Interaction between miR-142-3p and BDNF Val/Met Polymorphism Regulates Multiple Sclerosis Severity
Abstract
1. Introduction
2. Results
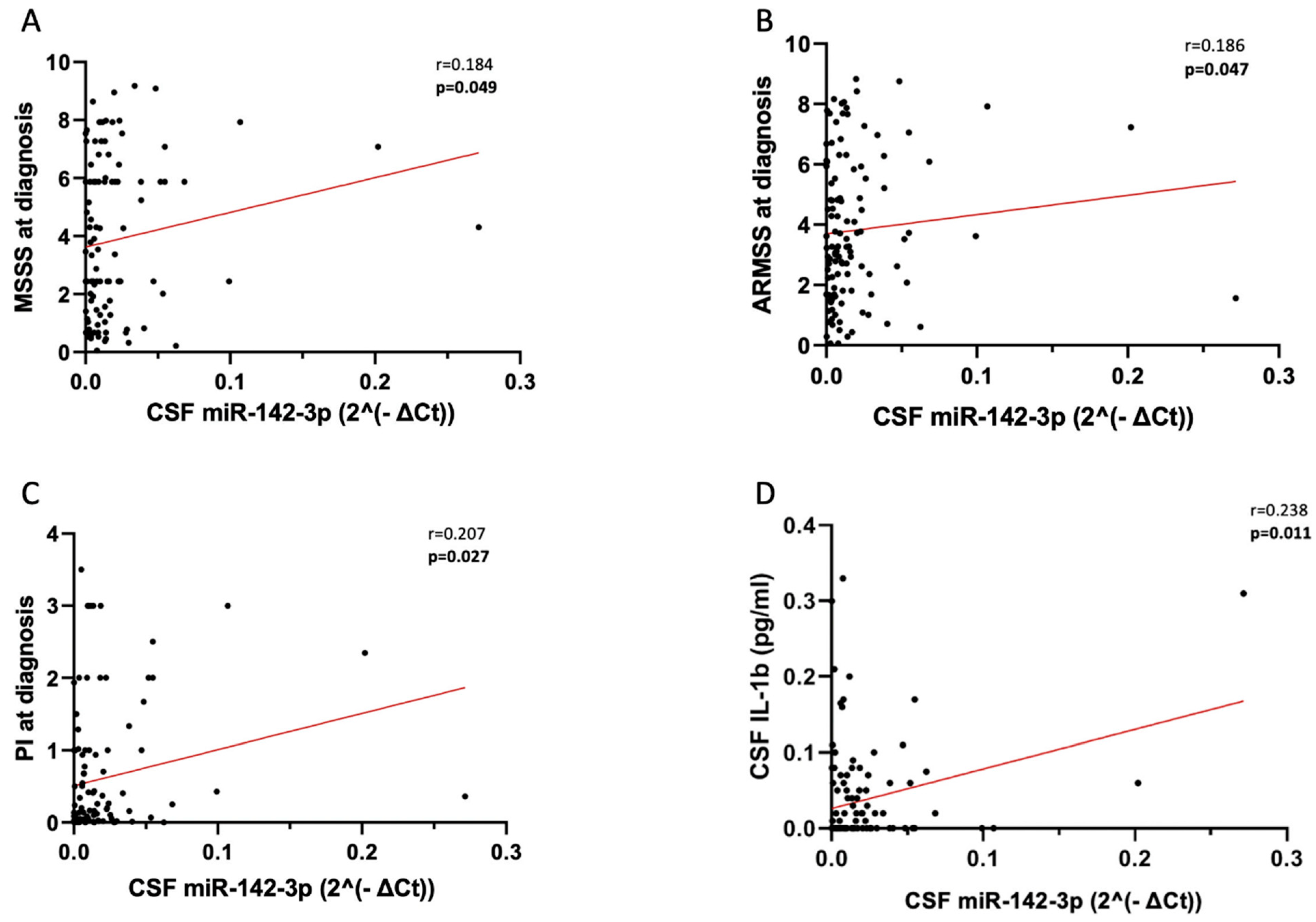
2.1. CSF miR-142-3p Associates with Adverse Clinical Signs of RRMS
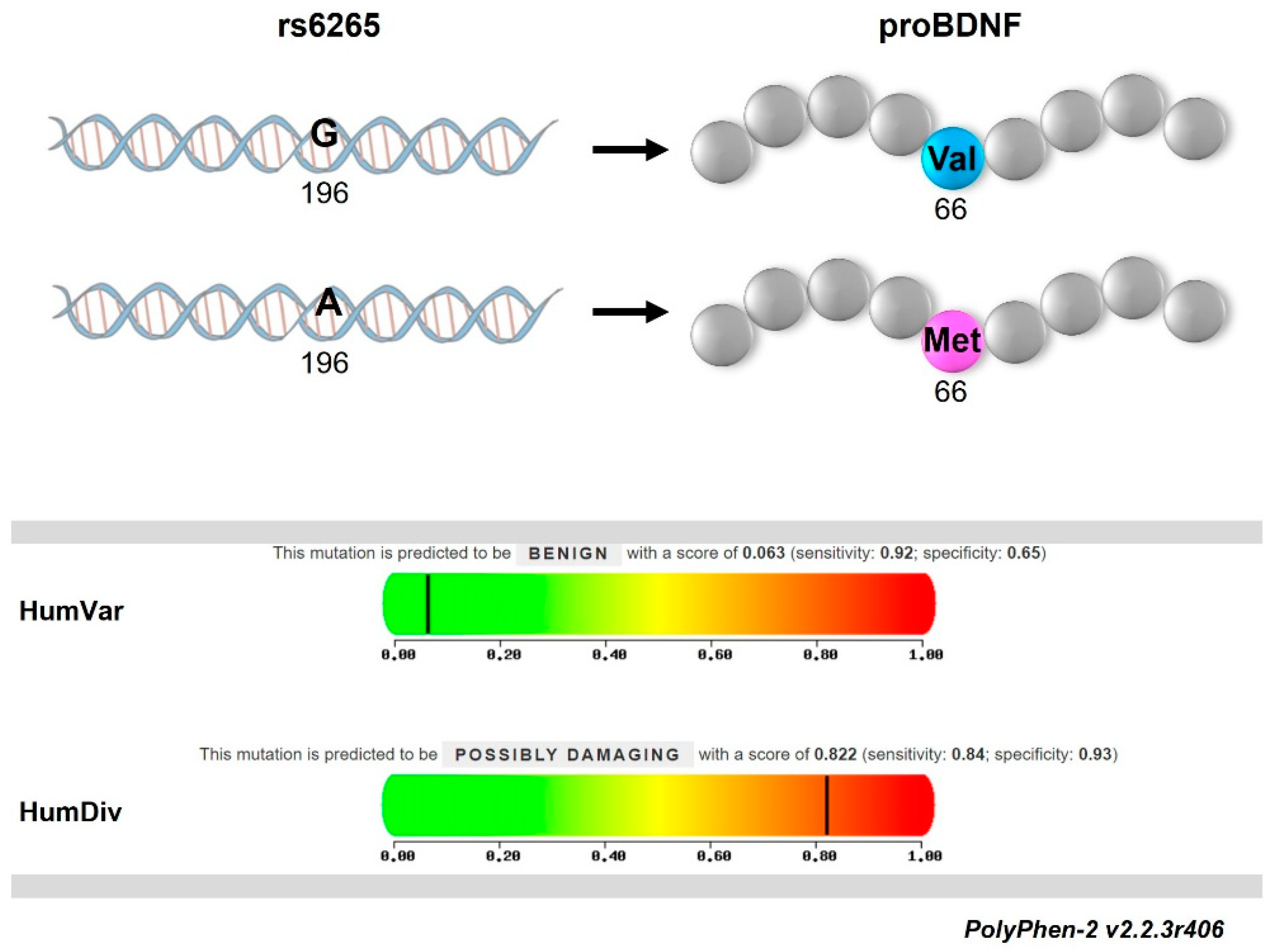
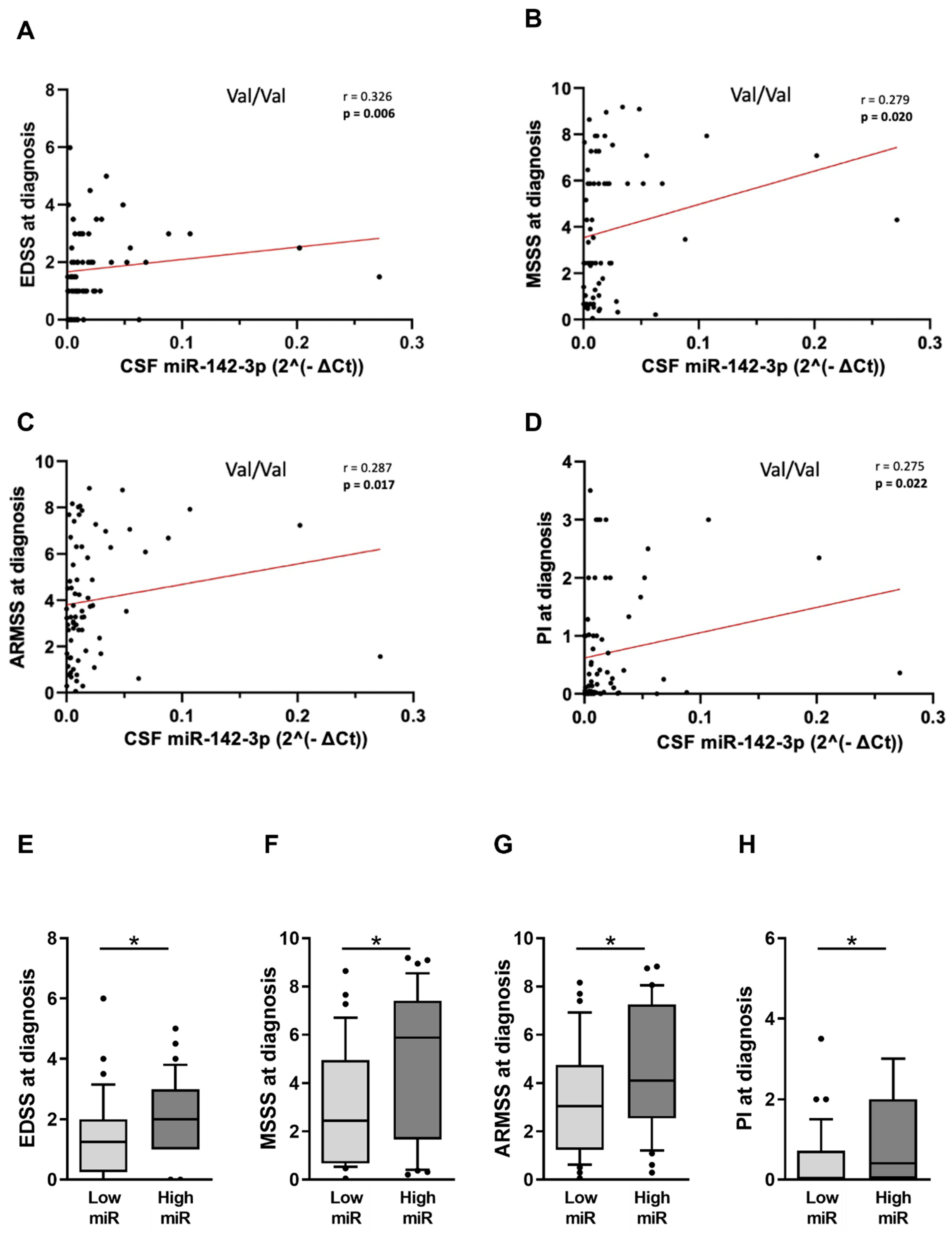
2.2. Met66-Allele Is Not Associated with the Risk of RRMS Onset but Can Contribute to Disease Course Heterogeneity
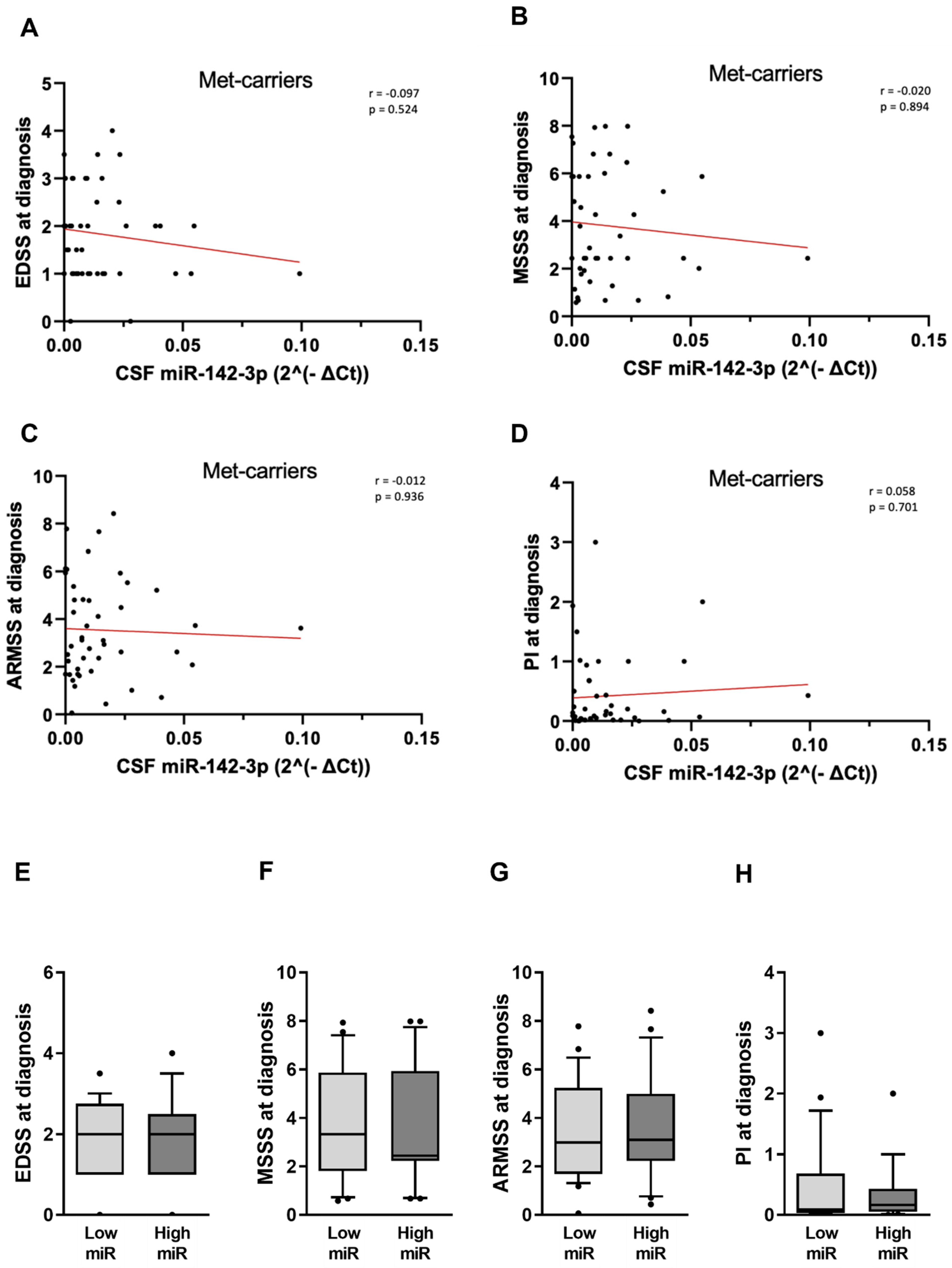
2.3. The IL1β-miR-142-3p Axis Is Disrupted in pwRRMS Carrying Met-Allele
2.4. The Detrimental Effect of miR-142-3p on Clinical Parameters Is Impaired in pwRRMS Carrying Met-Allele
3. Discussion
4. Materials and Methods
4.1. Patients with RRMS (pwRRMS)
4.2. CSF Collection and Analysis
4.3. SNP Val66Met Analysis
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Schmitz, F. Synapse Dysfunctions in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 1639. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Shams, H.; Didonna, A.; Baranzini, S.E.; Cree, B.A.C.; Hauser, S.L.; Henry, R.G.; Oksenberg, J.R. Integration of epigenetic and genetic profiles identifies multiple sclerosis disease-critical cell types and genes. Commun. Biol. 2023, 6, 342. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, G.; Gentile, A.; Musella, A.; Fresegna, D.; De Vito, F.; Bullitta, S.; Sepman, H.; Marfia, G.A.; Centonze, D. Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat. Rev. Neurol. 2015, 11, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, G.; De Vito, F.; Musella, A.; Gentile, A.; Bullitta, S.; Fresegna, D.; Sepman, H.; Di Sanza, C.; Haji, N.; Mori, F.; et al. miR-142-3p Is a Key Regulator of IL-1β-Dependent Synaptopathy in Neuroinflammation. J. Neurosci. 2017, 37, 546–561. [Google Scholar] [CrossRef] [PubMed]
- De Vito, F.; Musella, A.; Fresegna, D.; Rizzo, F.R.; Gentile, A.; Bassi, M.S.; Gilio, L.; Buttari, F.; Procaccini, C.; Colamatteo, A.; et al. MiR-142-3p regulates synaptopathy-driven disease progression in multiple sclerosis. Neuropathol. Appl. Neurobiol. 2022, 48, e12765. [Google Scholar] [CrossRef] [PubMed]
