire), lifestyle, and medical information. Higher n − 3 PUFA uptake was connected with a lower risk of conventional adenomas (OR, 0.89; 95% CI, 0.84–0.95) and serrated polyps (OR, 0.90; 95% CI, 0.84–0.96) [16].
Several studies performed on mouse models of CRC underline the protective effect of n − 3 PUFAs. A study using human cancer xenografts in mice found that lower fat intake led to a decrease in tumor mass, which was even more pronounced (up to 90%) with n − 3 PUFA supplementation with lower levels of angiogenesis-associated gene expression in the colon tumors in the n − 3 PUFA-treated animals [17]. In the well-established azoxymethane (AOM)/dextran sodium sulfate (DSS) colon tumor mice model, animals fed with EPA (20:5 n − 3) showed decreased tumor incidence and size [18]. AOM and DSS were used for their ability to chemically induce DNA damage and cause colonic epithelial inflammation, leading to fast tumor formation. Results show that EPA (20:5 n − 3) decreased cell proliferation, PGE2 levels, and expression of nuclear β-catenin while increasing cell apoptosis in the model. These data match the results of our own study examining the effect of endogenously increased n − 3 PUFA levels in the fat-1 mouse model on CRC induction and development. The transgenic mice used in this study carry the fat-1 gene from the roundworm Caenorhabditis elegans, which encodes for a fatty acid n − 3 desaturase. Therefore, fat-1 mice can endogenously generate n − 3 PUFAs from n − 6 PUFAs, changing the n − 6/n − 3 PUFA ratio from values around 30/1 to approximately 1–5/1. Using the AOM and DSS model of CRC induction, we could demonstrate that these endogenously increased tissue levels of n − 3 PUFAs and almost balanced n − 6/n − 3- PUFA ratio lower the incidence and growth rate of colon tumors in fat—1 mice [19].
However, there is also inconsistent evidence, with some studies describing no or even negative effects of n − 3 PUFAs, as reviewed in [20], and whether the observed effects can be translated into practical recommendations for CRC prevention remains an open question. A currently ongoing study is assessing the effect of EPA (20:5 n − 3) as an adjunctive therapy in CRC patients with liver metastases undergoing partial liver resection with curative intent [21].
4. Nonsteroidal Anti-Inflammatory Drugs Prevent Colorectal Cancer
In 1988, a paper describing an inverse relation between acetylsalicylic acid (ASA) intake and CRC risk was published [22]. This was confirmed by multiple studies in the following decades, reporting CRC risk reduction rates ranging from 24 to 28% due to ASA use [23,24,25]. ASA and other nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit both COX-1 and 2, thus reducing the amounts of prostaglandins produced. Specifically, the lowering of PGE2 levels, one of the main products of COX-2, is relevant as it has well-known pro-inflammatory and tumorigenic properties [26].
The protective effect is not limited to ASA but is also found with other COX-inhibiting NSAIDS, as demonstrated in a Danish-population-based case control study that saw a substantially decreased CRC risk in people using nonaspirin NSAIDs long term [27]. An Ohio study further specified these findings regarding different substances and their influence on CRC risk [28], with an odds ratio (OR) of 0.28 for ibuprofen or naproxen, and an OR of 0.28 for selective COX-2 inhibitors, showing that these substances have a substantial impact on CRC risk that is comparable to ASA (OR 0.33).
ASA, like many other NSAIDs, has a much higher affinity for COX-1 than 2 but is unique in its ability to disable them permanently and therefore inhibit blood clotting. Interestingly, this could prove helpful as well because of recent discoveries that described an increased platelet activation in CRC and linked it to several major steps of cancer progression. These include platelet-induced vessel and endothelial proliferation, cloaking of intravascular cancer cells, and even platelet stroma interactions that contribute to the inflammatory milieu [29].
Some studies indicate that acetylation by ASA changes COX-2 to no longer produce prostaglandins like PGE2 but anti-inflammatory lipid mediators such as aspirin-triggered lipoxins (derived from AA 20:4 n − 6) and resolvins (derived from n − 3 PUFAs) [30,31]. However, a recently published study did not see any evidence supporting the production of EPA-derived pro-resolving mediators [32]. Even individuals receiving both ASA and EPA supplements did not show any synthesis of ASA-triggered 15-epi-LXA4 or RvE1 in plasma or colon mucosa. This was also the case in an AOM/DSS mouse model study conducted by us in 2020, where ASA was administered in a dosage that is comparable to low-dose treatment in humans: although ASA exhibited all its established COX-inhibition-related effects such as attenuated platelet activation and decreased PGE2 formation, formation of ASA-triggered lipid mediators was not detectable [33].
Recommendations regarding regular ASA use for prevention of CRC are discussed controversially: while the United States Preventive Services Task Force issued a recommendation in its 2016 statement regarding its use in older adults [34], the update in 2022 did not uphold this [35], even though this is a matter of discussion and interpretation [36]. Regarding ASA treatment after diagnosis of CRC, survival benefit was only observed in patients with mutated PIK3CA (the phosphatidylinositol-4,5-bisphosphonate 3-kinase, catalytic subunit alpha polypeptide gene). PIK3CA mutations are present in 15–20% of CRC entities and play a key role in cancer development and progression, as we describe further in the following paragraphs [37]. Regular use of ASA increased CRC-specific and overall survival in these patients, while wild-type PIK3CA cancer patients did not benefit [38].
5. Lipid Mediators and Colorectal Cancer
PGE2 is the most important prostaglandin suppressed by NSAID treatment. It is derived from AA (through oxygenation by COX-1 or COX-2 and further modification by a PGE isomerase). Levels of PGE2 are increased in multiple cancer entities and promote carcinogenesis and metastasis in CRC. There are four different subtypes of PGE2 G-protein-coupled receptors: EP1, EP2, EP3, and EP4 [39]. Activation of EP receptors 2 and 4 can upregulate the PI3K/Akt pathway (Figure 1), which is involved in cell proliferation, survival, and differentiation [40]. With activation of the PI3K/Akt pathway, β-catenin translocates into the nucleus, and COX-2 transcription and translation are triggered [41] (Figure 1), resulting in CRC cell migration and metastases [42].
