The Functional Roles of the Src Homology 2 Domain-Containing Inositol 5-Phosphatases SHIP1 and SHIP2 in the Pathogenesis of Human Diseases
Abstract
1. Introduction
2. The Structure and Function of SH2 Domain-Containing Inositol Phosphatases
2.1. General Structure of SH2 Domain-Containing Inositol Phosphatases
2.2. Structural Similarities and Differences between the SHIP Paralogs
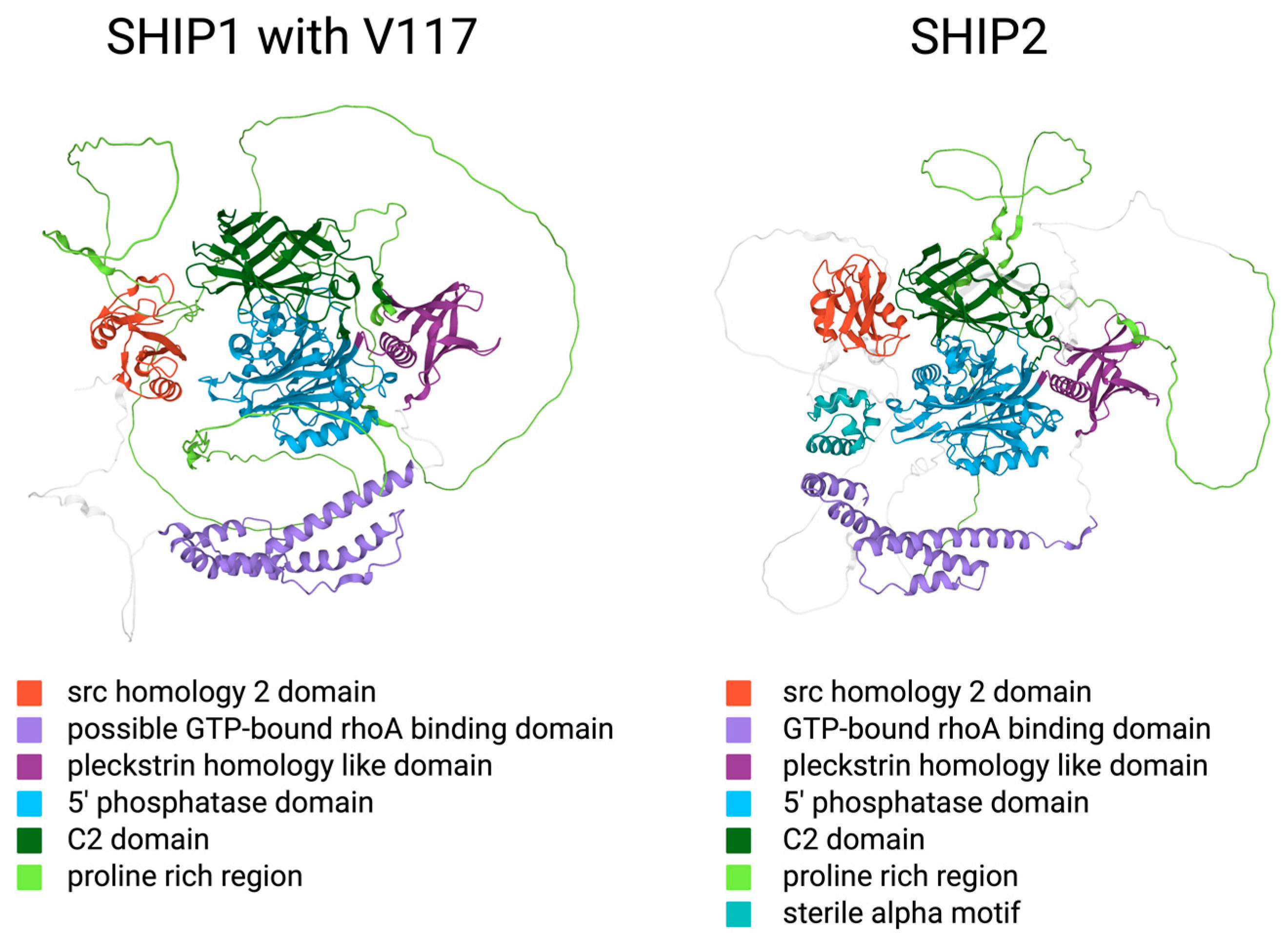
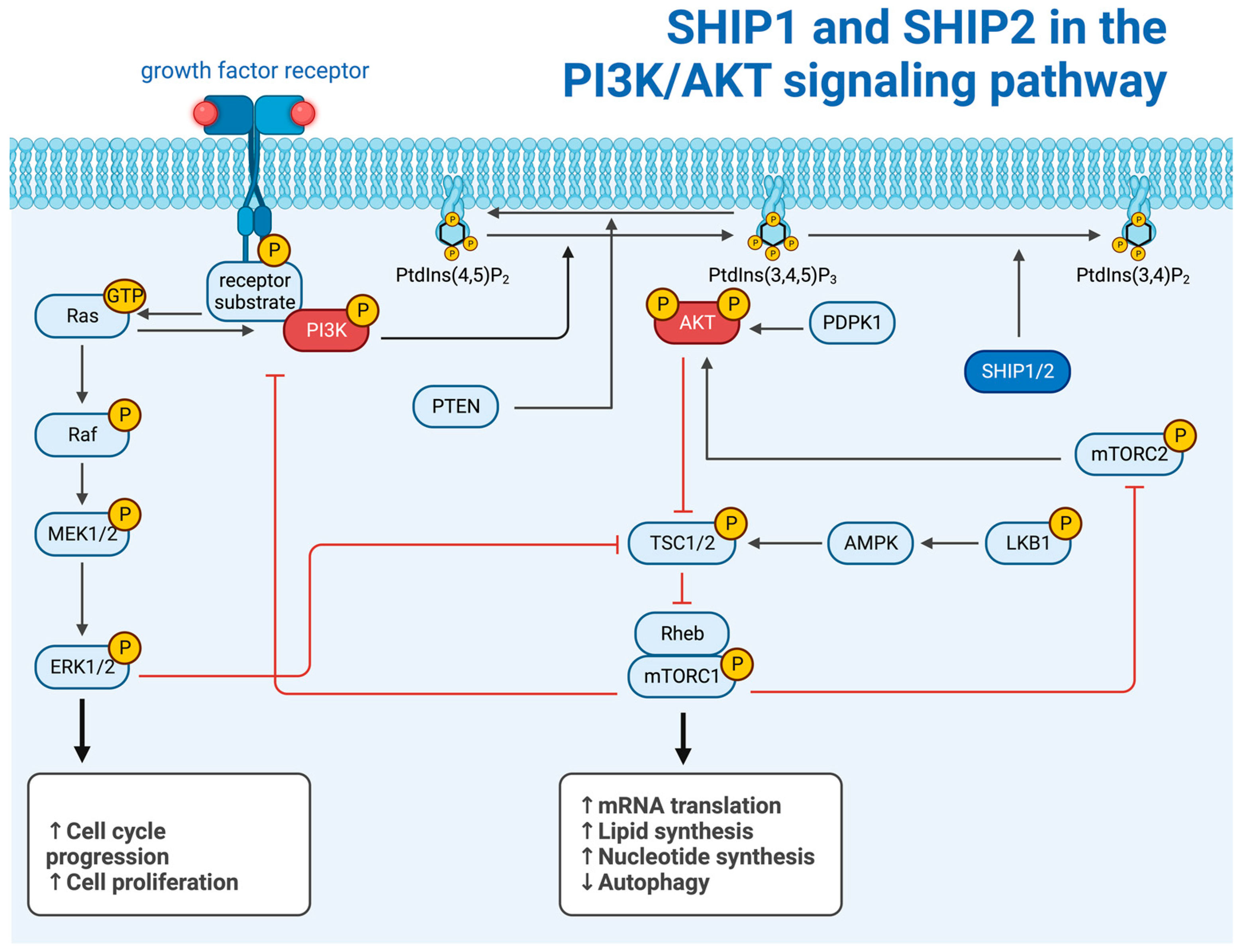
2.3. The Intracellular Function of SHIP1 and SHIP2
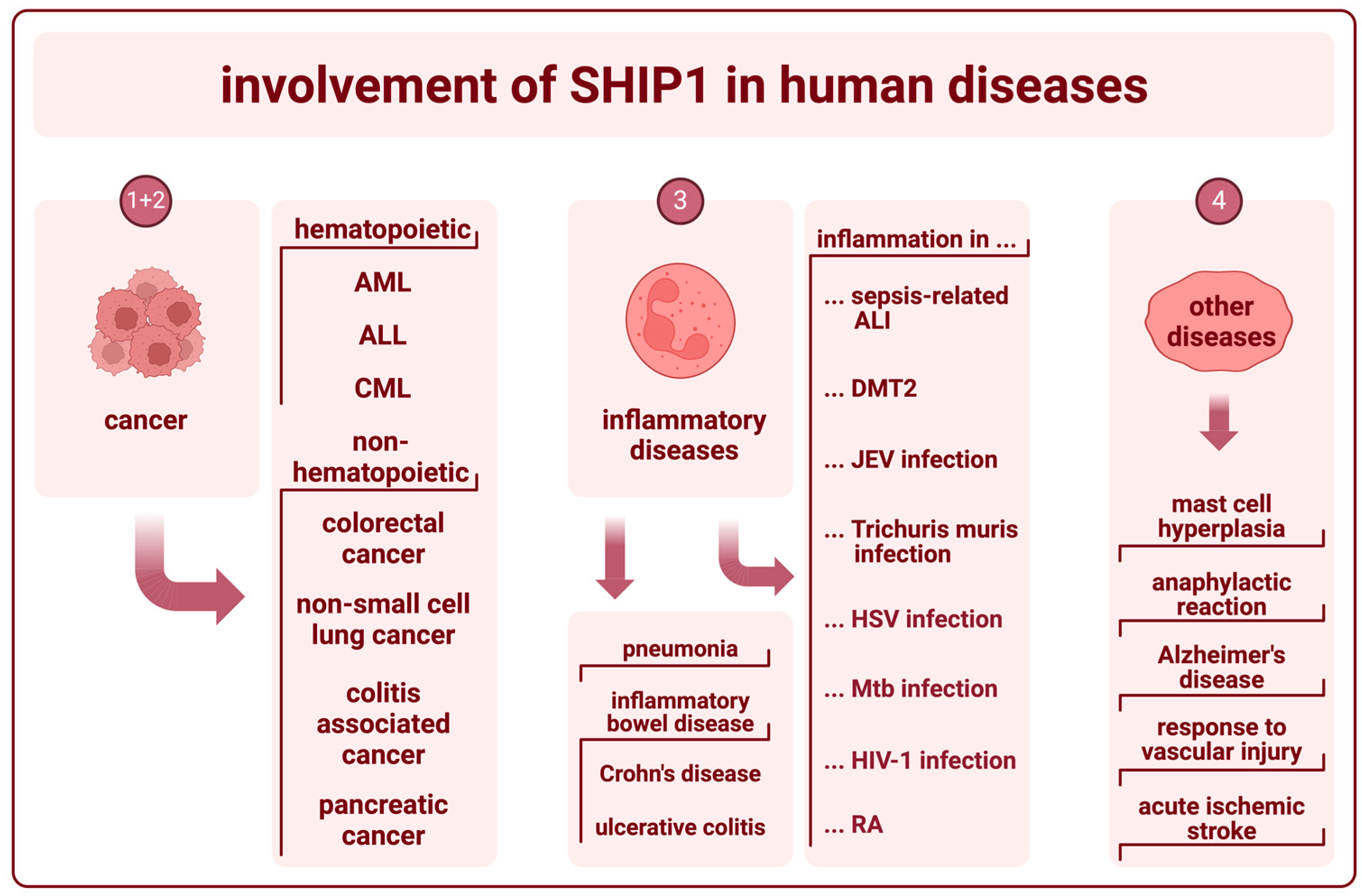
3. The Role of SHIP1 in Human Diseases
3.1. SHIP1 in Hematopoietic Cancer
3.2. SHIP1 in Other Types of Cancer
3.3. SHIP1 in Inflammatory Diseases
3.4. SHIP1 in Other Diseases
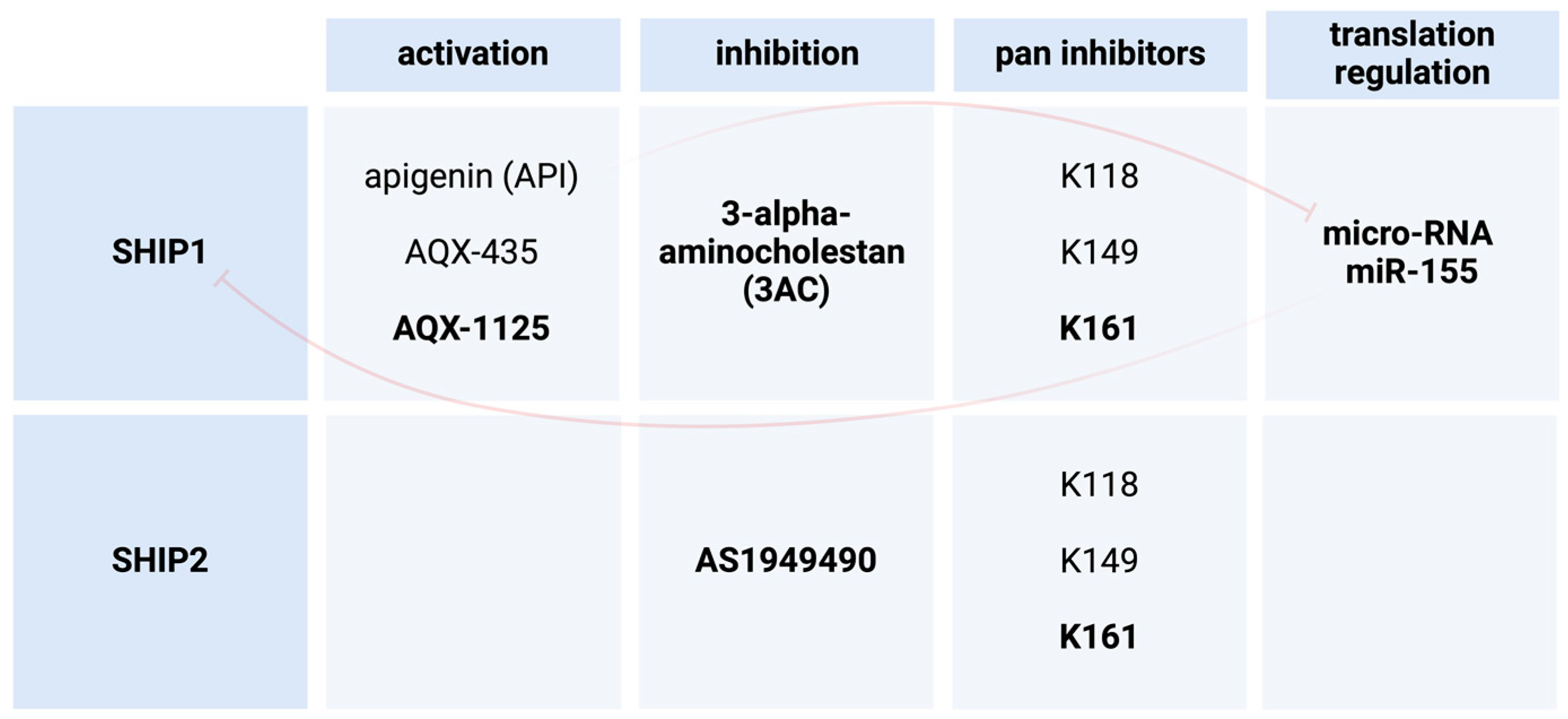
4. SHIP1 Modulation as a Possible Treatment in Human Diseases
4.1. 3AC as a SHIP1 Inhibitor in Leukemia
4.2. 3AC as a SHIP1 Inhibitor in Leishmania Major and Leishmania Donovani
4.3. SHIP1 Activation through miRNA Inhibition
4.4. AQX-435 as a SHIP1 Activator in B Cell Neoplasms
4.5. SHIP1 Hyper-Activation in CLL
4.6. AQX-1125 as a Small Molecule Activator of SHIP1
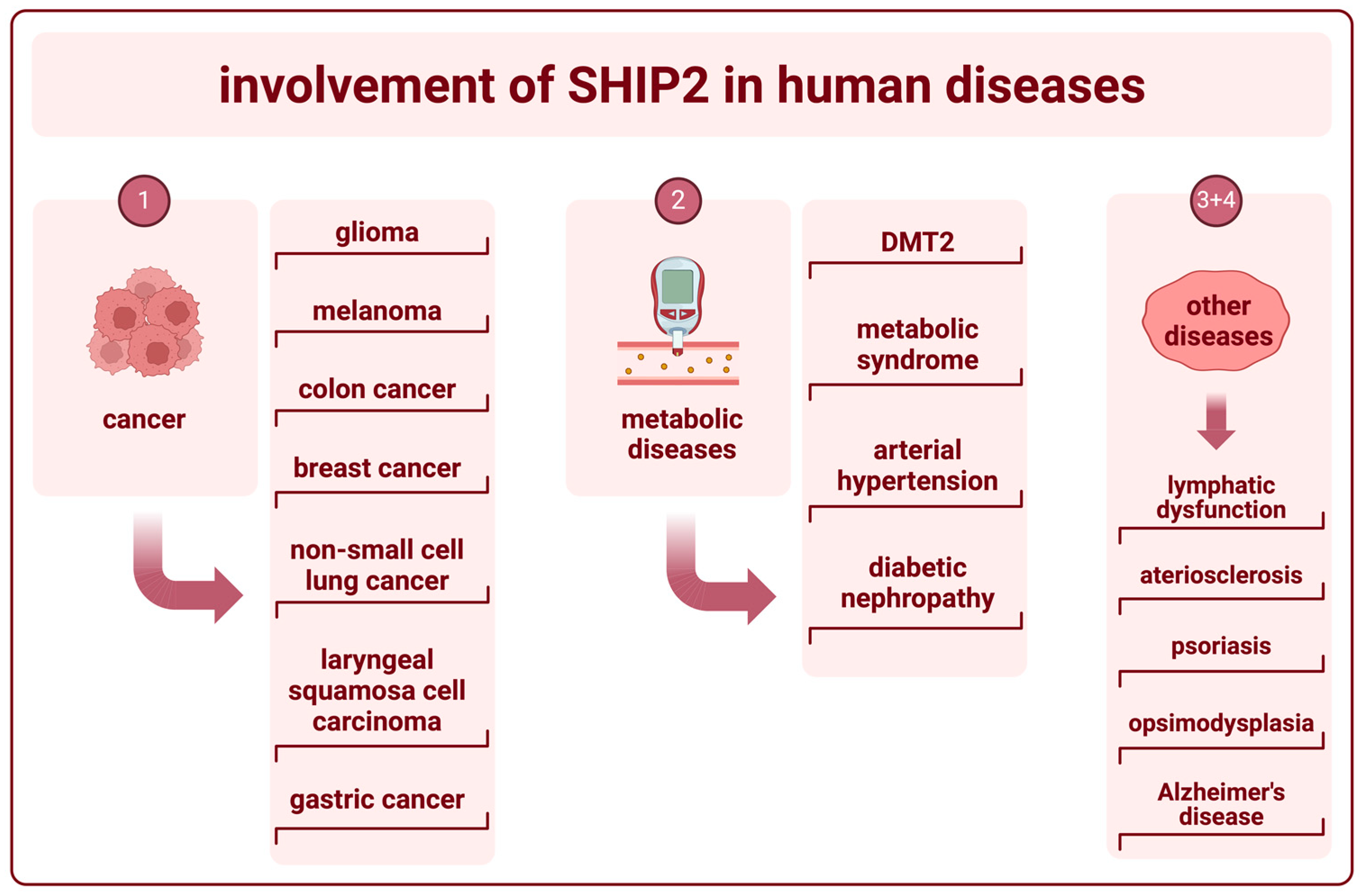
5. The Role of SHIP2 in Human Diseases
5.1. SHIP2 in Cancer
5.2. SHIP2 in Metabolic Diseases
5.3. The Role of SHIP2 in Vascular Function
5.4. SHIP2 in Other Diseases
6. SHIP2 Modulation as a Treatment in Human Diseases
6.1. AS1949490 as a SHIP2 Inhibitor in DMT2
6.2. Pan-SHIP1/SHIP2 Inhibitor K161 in AD
7. Discussion and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedicone, C.; Meyer, S.T.; Chisholm, J.D.; Kerr, W.G. Targeting SHIP1 and SHIP2 in Cancer. Cancers 2021, 13, 890. [Google Scholar] [CrossRef] [PubMed]