- De Vito, F.; Balletta, S.; Caioli, S.; Musella, A.; Guadalupi, L.; Vanni, V.; Fresegna, D.; Bassi, M.S.; Gilio, L.; Sanna, K.; et al. MiR-142-3p is a Critical Modulator of TNF-Mediated Neuronal Toxicity in Multiple Sclerosis. Curr. Neuropharmacol. 2023, 21, 2567–2582. [Google Scholar] [CrossRef] [PubMed]
- Junker, A.; Krumbholz, M.; Eisele, S.; Mohan, H.; Augstein, F.; Bittner, R.; Lassmann, H.; Wekerle, H.; Hohlfeld, R.; Meinl, E. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 2009, 132, 3342–3352. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, J.; Zhong, Y.; Jiang, L.; Mu, P.; Li, Y.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Expression, Regulation and Function of MicroRNAs in Multiple Sclerosis. Int. J. Med. Sci. 2014, 11, 810–818. [Google Scholar] [CrossRef]
- Gupta, N.; Jadhav, S.; Tan, K.-L.; Saw, G.; Mallilankaraman, K.B.; Dheen, S.T. miR-142-3p Regulates BDNF Expression in Activated Rodent Microglia Through Its Target CAMK2A. Front. Cell. Neurosci. 2020, 14, 132. [Google Scholar] [CrossRef]
- Schirò, G.; Iacono, S.; Ragonese, P.; Aridon, P.; Salemi, G.; Balistreri, C.R. A Brief Overview on BDNF-Trk Pathway in the Nervous System: A Potential Biomarker or Possible Target in Treatment of Multiple Sclerosis? Front. Neurol. 2022, 13, 917527. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Le Beau, M.M.; Espinosa, R.; Ip, N.Y.; Belluscio, L.; de la Monte, S.M.; Squinto, S.; Furth, M.E.; Yancopoulos, G.D. Human and rat brain-derived neurotrophic factor and neurotrophin-3: Gene structures, distributions, and chromosomal localizations. Genomics 1991, 10, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef] [PubMed]