A reduction in PGE2 might be even more relevant regarding its role in cancer stem cell (CSC) development and metastasis (Figure 1). A study published in 2015 found that PGE2 increased CSC numbers and migration leading to higher liver metastasis rates in mice. This effect could be decreased by a COX-2 blockade or knockdown of mediators like phosphoinositide 3-kinase (PI3K), EP4, or nuclear factor (NF)-κB. As such, PGE2 causes these effects through the EP4-PI3K and EP4-mitogen-activated protein kinase (MEK)-activated NF-κB [43].
Another significant step in tumor growth and metastasis that could be influenced by PGE2 is angiogenesis (Figure 2). It was demonstrated that PGE2 is able to increase angiogenesis due to C-X-C motif chemokine ligand 1 (CXCL1)-induction in CRC cells [44]. Additionally, it was shown to induce angiopoietin-2 expression in human endothelial cells and therefore increase an important vascular growth factor [45]. Moreover, PGE2 not only impacts tumor-associated signaling pathways but also immune cells in its microenvironment and the immune response toward cancer cells. When bound to EP4, it induces the differentiation of immunosuppressive M2 macrophages while reducing immunostimulatory M1 macrophages (Figure 2). Not only does this inhibit an efficient reaction toward these cancer cells, but it also impairs possible immune-checkpoint inhibition treatment [46].
Another prostaglandin that has been implicated in CRC biology is PGJ2 in Kirsten rat sarcoma viral oncogene homolog (KRAS)-mutated CRC cells. 15-d-PGJ mediates the formation of stress granules that are an essential tool in KRAS-mutated cell stress resistance. This allows the tumor cells to survive even when physiological proliferative barriers are lost and sometimes even when chemotherapy is administered while continuing to multiply uncontrollably. Further knowledge concerning the mechanisms behind this stress resistance and possible ways of inhibition could therefore ameliorate the prognosis of the 35–45% of CRC patients with KRAS mutations [47,48]. In addition, elevated thromboxane A2 (TXA2) levels have also been established as a key player in CRC pathogenesis, and their inhibition could reduce malignant potential and slow down the spreading of CRC cells [49]. The effect of TXA2 is primarily achieved by activating platelets via G-protein-coupled receptors that lead to platelet aggregation and release of other mediators, promoting cell growth and migration [50]. Additionally, TXA2 might act in a pro-tumorigenic manner by upregulating Kv7.1 ionic potassium (K+) channels (also called KCNQ1 and KvLQT1) that participate in cell cycle progression and proliferation, via the cAMP pathway [51].
Oxylipins that are produced from AA (20:4 n − 6) through different LOX-enzymes also influence tumor development and progression in distinct and partly opposing ways, as shown in Figure 3 and presented in the following paragraphs. Enzymatic metabolism of AA (20:4 n − 6) by 12-LOX, for instance, generates 12-HETE. Colorectal adenocarcinoma cells secrete 12-HETE, causing the retraction of cancer-associated fibroblasts and thus opening up entry gates to the adjacent stroma [52]. A biomimetic of LXA4, on the other hand, was found to inhibit the inflammatory state of the tumor microenvironment via the downregulation of ERK and the PI3K/AKT pathway in human dTHP-1 CRC cells. It furthermore decreased the level of tumor-associated neutrophils and myeloid-derived suppressor cells and increased T-cell recruitment intratumorally in a mouse xenograft colorectal carcinoma model [53,54]. Mast cells are another key player in immunity and have been suggested as a positive prognostic factor in CRC. Recent studies have indicated that Leukotriene B4 (LTB4) derived from these mast cells is essential for CD8+ recruitment, and mice lacking the LTB4 receptor had an increase in colon tumor progression and tumor-induced mortality [55]. Another LOX-derived oxylipin named LTC4 was found to induce the tumor suppressor 15-PGDH (15-hydroxyprostaglandin dehydrogenase), which leads to the downregulation of glioma-associated oncogene (GLI1) expression in a PKA (protein kinase A)-dependent manner, contributing to differentiation in CRC cells (Figure 3) [56]. 15-LOX-1 is downregulated in CRC cells, leading to lower levels of linoleic acid (LA) (18:2 n − 6)-derived 13-hydroxyoctadecadienoic acid (13-HODE) [57,58]. These lower levels of 13-HODE might contribute to tumor growth, as 13-HODE supplementation was shown in these studies to have an anti-proliferative effect on colon cancer cells.
Resolvin D1 (RvD1), derived from DHA (22:6 n − 3), has been studied regarding its effects on CRC and associated inflammation. It was reported that RvD1 possesses protective properties which are mediated through the blockade of IL-6 receptors, JAK2/STAT3, and following Cyclin D1 downregulation [59]. Additionally, a study demonstrated that RvD1 lowered the overexpression of c-Myc protein in HTC 116 human colon cancer cells through two separate mechanisms. First, it enhanced its ubiquitination and subsequent proteasomal degradation, and second, it inhibited its stabilization through extracellular-signal-regulated kinase-mediated phosphorylation by direct interaction with the ALX/FPR2 receptor [60].
Another DHA-derived lipid mediator important in the context of CRC is CYP P450-derived epoxydocosapentaenoic acids (EDPs). EDPs potently inhibit cancer growth, neovascularization, and metastasis both in vivo and in vitro [61], due to the inhibition of vascular endothelial growth factor (VEGF)- and fibroblast growth factor 2-induced angiogenesis (Figure 4). When combined with a low-dose inhibitor of the soluble epoxide hydrolase (sEH), thereby stabilizing the epoxy compounds, EDPs’ effects were further strengthened, leading to an approximately 70% decrease in tumor growth and metastasis [61]. This is in contrast to the effect of LA (18:2 n − 6)- and AA (20:4 n − 6)-derived epoxy compounds, which showed pro-tumorigenic effects in AOM/DSS-induced colon cancer in mice [62,63]. As depicted in Figure 4, these effects were caused by an increase in local VEGF secretion and receptors coupled with enhanced endothelial migration.
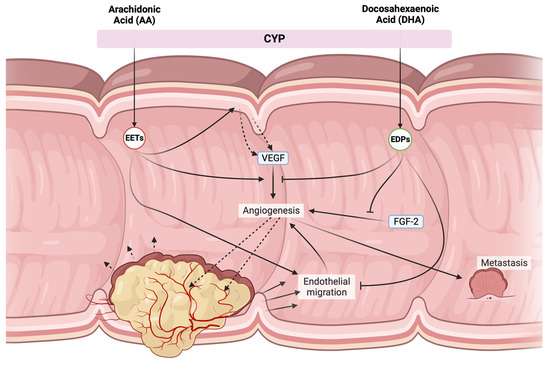
Figure 4.