- Suwa, A.; Kurama, T.; Shimokawa, T. SHIP2 and Its Involvement in Various Diseases. Expert Opin. Ther. Targets 2010, 14, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.P.; Erneux, C.; Potter, B.V.L. SHIP2: Structure, Function and Inhibition. ChemBioChem 2017, 18, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Rohrschneider, L.R.; Fuller, J.F.; Wolf, I.; Liu, Y.; Lucas, D.M. Structure, Function, and Biology of SHIP Proteins. Genes Dev. 2000, 14, 505–520. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium; Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Nalaskowski, M.M.; Ehm, P.; Rehbach, C.; Nelson, N.; Täger, M.; Modest, K.; Jücker, M. Nuclear Accumulation of SHIP1 Mutants Derived from AML Patients Leads to Increased Proliferation of Leukemic Cells. Cell. Signal. 2018, 49, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, R.; Fernandes, S.; Deuring, J.J.; de Haar, C.; Kuipers, E.J.; Vogelaar, L.; Middleton, F.A.; van der Woude, C.J.; Peppelenbosch, M.P.; Kerr, W.G.; et al. Analysis of SHIP1 Expression and Activity in Crohn’s Disease Patients. PLoS ONE 2017, 12, e0182308. [Google Scholar] [CrossRef]
- Fernandes, S.; Iyer, S.; Kerr, W.G. Role of SHIP1 in Cancer and Mucosal Inflammation: Role of SHIP1 in Cancer and Mucosal Inflammation. Ann. N. Y. Acad. Sci. 2013, 1280, 6–10. [Google Scholar] [CrossRef]
- Weinberg, E.D. Current Topics in Microbiology and Immunology. Volume 72. Microchem. J. 1977, 22, 590. [Google Scholar] [CrossRef]
- Husain, K.; Villalobos-Ayala, K.; Laverde, V.; Vazquez, O.A.; Miller, B.; Kazim, S.; Blanck, G.; Hibbs, M.L.; Krystal, G.; Elhussin, I.; et al. Apigenin Targets MicroRNA-155, Enhances SHIP-1 Expression, and Augments Anti-Tumor Responses in Pancreatic Cancer. Cancers 2022, 14, 3613. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, M.J.; Duan, M.; Armes, J.E.; Anderson, G.P.; Tarlinton, D.M.; Hibbs, M.L. Genetic Segregation of Inflammatory Lung Disease and Autoimmune Disease Severity in SHIP-1−/− Mice. J. Immunol. 2011, 186, 7164–7175. [Google Scholar] [CrossRef]