- An, J.J.; Gharami, K.; Liao, G.-Y.; Woo, N.H.; Lau, A.G.; Vanevski, F.; Torre, E.R.; Jones, K.R.; Feng, Y.; Lu, B.; et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 2008, 134, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Varendi, K.; Kumar, A.; Härma, M.-A.; Andressoo, J.-O. miR-1, miR-10b, miR-155, and miR-191 are novel regulators of BDNF. Cell Mol. Life Sci. 2014, 71, 4443–4456. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.G.; Irier, H.A.; Gu, J.; Tian, D.; Ku, L.; Liu, G.; Xia, M.; Fritsch, B.; Zheng, J.Q.; Dingledine, R.; et al. Distinct 3′UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF). Proc. Natl. Acad. Sci. USA 2010, 107, 15945–15950. [Google Scholar] [CrossRef]
- Pang, P.T.; Teng, H.K.; Zaitsev, E.; Woo, N.T.; Sakata, K.; Zhen, S.; Teng, K.K.; Yung, W.-H.; Hempstead, B.L.; Lu, B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 2004, 306, 487–491. [Google Scholar] [CrossRef]
- Nociti, V.; Romozzi, M. The Role of BDNF in Multiple Sclerosis Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 8447. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Cheeran, B.; Talelli, P.; Mori, F.; Koch, G.; Suppa, A.; Edwards, M.; Houlden, H.; Bhatia, K.; Greenwood, R.; Rothwell, J.C. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J. Physiol. 2008, 586, 5717–5725. [Google Scholar] [CrossRef]
- Mirowska-Guzel, D.; Mach, A.; Gromadzka, G.; Czlonkowski, A.; Czlonkowska, A. BDNF A196G and C270T gene polymorphisms and susceptibility to multiple sclerosis in the polish population. Gender differences. J. Neuroimmunol. 2008, 193, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Dolcetti, E.; Bruno, A.; Azzolini, F.; Gilio, L.; Moscatelli, A.; De Vito, F.; Pavone, L.; Iezzi, E.; Gambardella, S.; Giardina, E.; et al. The BDNF Val66Met Polymorphism (rs6265) Modulates Inflammation and Neurodegeneration in the Early Phases of Multiple Sclerosis. Genes 2022, 13, 332. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New Insights in the Biology of BDNF Synthesis and Release: Implications in CNS Function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef]
- Alrouji, M.; Manouchehrinia, A.; Gran, B.; Constantinescu, C.S. Effects of cigarette smoke on immunity, neuroinflammation and multiple sclerosis. J. Neuroimmunol. 2019, 329, 24–34. [Google Scholar] [CrossRef]