Opposing effects of CYP products derived from AA (20:4 n − 6) and DHA (22:6 n − 3). EETs mostly possess pro-tumorigenic effects, triggering local VEGF secretion and enhancing VEGF-receptor 2 expression, while also promoting endothelial migration allowing for further angiogenesis. Additionally, they might cause metastasis through VEGF release. EDPs suppress many of these effects by blocking both VEGF and FGF-2-dependent angiogenesis, as well as dampening endothelial cell migration. VEGF = vascular endothelial growth factor, CYP = cytochrome P450 enzymes, FGF2 = fibroblast growth factor 2, EETs = epoxyeicosatrienoic acids, EDPs = epoxyeicosatetraenoic acids.
6. Chemoprevention Strategies in Colorectal Cancer—Beyond NSAIDS
With commonly used NSAIDs, and particularly ASA, the concept of CRC chemoprevention has been proven, with the ongoing discussion of whether ASA administration might even become a widespread recommendation to lower CRC risk. As this NSAID effect is due to the modulation of lipid mediators, particularly PGE2 suppression, we questioned whether other commonly used drugs that have been implicated in the chemoprevention of CRC also have effects on lipid mediator formation.
Statins are another widely used group of substances that have been analyzed concerning their potential as cancer chemoprevention agents. As much as 35% of people in the US were taking them in 2018–2019 for their lipid-lowering properties that help prevent cardiovascular disease [64]. A case control study by Poynter et al. that included nearly 4.000 subjects showed an astonishing 43% reduction in CRC risk associated with 5 or more years of self-reported statin intake [65]. A retrospective cohort study of US veterans reported a risk reduction of 35% in a dose-dependent manner [66]. It has also been demonstrated that statins hindered growth and promoted apoptosis in human CRC cell lines [67]. Statins competitively inhibit the 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of the mevalonate pathway. This leads to a relative cholesterol deficiency inside the cell, encouraging it to produce higher quantities of LDL receptors, thus taking more LDL out of the bloodstream. However, the HMG-CoA reductase inhibition affects not just cholesterol levels but also other intermediates of the mevalonate pathway, including farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) [68]. Their inhibition is relevant as they are needed for post-translational modification (isoprenylation) and activation of many different cellular proteins [69]. The important ones are Ras and Rho, two small GTPases that are essential parts of signaling pathways for cell growth, gene expression, apoptosis and inflammation [70,71]. It has been shown that the dysregulation of the mevalonate pathway is able to drive cancer development [72].
Atorvastatin was shown to lower pro-inflammatory markers [73], and statins were shown to reduce CRP levels and pro-inflammatory cytokines [74] while upregulating CD4+ and CD25+ regulatory cells [75]. Additionally, atorvastatin inhibits platelet-dependent COX-2 expression in endothelial cells in a CD40-dependent manner [76]. This is relevant as platelets can enhance chemotaxis of inflammatory cells and vascular wall inflammation by releasing pro-inflammatory mediators [77], and inflammation modulation by statins might not only be beneficial for the treatment of the inflammatory process atherosclerosis [75] but might also influence carcinogenesis-associated inflammatory aspects. Furthermore, antioxidant effects [78] and effects on angiogenesis [79] and cell adhesion [80] have been described.
Interestingly, there is increasing evidence that statins increase AA (20:4 n − 6)-derived oxylipins. Several studies demonstrate that statins appear to increase the enzymatic activity of fatty acid desaturase 1 (FADS1), the rate-limiting enzyme of C20 PUFA synthesis from C18 precursors, such as the conversion from LA to AA c [81,82,83]. Indeed, we also observed increased levels of several AA-derived oxylipins, most notably PGD2 in the colon tissue of individuals receiving statin treatment compared with untreated subjects or subjects on ASA medication [84]. Interestingly, statins and ASA co-treatment blunted this increase. While most AA-derived oxylipins such as PGE2 are mostly pro-inflammatory (n − 6 pro-inflammatory oxylipins), PGD2 has been shown to hinder tumor progression, which might explain this apparent contradiction [85]. The impact of statins on AA (20:4 n − 6) metabolism and their anti-proliferative and inflammation-dampening role described above is still not completely understood.
Metformin is a frequently used drug for the therapy of type 2 diabetes, which is a risk factor for CRC [86]. A recent meta-analysis including 58 studies concluded that metformin users had a substantially lower incidence of colon adenomas, advanced adenomas, and CRC [87]. Additionally, it was shown that outcomes of metastatic CRC patients were improved, and overall survival, as well as CRC-specific survival, increased in those taking metformin. This was also confirmed in a Korean national cohort study including more than 320,000 people [88]. Participants with type-2 diabetes receiving metformin treatment had a lower risk of developing CRC not only compared with other type 2 diabetics but also compared with people without the condition. These findings highlight metformin’s effects that appear to surpass the dampening of a diabetes-associated CRC risk increase. One mechanism that might explain how this effect is mediated was explored in a bladder cancer model in which metformin was able to inhibit stem cell multiplication by reducing COX-2-mediated PGE2 and following STAT3 activation both in mouse bladder cancer and bladder cancer cell lines [89]. Changes in AA (20:4 n − 6)-derived oxylipins were also detected in studies investigating metabolic changes in healthy subjects receiving metformin [90,91]. Another study demonstrated that metformin was able to reduce EET formation [92], possibly by binding to the active site heme of CYP3A4, depleting cancer cells of AA-derived EETs and their pro-tumorigenic effects, such as angiogenesis and mTOR signaling [93].
7. Concomitant Medications and Immune Therapy
Given the importance of immune-modulating effects described for the chemopreventive approaches in CRC, often involving oxylipin pathways and particularly the PGE2 pathway, it is tempting to assume that these effects could become more important also in the context of prevention and treatment of cancer with advances in immune checkpoint inhibitor (ICI) therapy. A 2020 study was able to demonstrate that by reducing PGE2’s effect on immune cell (Figure 2) responsiveness to anti-PD-1, therapy in mice could be ameliorated [46]. This was achieved by blocking EP4 which led to a decreased function of immunosuppressive cells and enhanced cytotoxic T-cell-mediated tumor elimination. Tumor progression was reduced and survival in treated mice was prolonged. This shows how our understanding of oxylipins could help develop strategies to enhance already existing immunological therapy concepts.