- Kerr, W.G. Inhibitor and Activator: Dual Functions for SHIP in Immunity and Cancer: Divergent Roles for SHIP in Immune Signaling. Ann. N. Y. Acad. Sci. 2011, 1217, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Falasca, M. (Ed.) Phosphoinositides and Disease; Current Topics in Microbiology and Immunology; Springer: Dordrecht, The Netherlands, 2012; Volume 362, ISBN 978-94-007-5024-1. [Google Scholar]
- Zajac, D.J.; Simpson, J.; Zhang, E.; Parikh, I.; Estus, S. Expression of INPP5D Isoforms in Human Brain: Impact of Alzheimer’s Disease Neuropathology and Genetics. Genes 2023, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Metzner, A.; Precht, C.; Fehse, B.; Fiedler, W.; Stocking, C.; Günther, A.; Mayr, G.W.; Jücker, M. Reduced Proliferation of CD34+ Cells from Patients with Acute Myeloid Leukemia after Gene Transfer of INPP5D. Gene Ther. 2009, 16, 570–573. [Google Scholar] [CrossRef]
- Ehm, P.; Rietow, R.; Wegner, W.; Bußmann, L.; Kriegs, M.; Dierck, K.; Horn, S.; Streichert, T.; Horstmann, M.; Jücker, M. SHIP1 Is Present but Strongly Downregulated in T-ALL, and after Restoration Suppresses Leukemia Growth in a T-ALL Xenotransplantation Mouse Model. Cells 2023, 12, 1798. [Google Scholar] [CrossRef]
- Ooms, L.M.; Horan, K.A.; Rahman, P.; Seaton, G.; Gurung, R.; Kethesparan, D.S.; Mitchell, C.A. The Role of the Inositol Polyphosphate 5-Phosphatases in Cellular Function and Human Disease. Biochem. J. 2009, 419, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.; Kruse, V.; Fuhrmann, D.C.; Wolf, S.; Löber, J.; Alsouri, S.; Paglilla, N.; Lee, K.; Chapuy, B.; Brüne, B.; et al. SH2 Domain-Containing Inositol 5-Phosphatases Support the Survival of Burkitt Lymphoma Cells by Promoting Energy Metabolism. Haematologica 2024, 109, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Schaks, M.; Allgoewer, K.; Nelson, N.; Ehm, P.; Heumann, A.; Ewald, F.; Schumacher, U.; Simon, R.; Sauter, G.; Jücker, M. Ectopic Expression of Hematopoietic SHIP1 in Human Colorectal Cancer. Biomedicines 2020, 8, 215. [Google Scholar] [CrossRef]