- Pachner, A.R.; Steiner, I. The multiple sclerosis severity score (MSSS) predicts disease severity over time. J. Neurol. Sci. 2009, 278, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Poser, S.; Bauer, H.J.; Poser, W. Prognosis of multiple sclerosis. Acta Neurol. Scand. 1982, 65, 347–354. [Google Scholar] [CrossRef]
- Ramasamy, D.P.; Ramanathan, M.; Cox, J.L.; Antulov, R.; Weinstock-Guttman, B.; Bergsland, N.; Benedict, R.H.; Dwyer, M.G.; Minagar, A.; Zivadinov, R. Effect of Met66 allele of the BDNF rs6265 SNP on regional gray matter volumes in patients with multiple sclerosis: A voxel-based morphometry study. Pathophysiology 2011, 18, 53–60. [Google Scholar] [CrossRef]
- De Meo, E.; De Meo, E.; Portaccio, E.; Portaccio, E.; Prestipino, E.; Prestipino, E.; Nacmias, B.; Nacmias, B.; Bagnoli, S.; Bagnoli, S.; et al. Effect of BDNF Val66Met polymorphism on hippocampal subfields in multiple sclerosis patients. Mol. Psychiatry 2021, 27, 1010–1019. [Google Scholar] [CrossRef]
- Anastasia, A.; Deinhardt, K.; Chao, M.V.; Will, N.E.; Irmady, K.; Lee, F.S.; Hempstead, B.L.; Bracken, C. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat. Commun. 2013, 4, 2490. [Google Scholar] [CrossRef]
- Sarchielli, P.; Greco, L.; Stipa, A.; Floridi, A.; Gallai, V. Brain-derived neurotrophic factor in patients with multiple sclerosis. J. Neuroimmunol. 2002, 132, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Waschbisch, A.; Atiya, M.; Linker, R.A.; Potapov, S.; Schwab, S.; Derfuss, T. Glatiramer Acetate Treatment Normalizes Deregulated microRNA Expression in Relapsing Remitting Multiple Sclerosis. PLoS ONE 2011, 6, e24604. [Google Scholar] [CrossRef] [PubMed]
- Mildner, A.; Chapnik, E.; Manor, O.; Yona, S.; Kim, K.-W.; Aychek, T.; Varol, D.; Beck, G.; Itzhaki, Z.B.; Feldmesser, E.; et al. Mononuclear phagocyte miRNome analysis identifies miR-142 as critical regulator of murine dendritic cell homeostasis. Blood 2013, 121, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.J.; Wang, W.-L.; Reyes, E.Y.; Kumar, B.; Chen, C.-C.; Ramakrishna, C.; Cantin, E.M.; Vonderfecht, S.L.; Taganov, K.D.; Chau, N.; et al. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood 2015, 125, 3720–3730. [Google Scholar] [CrossRef] [PubMed]