When looking at the previously discussed chemoprevention agents, ASA treatment in particular might have synergistic effects in the context of immune checkpoint inhibitor therapy. COX activity was proposed as a main factor causing immune suppression across species, and when reduced, left mice CRC cells more susceptible to immune control [94]. This effect was again traced back to PGE2, as its immunosuppressive effects were essential for mutant BRAF mouse melanoma cells to grow in immune-competent organisms. Other substances similarly show promising results in animal studies. Metformin increased sensitivity towards PD-1 inhibition and increased CD8+ T-cell infiltration in lung cancer [95]. Additionally, the degradation of PD-L1 in mice breast cancer has been described [96]. Statins were shown to have comparable properties, as they were able to enhance T-cell activity and reduce PD-L1 expression in breast cancer. Atorvastatin supported the effect of a co-administered anti-PD-L1 therapy in vitro [97]. Recently, an experimental study was able to show that in animal models with dietary omega-3 (n − 3) polyunsaturated fatty acid supplementation and increases in their CYP-epoxyeicosanoids by pharmacologic inhibition of the sEH, the anti-tumor activity of ICI is enhanced [98].
While all of these results seem promising, data from human studies are currently often inconclusive: in contrast to the effect of steroids, ref. [99] baseline statin, and ASA (and beta-blocker), medications were associated with better tumor response to ICI treatment. Another study published in 2021 also found beneficial effects of low-dose ASA and/or statin administration in addition to ICI therapy [100]. However, a beneficial effect was not seen with COX inhibitors and ICI in another study of lung cancer [101]. Another recent study found higher rates of immune-adverse events with ASA treatment in patients undergoing ICI treatment [102]. On the other hand, there are also initial promising data with metformin plus ICI in melanoma [103], as well as metformin plus ICI in lung cancer [104,105].
This difference could be explained in part due to many different co-medications, making the elucidation of modulating effects difficult. Specifically, many cancer patients receive steroids as part of their combination therapies, which are powerful immune-modulating compounds that might well blunt all other effects—and were shown to have a worse outcome in combination with ICI therapy [99].
8. Conclusions and Perspectives
In this comprehensive review, we examined the current understanding of CRC prevention strategies, with a primary focus on inflammation modulation, particularly through the manipulation of PUFA-derived oxylipin pathways. Central to our discussion is the modulation of the PGE2 signaling pathway. We also discuss several other oxylipin pathways that have shown promise in thwarting carcinogenesis. While both epidemiological studies and experimental evidence have suggested a lowered CRC risk with n − 3 PUFA supplementation and therefore a change in lipid mediator composition, the establishment of clear intake or supplementation recommendations remains elusive.
The effectiveness of NSAIDs, particularly ASA, in preventing CRC is well-supported by robust data. However, ongoing debates persist regarding the optimal recommendations for ASA use in CRC prevention. One of the major challenges lies in treating a large number of patients over extended periods to prevent relatively rare events, while also considering the potential risks of side effects associated with agents such as ASA, statins, or metformin. This complexity is further compounded in Western countries, where colonoscopy-based screening strategies are already firmly established as preventive measures. However, the insights gleaned from our exploration of oxylipin pathways offer intriguing possibilities not only in CRC prevention but also in addressing other malignancies. Many gastrointestinal cancers and other malignancies exhibit an inflammatory component in their tumorigenesis process, suggesting the potential applicability of these approaches across various cancer types.
Moreover, as the landscape of cancer treatment evolves, with immune checkpoint inhibition (ICI) assuming a central role, there emerges an opportunity to extend oxylipin concepts from prevention to treatment optimization. Understanding the immunomodulating effects of CYP-, LOX-, and COX-derived oxylipins can help to employ them to increase the effectiveness of these therapeutic strategies. Future endeavors could benefit from analyzing oxylipin pathways within the context of immune-modulation-based cancer therapies, thereby paving the way for novel approaches to treatment optimization.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Figures were created with www.BioRender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Malvezzi, M.; Bertuccio, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2014. Ann. Oncol. 2014, 25, 1650–1656. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Strum, W.B. Colorectal Adenomas. N. Engl. J. Med. 2016, 374, 1065–1075. [Google Scholar] [CrossRef]