- Fu, Q.; Huang, Y.; Ge, C.; Li, Z.; Tian, H.; Li, Q.; Li, H.; Li, R.; Tao, X.; Xue, Y.; et al. SHIP1 Inhibits Cell Growth, Migration, and Invasion in Non-small Cell Lung Cancer through the PI3K/AKT Pathway. Oncol. Rep. 2019, 41, 2337–2350. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liao, M.; Wang, J. TLR4 Signaling in the Development of Colitis-Associated Cancer and Its Possible Interplay with microRNA-155. Cell Commun. Signal 2021, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Srivastava, N.; Sudan, R.; Middleton, F.A.; Shergill, A.K.; Ryan, J.C.; Kerr, W.G. SHIP1 Deficiency in Inflammatory Bowel Disease Is Associated With Severe Crohn’s Disease and Peripheral T Cell Reduction. Front. Immunol. 2018, 9, 1100. [Google Scholar] [CrossRef] [PubMed]
- Sudan, R.; Fernandes, S.; Srivastava, N.; Pedicone, C.; Meyer, S.T.; Chisholm, J.D.; Engelman, R.W.; Kerr, W.G. LRBA Deficiency Can Lead to Lethal Colitis That Is Diminished by SHIP1 Agonism. Front. Immunol. 2022, 13, 830961. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Yang, J.; Guo, S.; Zhao, G.; Wu, H.; Deng, G. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol. Ther. 2019, 27, 1758–1771. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.C.; Shakibakho, S.; Durrer, C.; Simtchouk, S.; Jawanda, K.K.; Cheung, S.T.; Mui, A.L.; Little, J.P. Hyporesponsiveness to the Anti-Inflammatory Action of Interleukin-10 in Type 2 Diabetes. Sci. Rep. 2016, 6, 21244. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, U.; Ding, Z.; Deng, S.; Ye, J.; Cao, S.; Chen, Z. Pathogenicity and Virulence of Japanese Encephalitis Virus: Neuroinflammation and Neuronal Cell Damage. Virulence 2021, 12, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, S.; Antignano, F.; Hughes, M.R.; Wang, S.K.H.; Snyder, K.; Sammis, G.M.; Kerr, W.G.; McNagny, K.M.; Zaph, C. Myeloid Cell-Specific Expression of Ship1 Regulates IL-12 Production and Immunity to Helminth Infection. Mucosal Immunol. 2012, 5, 535–543. [Google Scholar] [CrossRef][Green Version]