- Glémain, A.; Néel, M.; Néel, A.; André-Grégoire, G.; Gavard, J.; Martinet, B.; Le Bloas, R.; Riquin, K.; Hamidou, M.; Fakhouri, F.; et al. Neutrophil-derived extracellular vesicles induce endothelial inflammation and damage through the transfer of miRNAs. J. Autoimmun. 2022, 129, 102826. [Google Scholar] [CrossRef]
- Chaudhuri, A.D.; Yelamanchili, S.V.; Marcondes, M.C.G.; Fox, H.S. Up-regulation of microRNA-142 in simian immunodeficiency virus encephalitis leads to repression of sirtuin1. FASEB J. 2013, 27, 3720–3729. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Park, J.; Kim, J.-I.; Chang, M.-Y.; Lee, S.-H.; Cho, Y.-H.; Hwang, J. Lin28B and miR-142-3p regulate neuronal differentiation by modulating Staufen1 expression. Cell Death Differ. 2018, 25, 432–443. [Google Scholar] [CrossRef]
- Ikuma, Y.; Sakai, A.; Sakamoto, A.; Suzuki, H. Increased extracellular release of microRNAs from dorsal root ganglion cells in a rat model of neuropathic pain caused by peripheral nerve injury. PLoS ONE 2023, 18, e0280425. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; He, L.; Huang, J.; Liu, J.; Chen, W.; Zhong, J.; Wei, T.; Li, Z.; Zhu, J.; Lei, J. miR-142-3p encapsulated in T lymphocyte-derived tissue small extracellular vesicles induces Treg function defect and thyrocyte destruction in Hashimoto’s thyroiditis. BMC Med. 2023, 21, 206. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, J.; Lei, Z.; Shen, S.; Li, D.; Shen, G.; Zhang, G.; Feng, Z. miR-142-3p restricts cAMP production in CD4+CD25- T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. 2009, 10, 180–185. [Google Scholar] [CrossRef]
- Zhou, Q.; Haupt, S.; Prots, I.; Thümmler, K.; Kremmer, E.; Lipsky, P.E.; Schulze-Koops, H.; Skapenko, A. miR-142-3p is involved in CD25+ CD4 T cell proliferation by targeting the expression of glycoprotein A repetitions predominant. J. Immunol. 2013, 190, 6579–6588. [Google Scholar] [CrossRef] [PubMed]