- Lichtenstern, C.R.; Ngu, R.K.; Shalapour, S.; Karin, M. Immunotherapy, Inflammation and Colorectal Cancer. Cells 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.; Hawley, M.; Holinstat, M. The expansive role of oxylipins on platelet biology. J. Mol. Med. 2017, 95, 575–588. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Srinivasan, B.D. Eicosapentaenoic acid metabolism in human and rabbit anterior uvea. Prostaglandins 1986, 31, 1159–1164. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Oates, J.A. The 1982 Nobel Prize in Physiology or Medicine. Science 1982, 218, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Bartram, H.P.; Gostner, A.; Scheppach, W.; Reddy, B.S.; Rao, C.V.; Dusel, G.; Richter, F.; Richter, A.; Kasper, H. Effects of fish oil on rectal cell proliferation, mucosal fatty acids, and prostaglandin E2 release in healthy subjects. Gastroenterology 1993, 105, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Anti, M.; Marra, G.; Armelao, F.; Bartoli, G.M.; Ficarelli, R.; Percesepe, A.; De Vitis, I.; Maria, G.; Sofo, L.; Rapaccini, G.L.; et al. Effect of ω-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology 1992, 103, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.N.; Chavarro, J.E.; Lee, I.M.; Willett, W.C.; Ma, J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, K.; Ogino, S.; Giovannucci, E.L.; Chan, A.T.; Song, M. Association Between Risk Factors for Colorectal Cancer and Risk of Serrated Polyps and Conventional Adenomas. Gastroenterology 2018, 155, 355–373.e318. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hancock, R.L.; Mohammadpour, H.; McGregor, B.; Manalo, P.; Khaiboullina, S.; Hall, M.R.; Pardini, L.; Pardini, R.S. Influence of omega-3 fatty acids on the growth of human colon carcinoma in nude mice. Cancer Lett. 2002, 187, 169–177. [Google Scholar] [CrossRef]
- Piazzi, G.; D’Argenio, G.; Prossomariti, A.; Lembo, V.; Mazzone, G.; Candela, M.; Biagi, E.; Brigidi, P.; Vitaglione, P.; Fogliano, V.; et al. Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. Int. J. Cancer 2014, 135, 2004–2013. [Google Scholar] [CrossRef]
- Nowak, J.; Weylandt, K.H.; Habbel, P.; Wang, J.; Dignass, A.; Glickman, J.N.; Kang, J.X. Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis 2007, 28, 1991–1995. [Google Scholar] [CrossRef]
- Tu, M.; Wang, W.; Zhang, G.; Hammock, B.D. ω-3 Polyunsaturated Fatty Acids on Colonic Inflammation and Colon Cancer: Roles of Lipid-Metabolizing Enzymes Involved. Nutrients 2020, 12, 3301. [Google Scholar] [CrossRef]
- Hull, M.A.; Ow, P.L.; Ruddock, S.; Brend, T.; Smith, A.F.; Marshall, H.; Song, M.; Chan, A.T.; Garrett, W.S.; Yilmaz, O.; et al. Randomised, placebo-controlled, phase 3 trial of the effect of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid (EPA) on colorectal cancer recurrence and survival after surgery for resectable liver metastases: EPA for Metastasis Trial 2 (EMT2) study protocol. BMJ Open 2023, 13, e077427. [Google Scholar] [CrossRef]
- Kune, G.A.; Kune, S.; Watson, L.F. Colorectal cancer risk, chronic illnesses, operations, and medications: Case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988, 48, 4399–4404. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Wilson, M.; Elwin, C.E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Nan, H.; Hutter, C.M.; Lin, Y.; Jacobs, E.J.; Ulrich, C.M.; White, E.; Baron, J.A.; Berndt, S.I.; Brenner, H.; Butterbach, K.; et al. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA 2015, 313, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Fowkes, F.G.; Belch, J.F.; Ogawa, H.; Warlow, C.P.; Meade, T.W. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet 2011, 377, 31–41. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Prostaglandins and cancer. Gut 2006, 55, 115–122. [Google Scholar] [CrossRef]
- Friis, S.; Riis, A.H.; Erichsen, R.; Baron, J.A.; Sørensen, H.T. Low-Dose Aspirin or Nonsteroidal Anti-inflammatory Drug Use and Colorectal Cancer Risk: A Population-Based, Case-Control Study. Ann. Intern. Med. 2015, 163, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Beebe-Donk, J.; Alshafie, G.A. Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer 2008, 8, 237. [Google Scholar] [CrossRef]
- Bambace, N.M.; Holmes, C.E. The platelet contribution to cancer progression. J. Thromb. Haemost. 2011, 9, 237–249. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Endogenous pro-resolving and anti-inflammatory lipid mediators: A new pharmacologic genus. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S200–S215. [Google Scholar] [CrossRef]
- Weylandt, K.H.; Chiu, C.Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Fuller, H.; Race, A.D.; Fenton, H.; Burke, L.; Downing, A.; Williams, E.A.; Rees, C.J.; Brown, L.C.; Loadman, P.M.; Hull, M.A. Plasma and rectal mucosal oxylipin levels during aspirin and eicosapentaenoic acid treatment in the seAFOod polyp prevention trial. Prostaglandins Leukot. Essent. Fat. Acids 2023, 192, 102570. [Google Scholar] [CrossRef]
- Rohwer, N.; Kühl, A.A.; Ostermann, A.I.; Hartung, N.M.; Schebb, N.H.; Zopf, D.; McDonald, F.M.; Weylandt, K.H. Effects of chronic low-dose aspirin treatment on tumor prevention in three mouse models of intestinal tumorigenesis. Cancer Med. 2020, 9, 2535–2550. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Dehmer, S.P.; O’Keefe, L.R.; Grossman, E.S.; Maciosek, M.V. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. In Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: An Updated Decision Analysis for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2022. [Google Scholar]
- Chan, A.T. Aspirin and the USPSTF-What About Cancer? JAMA Oncol. 2022, 8, 1392–1394. [Google Scholar] [CrossRef]
- He, Y.; Van’t Veer, L.J.; Mikolajewska-Hanclich, I.; van Velthuysen, M.L.; Zeestraten, E.C.; Nagtegaal, I.D.; van de Velde, C.J.; Marijnen, C.A. PIK3CA mutations predict local recurrences in rectal cancer patients. Clin. Cancer Res. 2009, 15, 6956–6962. [Google Scholar] [CrossRef]