- Qin, Q.; Wang, Y.; Huang, X.; Jin, X. SHIP-1 Affects Herpetic Simplex Keratitis Prognosis by Mediating CD4+ T Lymphocytes Migration through PI3K Signaling and Transcription Factor KLF2 in the Cornea. Antivir. Res. 2022, 207, 105424. [Google Scholar] [CrossRef]
- Rothchild, A.C.; Sissons, J.R.; Shafiani, S.; Plaisier, C.; Min, D.; Mai, D.; Gilchrist, M.; Peschon, J.; Larson, R.P.; Bergthaler, A.; et al. MiR-155–Regulated Molecular Network Orchestrates Cell Fate in the Innate and Adaptive Immune Response to Mycobacterium Tuberculosis. Proc. Natl. Acad. Sci. USA 2016, 113, E6172–E6181. [Google Scholar] [CrossRef]
- Alter, G.; Suscovich, T.J.; Kleyman, M.; Teigen, N.; Streeck, H.; Zaman, M.T.; Meier, A.; Altfeld, M. Low Perforin and Elevated SHIP-1 Expression Is Associated with Functional Anergy of Natural Killer Cells in Chronic HIV-1 Infection. Aids 2006, 20, 1549–1551. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a Proinflammatory Regulator in Clinical and Experimental Arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef] [PubMed]
- Haddon, D.J.; Antignano, F.; Hughes, M.R.; Blanchet, M.-R.; Zbytnuik, L.; Krystal, G.; McNagny, K.M. SHIP1 Is a Repressor of Mast Cell Hyperplasia, Cytokine Production, and Allergic Inflammation In Vivo. J. Immunol. 2009, 183, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.P.; Lin, P.B.-C.; Dong, C.; Moutinho, M.; Casali, B.T.; Liu, Y.; Lamb, B.T.; Landreth, G.E.; Oblak, A.L.; Nho, K. INPP5D Expression Is Associated with Risk for Alzheimer’s Disease and Induced by Plaque-Associated Microglia. Neurobiol. Dis. 2021, 153, 105303. [Google Scholar] [CrossRef]