- Talebi, F.; Ghorbani, S.; Chan, W.F.; Boghozian, R.; Masoumi, F.; Ghasemi, S.; Vojgani, M.; Power, C.; Noorbakhsh, F. MicroRNA-142 regulates inflammation and T cell differentiation in an animal model of multiple sclerosis. J. Neuroinflamm. 2017, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Erkal, B.; Korkut, S.V. Identification of miRNAs and their potential effects on multiple sclerosis related pathways using ın silico analysis. Mult. Scler. Relat. Disord. 2022, 59, 103642. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhang, X.; Jia, H.; Li, D.; Zhang, B.; Zhang, H.; Hong, M.; Jiang, T.; Jiang, Q.; Lu, J.; et al. An oncogenic role of miR-142-3p in human T-cell acute lymphoblastic leukemia (T-ALL) by targeting glucocorticoid receptor-α and cAMP/PKA pathways. Leukemia 2012, 26, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Tobón, K.E.; Chang, D.; Kuzhikandathil, E.V. MicroRNA 142-3p mediates post-transcriptional regulation of D1 dopamine receptor expression. PLoS ONE 2012, 7, e49288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Färber, K.; Pannasch, U.; Kettenmann, H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol. Cell. Neurosci. 2005, 29, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.T.; Vickers, J.C.; Stuart, K.E.; Cechova, K.; Ward, D.D. The BDNF Val66Met Polymorphism Modulates Resilience of Neurological Functioning to Brain Ageing and Dementia: A Narrative Review. Brain Sci. 2020, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Manna, I.; Iaccino, E.; Dattilo, V.; Barone, S.; Vecchio, E.; Mimmi, S.; Filippelli, E.; Demonte, G.; Polidoro, S.; Granata, A.; et al. Exosome-associated miRNA profile as a prognostic tool for therapy response monitoring in multiple sclerosis patients. FASEB J. 2018, 32, 4241–4246. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]

| n | 114 | |
|---|---|---|
| Age at MS onset, years | Median (IQR) | 30.8 (24.6–40.9) |