- Liao, X.; Lochhead, P.; Nishihara, R.; Morikawa, T.; Kuchiba, A.; Yamauchi, M.; Imamura, Y.; Qian, Z.R.; Baba, Y.; Shima, K.; et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N. Engl. J. Med. 2012, 367, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef]
- Yen, J.-H.; Kocieda, V.P.; Jing, H.; Ganea, D. Prostaglandin E2 Induces Matrix Metalloproteinase 9 Expression in Dendritic Cells through Two Independent Signaling Pathways Leading to Activator Protein 1 (AP-1) Activation. J. Biol. Chem. 2011, 286, 38913–38923. [Google Scholar] [CrossRef] [PubMed]
- De Keijzer, S.; Meddens, M.B.M.; Torensma, R.; Cambi, A. The Multiple Faces of Prostaglandin E2 G-Protein Coupled Receptor Signaling during the Dendritic Cell Life Cycle. Int. J. Mol. Sci. 2013, 14, 6542–6555. [Google Scholar] [CrossRef]
- Hsu, H.H.; Lin, Y.M.; Shen, C.Y.; Shibu, M.A.; Li, S.Y.; Chang, S.H.; Lin, C.C.; Chen, R.J.; Viswanadha, V.P.; Shih, H.N.; et al. Prostaglandin E2-Induced COX-2 Expressions via EP2 and EP4 Signaling Pathways in Human LoVo Colon Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1132. [Google Scholar] [CrossRef]
- Wang, D.; Fu, L.; Sun, H.; Guo, L.; DuBois, R.N. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology 2015, 149, 1884–1895.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Brown, J.; Daikoku, T.; Ning, W.; Shi, Q.; Richmond, A.; Strieter, R.; Dey, S.K.; DuBois, R.N. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J. Exp. Med. 2006, 203, 941–951. [Google Scholar] [CrossRef]
- Szymczak, M.; Murray, M.; Petrovic, N. Modulation of angiogenesis by ω-3 polyunsaturated fatty acids is mediated by cyclooxygenases. Blood 2008, 111, 3514–3521. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yu, W.; He, J.; Liu, W.; Yang, J.; Lin, X.; Zhang, Y.; Wang, X.; Jiang, W.; Luo, J.; et al. Reprogramming immunosuppressive myeloid cells facilitates immunotherapy for colorectal cancer. EMBO Mol. Med. 2021, 13, e12798. [Google Scholar] [CrossRef] [PubMed]
- Grabocka, E.; Bar-Sagi, D. Mutant KRAS Enhances Tumor Cell Fitness by Upregulating Stress Granules. Cell 2016, 167, 1803–1813.e12. [Google Scholar] [CrossRef]
- Dinu, D.; Dobre, M.; Panaitescu, E.; Birla, R.; Iosif, C.; Hoara, P.; Caragui, A.; Boeriu, M.; Constantinoiu, S.; Ardeleanu, C. Prognostic significance of KRAS gene mutations in colorectal cancer—Preliminary study. J. Med. Life 2014, 7, 581–587. [Google Scholar]
- Li, H.; Liu, K.; Boardman, L.A.; Zhao, Y.; Wang, L.; Sheng, Y.; Oi, N.; Limburg, P.J.; Bode, A.M.; Dong, Z. Circulating Prostaglandin Biosynthesis in Colorectal Cancer and Potential Clinical Significance. EBioMedicine 2015, 2, 165–171. [Google Scholar] [CrossRef]
- Sakai, H.; Suzuki, T.; Takahashi, Y.; Ukai, M.; Tauchi, K.; Fujii, T.; Horikawa, N.; Minamimura, T.; Tabuchi, Y.; Morii, M.; et al. Upregulation of thromboxane synthase in human colorectal carcinoma and the cancer cell proliferation by thromboxane A2. FEBS Lett. 2006, 580, 3368–3374. [Google Scholar] [CrossRef]
- Shimizu, T.; Fujii, T.; Takahashi, Y.; Takahashi, Y.; Suzuki, T.; Ukai, M.; Tauchi, K.; Horikawa, N.; Tsukada, K.; Sakai, H. Up-regulation of Kv7.1 channels in thromboxane A2-induced colonic cancer cell proliferation. Pflugers Arch. 2014, 466, 541–548. [Google Scholar] [CrossRef]
- Stadler, S.; Nguyen, C.H.; Schachner, H.; Milovanovic, D.; Holzner, S.; Brenner, S.; Eichsteininger, J.; Stadler, M.; Senfter, D.; Krenn, L.; et al. Colon cancer cell-derived 12(S)-HETE induces the retraction of cancer-associated fibroblast via MLC2, RHO/ROCK and Ca2+ signalling. Cell Mol. Life Sci. 2017, 74, 1907–1921. [Google Scholar] [CrossRef]
- Dong, T.; Dave, P.; Yoo, E.; Ebright, B.; Ahluwalia, K.; Zhou, E.; Asante, I.; Salimova, M.; Pei, H.; Lin, T.; et al. NAP1051, a Lipoxin A4 Biomimetic Analogue, Demonstrates Antitumor Activity Against the Tumor Microenvironment. Mol. Cancer Ther. 2021, 20, 2384–2397. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, J.; Huang, W.; Xu, Q.; Ye, D.; Sun, R.; Zhang, D. Colorectal Cancer Is Associated with a Deficiency of Lipoxin A4, an Endogenous Anti-inflammatory Mediator. J. Cancer 2019, 10, 4719–4730. [Google Scholar] [CrossRef] [PubMed]
- Bodduluri, S.R.; Mathis, S.; Maturu, P.; Krishnan, E.; Satpathy, S.R.; Chilton, P.M.; Mitchell, T.C.; Lira, S.; Locati, M.; Mantovani, A.; et al. Mast Cell-Dependent CD8+ T-cell Recruitment Mediates Immune Surveillance of Intestinal Tumors in ApcMin/+ Mice. Cancer Immunol. Res. 2018, 6, 332–347. [Google Scholar] [CrossRef]
- Satapathy, S.R.; Topi, G.; Osman, J.; Hellman, K.; Ek, F.; Olsson, R.; Sime, W.; Mehdawi, L.M.; Sjolander, A. Tumour suppressor 15-hydroxyprostaglandin dehydrogenase induces differentiation in colon cancer via GLI1 inhibition. Oncogenesis 2020, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Shureiqi, I.; Wojno, K.J.; Poore, J.A.; Reddy, R.G.; Moussalli, M.J.; Spindler, S.A.; Greenson, J.K.; Normolle, D.; Hasan, A.A.; Lawrence, T.S.; et al. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis 1999, 20, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Nixon, J.B.; Kim, K.S.; Lamb, P.W.; Bottone, F.G.; Eling, T.E. 15-Lipoxygenase-1 has anti-tumorigenic effects in colorectal cancer. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 7–15. [Google Scholar] [CrossRef]
- Lee, H.N.; Choi, Y.S.; Kim, S.H.; Zhong, X.; Kim, W.; Park, J.S.; Saeidi, S.; Han, B.W.; Kim, N.; Lee, H.S.; et al. Resolvin D1 suppresses inflammation-associated tumorigenesis in the colon by inhibiting IL-6-induced mitotic spindle abnormality. FASEB J. 2021, 35, e21432. [Google Scholar] [CrossRef]
