Combinatory Nanovesicle with siRNA-Loaded Extracellular Vesicle and IGF-1 for Osteoarthritis Treatments
Abstract
1. Introduction
2. Results and Discussion
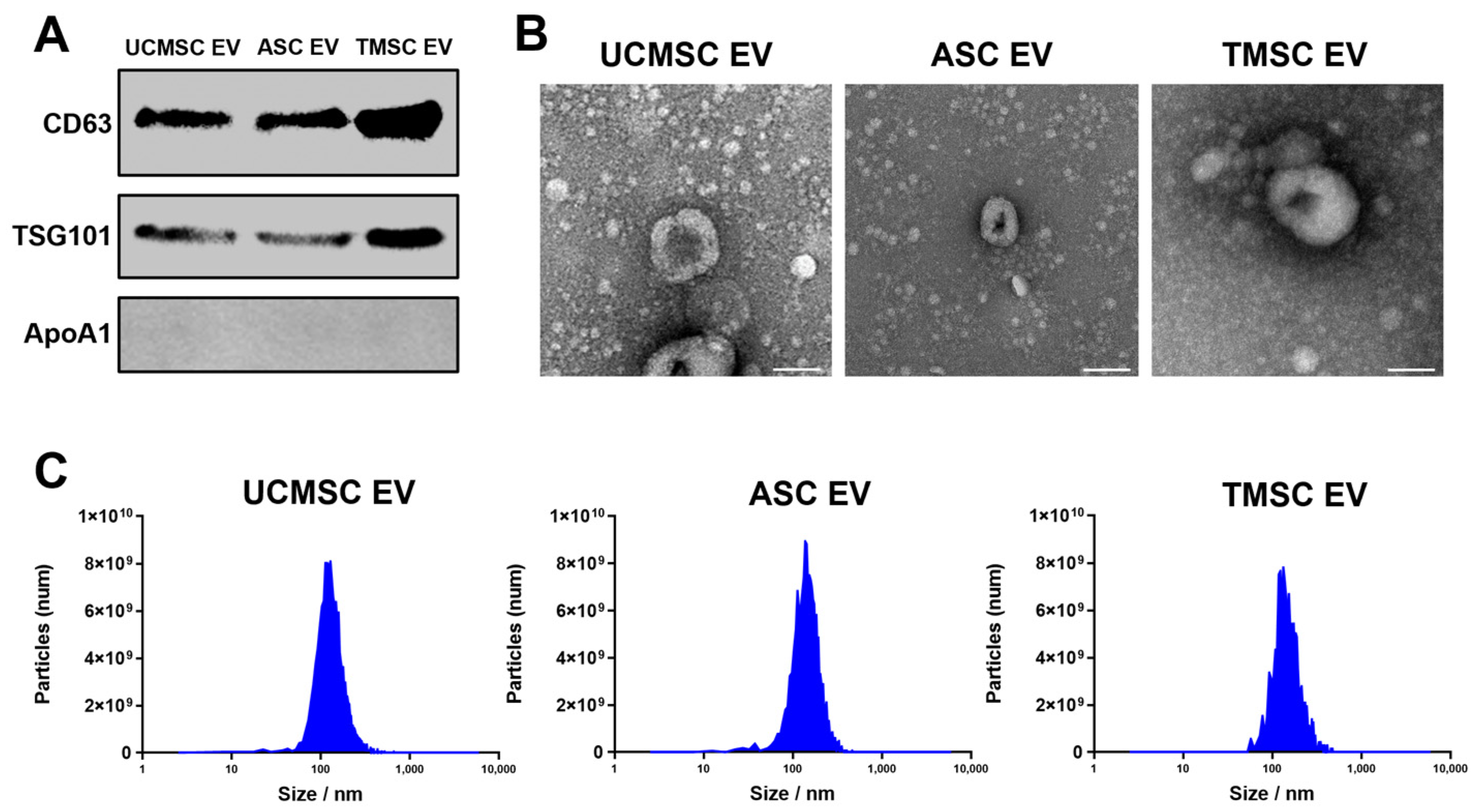