| Age at diagnosis, years | Median (IQR) | 34.4 (27.4–45.3) |
| Sex, F | n (%) | 78 (68.4) |
| Radiological activity, yes | n/tot (%) | 45/109 (41.3) § |
| OCB, yes | n/tot (%) | 98/114 (86) |
| Disease duration, months | Median (IQR) | 6.7 (1.1–36.7) |
| EDSS, value | Median (IQR) | 1.75 (1–2.5) |
| MSSS, value | Median (IQR) | 3.1 (1.4–5.9) |
| ARMSS, value | Median (IQR) | 3.3 (1.8–5.9) |
| PI, value | Median (IQR) | 0.2 (0.02–1.0) |
| RR-MS | N | Val/Val (69) | Low miR-142-3p Levels (36) | High miR-142-3p Levels (33) | p |
|---|---|---|---|---|---|
| Age at diagnosis, years | Median (IQR) | 31.3 (25.1–41.7) | 31.2 (26.5–44.8) | 31.3 (24.0–41.7) | 0.581 |
| Sex, F | N (%) | 49 (71) | 27 (75.0) | 22 (66.7) | 0.310 |
| Radiological activity, yes | N/tot (%) | 27/65 (41.5) § | 12/33 (36.4) § | 15/32 (46.9) § | 0.272 |
| OCB, yes | N/tot (%) | 62/69 (89.9) | 32/36 (88.9) | 30/33 (90.9) | 0.550 |
| Disease duration, years | Median (IQR) | 4.1 (1–35.7) | 4.3 (1.0–45.8) | 4 (1.0–34.1) | 0.738 |
| EDSS, value | Median (IQR) | 1.5 (1–3) | 1 (0–2) | 2 (1–3) | 0.013 * |
| MSSS, value | Median (IQR) | 3.3 (1.0–5.9) | 2.4 (0.7–4.9) | 5.9 (1.7–7.4) | 0.022 * |
| ARMSS, value | Median (IQR) | 3.5 (1.7–6.3) | 3.0 (1.2–4.7) | 4.1 (2.5–7.3) | 0.026 * |
| PI, value | Median (IQR) | 0.2 (0.0–1) | 0.1 (0.0–0.7) | 0.4 (0.1–2.0) | 0.011 * |
| miR-142-3p | Median (IQR) | 0.009 (0.004–0.02) | 0.004 (0.001–0.007) | 0.02 (0.010–0.040) | 0.001 * |
| RR-MS | N | Met-Allele Carriers (45) | Low miR-142-3p Levels (24) | High miR-142-3p Levels (21) | p |
|---|---|---|---|---|---|
| Age at diagnosis, years | Median (IQR) | 39.8 (31.5–47.2) | 43.0 (34.1–47.9) | 34.3 (30.4–43.7) | 0.195 |
| Sex, F | N (%) | 29 (64.4) | 15 (62.5) | 14 (66.7) | 0.509 |
| Radiological activity, yes | N/tot (%) | 18/44 (40.9) § | 7/23 (30.4) § | 11/21 (52.4) | 0.121 |
| OCB, yes | N/tot (%) | 36/45 (80) | 20/24 (83.3) | 16/21 (76.2) | 0.410 |
| Disease duration, years | Median (IQR) | 12.7 (2.4–36.9) | 18.0 (2.9–37.0) | 12.7 (2.3–30.9) | 0.480 |