- Zhong, X.; Lee, H.N.; Surh, Y.J. RvD1 inhibits TNFα-induced c-Myc expression in normal intestinal epithelial cells and destabilizes hyper-expressed c-Myc in colon cancer cells. Biochem. Biophys. Res. Commun. 2018, 496, 316–323. [Google Scholar] [CrossRef]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.Y.; Stephen Lee, K.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Edin, M.L.; Wang, Y.; Luo, Y.; Wan, D.; Yang, H.; Song, C.Q.; Xue, W.; Sanidad, K.Z.; et al. Targeted Metabolomics Identifies the Cytochrome P450 Monooxygenase Eicosanoid Pathway as a Novel Therapeutic Target of Colon Tumorigenesis. Cancer Res. 2019, 79, 1822–1830. [Google Scholar] [CrossRef]
- Panigrahy, D.; Edin, M.L.; Lee, C.R.; Huang, S.; Bielenberg, D.R.; Butterfield, C.E.; Barnés, C.M.; Mammoto, A.; Mammoto, T.; Luria, A.; et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J. Clin. Investig. 2012, 122, 178–191. [Google Scholar] [CrossRef]
- Matyori, A.; Brown, C.P.; Ali, A.; Sherbeny, F. Statins utilization trends and expenditures in the U.S. before and after the implementation of the 2013 ACC/AHA guidelines. Saudi Pharm. J. 2023, 31, 795–800. [Google Scholar] [CrossRef]
- Poynter, J.N.; Gruber, S.B.; Higgins, P.D.; Almog, R.; Bonner, J.D.; Rennert, H.S.; Low, M.; Greenson, J.K.; Rennert, G. Statins and the risk of colorectal cancer. N. Engl. J. Med. 2005, 352, 2184–2192. [Google Scholar] [CrossRef]
- Farwell, W.R.; Scranton, R.E.; Lawler, E.V.; Lew, R.A.; Brophy, M.T.; Fiore, L.D.; Gaziano, J.M. The association between statins and cancer incidence in a veterans population. J. Natl. Cancer Inst. 2008, 100, 134–139. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Napoli, C.; Lerman, A. Statin effects beyond lipid lowering—Are they clinically relevant? Eur. Heart J. 2003, 24, 225–248. [Google Scholar] [CrossRef]
- Gazzerro, P.; Proto, M.C.; Gangemi, G.; Malfitano, A.M.; Ciaglia, E.; Pisanti, S.; Santoro, A.; Laezza, C.; Bifulco, M. Pharmacological actions of statins: A critical appraisal in the management of cancer. Pharmacol. Rev. 2012, 64, 102–146. [Google Scholar] [CrossRef] [PubMed]
- Demierre, M.F.; Higgins, P.D.; Gruber, S.B.; Hawk, E.; Lippman, S.M. Statins and cancer prevention. Nat. Rev. Cancer 2005, 5, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Pylayeva-Gupta, Y.; Grabocka, E.; Bar-Sagi, D. RAS oncogenes: Weaving a tumorigenic web. Nat. Rev. Cancer 2011, 11, 761–774. [Google Scholar] [CrossRef]
- Rathinam, R.; Berrier, A.; Alahari, S.K. Role of Rho GTPases and their regulators in cancer progression. Front. Biosci. (Landmark Ed.) 2011, 16, 2561–2571. [Google Scholar] [CrossRef]
- Duncan, R.E.; El-Sohemy, A.; Archer, M.C. Mevalonate promotes the growth of tumors derived from human cancer cells in vivo and stimulates proliferation in vitro with enhanced cyclin-dependent kinase-2 activity. J. Biol. Chem. 2004, 279, 33079–33084. [Google Scholar] [CrossRef] [PubMed]
- Ascer, E.; Bertolami, M.C.; Venturinelli, M.L.; Buccheri, V.; Souza, J.; Nicolau, J.C.; Ramires, J.A.; Serrano, C.V., Jr. Atorvastatin reduces proinflammatory markers in hypercholesterolemic patients. Atherosclerosis 2004, 177, 161–166. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Mausner-Fainberg, K.; Luboshits, G.; Mor, A.; Maysel-Auslender, S.; Rubinstein, A.; Keren, G.; George, J. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis 2008, 197, 829–839. [Google Scholar] [CrossRef]
- Mosheimer, B.A.; Kaneider, N.C.; Feistritzer, C.; Djanani, A.; Sturn, D.H.; Patsch, J.R.; Wiedermann, C.J. CD40-ligand-dependent induction of COX-2 gene expression in endothelial cells by activated platelets: Inhibitory effects of atorvastatin. Blood Coagul. Fibrinolysis 2005, 16, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Margaritis, M.; Lee, R.; Channon, K.; Antoniades, C. Statins as anti-inflammatory agents in atherogenesis: Molecular mechanisms and lessons from the recent clinical trials. Curr. Pharm. Des. 2012, 18, 1519–1530. [Google Scholar] [CrossRef]
- Davignon, J.; Jacob, R.F.; Mason, R.P. The antioxidant effects of statins. Coron. Artery Dis. 2004, 15, 251–258. [Google Scholar] [CrossRef]
- Elewa, H.F.; El-Remessy, A.B.; Somanath, P.R.; Fagan, S.C. Diverse effects of statins on angiogenesis: New therapeutic avenues. Pharmacotherapy 2010, 30, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.J.; Lob, S.; Lindau, D.; Horzer, H.; Guckel, B.; Klein, G.; Glatzle, J.; Rammensee, H.G.; Brucher, B.L.; Konigsrainer, A. Simvastatin reduces tumor cell adhesion to human peritoneal mesothelial cells by decreased expression of VCAM-1 and β1 integrin. Int. J. Oncol. 2011, 39, 1593–1600. [Google Scholar] [CrossRef]
- Tanaka, S.; Ishihara, N.; Suzuki, S.; Watanabe, Y.; Nagayama, D.; Yamaguchi, T.; Ohira, M.; Saiki, A.; Tanaka, T.; Tatsuno, I. Fatty acid desaturase 2 is up-regulated by the treatment with statin through geranylgeranyl pyrophosphate-dependent Rho kinase pathway in HepG2 cells. Sci. Rep. 2019, 9, 10009. [Google Scholar] [CrossRef]
- Wang, C.; Enssle, J.; Pietzner, A.; Schmöcker, C.; Weiland, L.; Ritter, O.; Jaensch, M.; Elbelt, U.; Pagonas, N.; Weylandt, K.H. Essential Polyunsaturated Fatty Acids in Blood from Patients with and without Catheter-Proven Coronary Artery Disease. Int. J. Mol. Sci. 2022, 23, 766. [Google Scholar] [CrossRef] [PubMed]