- Séverin, S.; Gratacap, M.-P.; Lenain, N.; Alvarez, L.; Hollande, E.; Penninger, J.M.; Gachet, C.; Plantavid, M.; Payrastre, B. Deficiency of Src Homology 2 Domain–Containing Inositol 5-Phosphatase 1 Affects Platelet Responses and Thrombus Growth. J. Clin. Investig. 2007, 117, 944–952. [Google Scholar] [CrossRef]
- Tu, W.-J.; Liu, X.-Y.; Dong, H.; Yu, Y.; Wang, Y.; Chen, H. Phosphatidylinositol-3,4,5-Trisphosphate 5-Phosphatase 1: A Meaningful and Independent Marker to Predict Stroke in the Chinese Population. J. Mol. Neurosci. 2014, 52, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.; Fuhler, G.M.; Iyer, S.; Smith, M.J.; Park, M.-Y.; Paraiso, K.H.T.; Engelman, R.W.; Kerr, W.G. SHIP1 Inhibition Increases Immunoregulatory Capacity and Triggers Apoptosis of Hematopoietic Cancer Cells. J. Immunol. 2010, 184, 3582–3589. [Google Scholar] [CrossRef]
- Lemm, E.A.; Valle-Argos, B.; Smith, L.D.; Richter, J.; Gebreselassie, Y.; Carter, M.J.; Karolova, J.; Svaton, M.; Helman, K.; Weston-Bell, N.J.; et al. Preclinical Evaluation of a Novel SHIP1 Phosphatase Activator for Inhibition of PI3K Signaling in Malignant B Cells. Clin. Cancer Res. 2020, 26, 1700–1711. [Google Scholar] [CrossRef]
- Ecker, V.; Stumpf, M.; Brandmeier, L.; Neumayer, T.; Pfeuffer, L.; Engleitner, T.; Ringshausen, I.; Nelson, N.; Jücker, M.; Wanninger, S.; et al. Targeted PI3K/AKT-Hyperactivation Induces Cell Death in Chronic Lymphocytic Leukemia. Nat. Commun. 2021, 12, 3526. [Google Scholar] [CrossRef]
- Nickel, J.C.; Egerdie, B.; Davis, E.; Evans, R.; Mackenzie, L.; Shrewsbury, S.B. A Phase II Study of the Efficacy and Safety of the Novel Oral SHIP1 Activator AQX-1125 in Subjects with Moderate to Severe Interstitial Cystitis/Bladder Pain Syndrome. J. Urol. 2016, 196, 747–754. [Google Scholar] [CrossRef]