| EDSS, value | Median (IQR) | 2 (1–2.5) | 2 (1–3) | 2 (2–2.5) | 0.716 |
| MSSS, value | Median (IQR) | 2.9 (2.0–5.9) | 3.3 (1.8–5.9) | 2.4 (2.2–5.9) | 0.973 |
| ARMSS, value | Median (IQR) | 3.1 (1.9–5.0) | 3.0 (1.7–5.2) | 3.1 (2.2–5.0) | 0.794 |
| PI, value | Median (IQR) | 0.1 (0.0–0.6) | 0.1 (0.0–0.7) | 0.2 (0.1–0.4) | 0.640 |
| miR-142-3p | Median (IQR) | 0.01 (0.003–0.2) | 0.003 (0.001–0.006) | 0.02 (0.010–0.040) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolcetti, E.; Musella, A.; Balletta, S.; Gilio, L.; Bruno, A.; Stampanoni Bassi, M.; Lauritano, G.; Buttari, F.; Fresegna, D.; Tartacca, A.; et al. Interaction between miR-142-3p and BDNF Val/Met Polymorphism Regulates Multiple Sclerosis Severity. Int. J. Mol. Sci. 2024, 25, 5253. https://doi.org/10.3390/ijms25105253
Dolcetti E, Musella A, Balletta S, Gilio L, Bruno A, Stampanoni Bassi M, Lauritano G, Buttari F, Fresegna D, Tartacca A, et al. Interaction between miR-142-3p and BDNF Val/Met Polymorphism Regulates Multiple Sclerosis Severity. International Journal of Molecular Sciences. 2024; 25(10):5253. https://doi.org/10.3390/ijms25105253
Chicago/Turabian StyleDolcetti, Ettore, Alessandra Musella, Sara Balletta, Luana Gilio, Antonio Bruno, Mario Stampanoni Bassi, Gianluca Lauritano, Fabio Buttari, Diego Fresegna, Alice Tartacca, and et al. 2024. "Interaction between miR-142-3p and BDNF Val/Met Polymorphism Regulates Multiple Sclerosis Severity" International Journal of Molecular Sciences 25, no. 10: 5253. https://doi.org/10.3390/ijms25105253
APA StyleDolcetti, E., Musella, A., Balletta, S., Gilio, L., Bruno, A., Stampanoni Bassi, M., Lauritano, G., Buttari, F., Fresegna, D., Tartacca, A., Mariani, F., Palmerio, F., Rovella, V., Ferese, R., Gambardella, S., Giardina, E., Finardi, A., Furlan, R., Mandolesi, G., ... De Vito, F. (2024). Interaction between miR-142-3p and BDNF Val/Met Polymorphism Regulates Multiple Sclerosis Severity. International Journal of Molecular Sciences, 25(10), 5253. https://doi.org/10.3390/ijms25105253