- Garshick, M.S.; Block, R.; Drenkova, K.; Tawil, M.; James, G.; Brenna, J.T. Statin therapy upregulates arachidonic acid status via enhanced endogenous synthesis in patients with plaque psoriasis. Prostaglandins Leukot. Essent. Fat. Acids 2022, 180, 102428. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, H.; Schmocker, C.; Hartmann, D.; Rohwer, N.; Rund, K.; Kutzner, L.; Nolte, F.; Ostermann, A.I.; Schebb, N.H.; Weylandt, K.H. Aspirin alone and combined with a statin suppresses eicosanoid formation in human colon tissue. J. Lipid Res. 2018, 59, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Jara-Gutiérrez, Á.; Baladrón, V. The Role of Prostaglandins in Different Types of Cancer. Cells 2021, 10, 1487. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.W.; Jiang, A.A.; Toh, E.M.S.; Ng, C.H.; Ong, Z.H.; Peng, S.; Tham, H.Y.; Sundar, R.; Chong, C.S.; Khoo, C.M. Metformin and colorectal cancer: A systematic review, meta-analysis and meta-regression. Int. J. Colorectal Dis. 2020, 35, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-w.; Choi, E.-A.; Kim, Y.-S.; Kim, Y.; You, H.-S.; Han, Y.-E.; Kim, H.-S.; Bae, Y.-J.; Kim, J.; Kang, H.-T. Metformin usage and the risk of colorectal cancer: A national cohort study. Int. J. Color. Dis. 2021, 36, 303–310. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, W.; Tong, D.; Liu, G.; Lan, W.; Zhang, D.; Xiao, H.; Zhang, Y.; Huang, Z.; Yang, J.; et al. Metformin represses bladder cancer progression by inhibiting stem cell repopulation via COX2/PGE2/STAT3 axis. Oncotarget 2016, 7, 28235–28246. [Google Scholar] [CrossRef] [PubMed]
- Dahabiyeh, L.A.; Mujammami, M.; Arafat, T.; Benabdelkamel, H.; Alfadda, A.A.; Abdel Rahman, A.M. A Metabolic Pattern in Healthy Subjects Given a Single Dose of Metformin: A Metabolomics Approach. Front. Pharmacol. 2021, 12, 705932. [Google Scholar] [CrossRef]
- Dahabiyeh, L.A.; Mujammami, M.; AlMalki, R.H.; Arafat, T.; Benabdelkamel, H.; Alfadda, A.A.; Abdel Rahman, A.M. Lipids Alterations Associated with Metformin in Healthy Subjects: An Investigation Using Mass Spectrometry Shotgun Approach. Int. J. Mol. Sci. 2022, 23, 11478. [Google Scholar] [CrossRef]
- Guo, Z.; Sevrioukova, I.F.; Denisov, I.G.; Zhang, X.; Chiu, T.L.; Thomas, D.G.; Hanse, E.A.; Cuellar, R.A.D.; Grinkova, Y.V.; Langenfeld, V.W.; et al. Heme Binding Biguanides Target Cytochrome P450-Dependent Cancer Cell Mitochondria. Cell Chem. Biol. 2017, 24, 1259–1275.e1256. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Johnson, V.; Barrera, J.; Porras, M.; Hinojosa, D.; Hernández, I.; McGarrah, P.; Potter, D.A. Targeting cytochrome P450-dependent cancer cell mitochondria: Cancer associated CYPs and where to find them. Cancer Metastasis Rev. 2018, 37, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Zelenay, S.; van der Veen, A.G.; Böttcher, J.P.; Snelgrove, K.J.; Rogers, N.; Acton, S.E.; Chakravarty, P.; Girotti, M.R.; Marais, R.; Quezada, S.A.; et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 2015, 162, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, C.; Zhang, K.; Lin, C.; Wu, F.; Tang, X.; Wu, D.; Dou, Y.; Han, R.; Wang, Y.; et al. Metformin Combining PD-1 Inhibitor Enhanced Anti-Tumor Efficacy in STK11 Mutant Lung Cancer Through AXIN-1-Dependent Inhibition of STING Ubiquitination. Front. Mol. Biosci. 2022, 9, 780200. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Yang, W.H.; Xia, W.; Wei, Y.; Chan, L.C.; Lim, S.O.; Li, C.W.; Kim, T.; Chang, S.S.; Lee, H.H.; et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol. Cell 2018, 71, 606–620.e607. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.J.; Lee, C.H.; Bae, J.H.; Park, J.M.; Park, S.S.; Baek, M.C. Atorvastatin Enhances the Efficacy of Immune Checkpoint Therapy and Suppresses the Cellular and Extracellular Vesicle PD-L1. Pharmaceutics 2022, 14, 1660. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.G.; Wang, W.; Rothenberger, E.; Yang, J.; Gilligan, M.M.; Kipper, F.C.; Attaya, A.; Gartung, A.; Hwang, S.H.; Gillespie, M.J.; et al. Enhancing cancer immunotherapy via inhibition of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA 2024, 121, e2314085121. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; Rastelli, F.; Bisonni, R.; Santini, D.; et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J. Immunother. Cancer 2020, 8, e001361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Chen, S.; Li, Z.; Chen, J.; Li, W. The effect of concomitant use of statins, NSAIDs, low-dose aspirin, metformin and beta-blockers on outcomes in patients receiving immune checkpoint inhibitors: A systematic review and meta-analysis. Oncoimmunology 2021, 10, 1957605. [Google Scholar] [CrossRef]
- Araki, T.; Kanda, S.; Ide, T.; Sonehara, K.; Komatsu, M.; Tateishi, K.; Minagawa, T.; Kiniwa, Y.; Kawakami, S.; Nomura, S.; et al. Antiplatelet drugs may increase the risk for checkpoint inhibitor-related pneumonitis in advanced cancer patients. ESMO Open 2023, 8, 102030. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Z.; Li, R.; Huang, R.; Peng, X. The association between aspirin use and immune-related adverse events in specific cancer patients receiving ICIs therapy: Analysis of the FAERS database. Front. Pharmacol. 2023, 14, 1259628. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.Z.; Mercado, R.R.; Shirai, K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J. Immunother. Cancer 2018, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.Z.; Dragnev, K.; Sarwar, T.; Shirai, K. Clinical outcomes in non-small-cell lung cancer patients receiving concurrent metformin and immune checkpoint inhibitors. Lung Cancer Manag. 2019, 8, Lmt11. [Google Scholar] [CrossRef]
- Yang, J.; Kim, S.H.; Jung, E.H.; Kim, S.-A.; Suh, K.J.; Lee, J.Y.; Kim, J.-W.; Kim, J.W.; Lee, J.-O.; Kim, Y.J.; et al. The effect of metformin or dipeptidyl peptidase 4 inhibitors on clinical outcomes in metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. Thoracic Cancer 2023, 14, 52–60. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).