- Mokni, M. Leishmanioses cutanées. Ann. Dermatol. Vénéréologie 2019, 146, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Nagar, R.; Brandt, C.; Harcourt, K.; Clare, S.; Ferguson, M.A.J.; Wright, G.J. The Leishmania Donovani Ortholog of the Glycosylphosphatidylinositol Anchor Biosynthesis Cofactor PBN1 Is Essential for Host Infection. mBio 2022, 13, e00433-22. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, B.P.; Das, S.; Bodhale, N.; Prakash Pandey, S.; Sudan, R.; Srivastava, N.; Chisholm, J.D.; Kerr, W.G.; Majumdar, S.; Saha, B. SHIP1 Inhibition via 3-Alpha-Amino-Cholestane Enhances Protection against Leishmania Infection. Cytokine 2023, 171, 156373. [Google Scholar] [CrossRef] [PubMed]
- Stenton, G.R.; Mackenzie, L.F.; Tam, P.; Cross, J.L.; Harwig, C.; Raymond, J.; Toews, J.; Wu, J.; Ogden, N.; MacRury, T.; et al. Characterization of AQX-1125, a Small-molecule SHIP1 Activator: Part 1. Effects on Inflammatory Cell Activation and Chemotaxis in Vitro and Pharmacokinetic Characterization in Vivo. Br. J. Pharmacol. 2013, 168, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); Identifier: NCT02324972. Pilot Efficacy and Safety Study of AQX-1125 in Atopic Dermatitis (KINSHIP). Available online: https://clinicaltrials.gov/study/NCT02324972?cond=ship1&rank=1 (accessed on 9 April 2024).
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); Identifier: NCT01882543. Efficacy and Safety of AQX-1125 in IC/BPS (LEADERSHIP). Available online: https://clinicaltrials.gov/study/NCT01882543?cond=ship1&rank=2 (accessed on 9 April 2024).
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); Identifier: NCT01954628. Efficacy and Safety of AQX-1125 in Unstable COPD (FLAGSHIP). Available online: https://clinicaltrials.gov/study/NCT01954628?cond=ship1&rank=3 (accessed on 9 April 2024).
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).; Identifier: NCT02858453. Efficacy and Safety of 2 Doses of AQX-1125 in Subjects With Interstitial Cystitis/Bladder Pain Syndrome (LEADERSHIP 301). Available online: https://clinicaltrials.gov/study/NCT02858453?cond=ship1&rank=4 (accessed on 9 April 2024).
- Elong Edimo, W.; Derua, R.; Janssens, V.; Nakamura, T.; Vanderwinden, J.-M.; Waelkens, E.; Erneux, C. Evidence of SHIP2 Ser132 Phosphorylation, Its Nuclear Localization and Stability. Biochem. J. 2011, 439, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fu, M.; Ding, Y.; Weng, Y.; Fan, W.; Pu, X.; Ge, Z.; Zhan, F.; Ni, H.; Zhang, W.; et al. High SHIP2 Expression Indicates Poor Survival in Colorectal Cancer. Dis. Markers 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Csolle, M.P.; Ooms, L.M.; Papa, A.; Mitchell, C.A. PTEN and Other PtdIns(3,4,5)P3 Lipid Phosphatases in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 9189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Y.; Tan, G. Prognostic Value of Elevated SHIP2 Expression in Laryngeal Squamous Cell Carcinoma. Arch. Med. Res. 2011, 42, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Murata-Kamiya, N.; Hatakeyama, M. Helicobacter Pylori CagA Oncoprotein Interacts with SHIP2 to Increase Its Delivery into Gastric Epithelial Cells. Cancer Sci. 2020, 111, 1596–1606. [Google Scholar] [CrossRef]
- Hatakeyama, M. Structure and Function of Helicobacter Pylori CagA, the First-Identified Bacterial Protein Involved in Human Cancer. Proc. Jpn. Academy. Ser. B Phys. Biol. Sci. 2017, 93, 196–219. [Google Scholar] [CrossRef]
- Marion, E.; Kaisaki, P.J.; Pouillon, V.; Gueydan, C.; Levy, J.C.; Bodson, A.; Krzentowski, G.; Daubresse, J.-C.; Mockel, J.; Behrends, J.; et al. The Gene INPPL1, Encoding the Lipid Phosphatase SHIP2, Is a Candidate for Type 2 Diabetes In Rat and Man. Diabetes 2002, 51, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Hyvönen, M.E.; Ihalmo, P.; Forsblom, C.; Thorn, L.; Sandholm, N.; Lehtonen, S.; Groop, P.-H. INPPL1 Is Associated with the Metabolic Syndrome in Men with Type 1 Diabetes, but Not with Diabetic Nephropathy: INPPL1 Is Associated with the Metabolic Syndrome in Type 1 Diabetes. Diabet. Med. 2012, 29, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Agollah, G.D.; Gonzalez-Garay, M.L.; Rasmussen, J.C.; Tan, I.-C.; Aldrich, M.B.; Darne, C.; Fife, C.E.; Guilliod, R.; Maus, E.A.; King, P.D.; et al. Evidence for SH2 Domain-Containing 5′-Inositol Phosphatase-2 (SHIP2) Contributing to a Lymphatic Dysfunction. PLoS ONE 2014, 9, e112548. [Google Scholar] [CrossRef] [PubMed]
- DeKroon, R.; Robinette, J.B.; Hjelmeland, A.B.; Wiggins, E.; Blackwell, M.; Mihovilovic, M.; Fujii, M.; York, J.; Hart, J.; Kontos, C.; et al. APOE4-VLDL Inhibits the HDL-Activated Phosphatidylinositol 3-Kinase/Akt Pathway via the Phosphoinositol Phosphatase SHIP2. Circ. Res. 2006, 99, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Zibert, J.R.; Løvendorf, M.B.; Litman, T.; Olsen, J.; Kaczkowski, B.; Skov, L. MicroRNAs and Potential Target Interactions in Psoriasis. J. Dermatol. Sci. 2010, 58, 177–185. [Google Scholar] [CrossRef]
- Ando, K.; Erneux, C.; Homa, M.; Houben, S.; de Fisenne, M.-A.; Brion, J.-P.; Leroy, K. Dysregulation of Phosphoinositide 5-Phosphatases and Phosphoinositides in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 614855. [Google Scholar] [CrossRef] [PubMed]
- Suwa, A.; Yamamoto, T.; Sawada, A.; Minoura, K.; Hosogai, N.; Tahara, A.; Kurama, T.; Shimokawa, T.; Aramori, I. Discovery and Functional Characterization of a Novel Small Molecule Inhibitor of the Intracellular Phosphatase, SHIP2: Discovery of First Small Molecule SHIP2 Inhibitor. Br. J. Pharmacol. 2009, 158, 879–887. [Google Scholar] [CrossRef]
- Pedicone, C.; Fernandes, S.; Dungan, O.M.; Dormann, S.M.; Viernes, D.R.; Adhikari, A.A.; Choi, L.B.; De Jong, E.P.; Chisholm, J.D.; Kerr, W.G. Pan-SHIP1/2 Inhibitors Promote Microglia Effector Functions Essential for CNS Homeostasis. J. Cell Sci. 2020, 133, jcs238030. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, S.M.; Jücker, M. The Functional Roles of the Src Homology 2 Domain-Containing Inositol 5-Phosphatases SHIP1 and SHIP2 in the Pathogenesis of Human Diseases. Int. J. Mol. Sci. 2024, 25, 5254. https://doi.org/10.3390/ijms25105254
Müller SM, Jücker M. The Functional Roles of the Src Homology 2 Domain-Containing Inositol 5-Phosphatases SHIP1 and SHIP2 in the Pathogenesis of Human Diseases. International Journal of Molecular Sciences. 2024; 25(10):5254. https://doi.org/10.3390/ijms25105254
Chicago/Turabian StyleMüller, Spike Murphy, and Manfred Jücker. 2024. "The Functional Roles of the Src Homology 2 Domain-Containing Inositol 5-Phosphatases SHIP1 and SHIP2 in the Pathogenesis of Human Diseases" International Journal of Molecular Sciences 25, no. 10: 5254. https://doi.org/10.3390/ijms25105254
APA StyleMüller, S. M., & Jücker, M. (2024). The Functional Roles of the Src Homology 2 Domain-Containing Inositol 5-Phosphatases SHIP1 and SHIP2 in the Pathogenesis of Human Diseases. International Journal of Molecular Sciences, 25(10), 5254. https://doi.org/10.3390/ijms25105254