2.1. Characterization of Extracellular Vesicles (EVs)
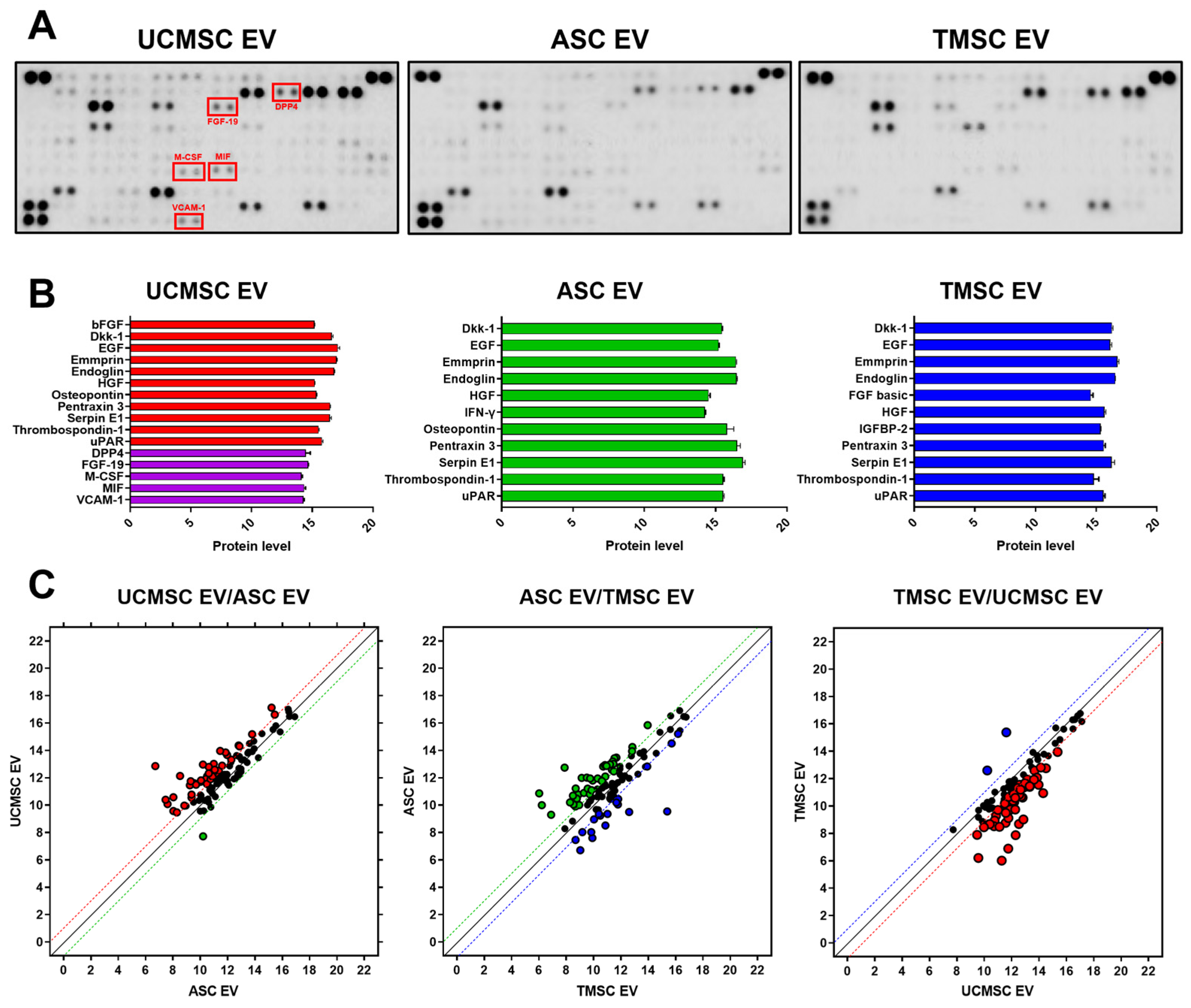
2.2. Comparative Analysis for Internal Composition of EVs
2.3. Bioinformatics Analysis for Estimating EV Functionality
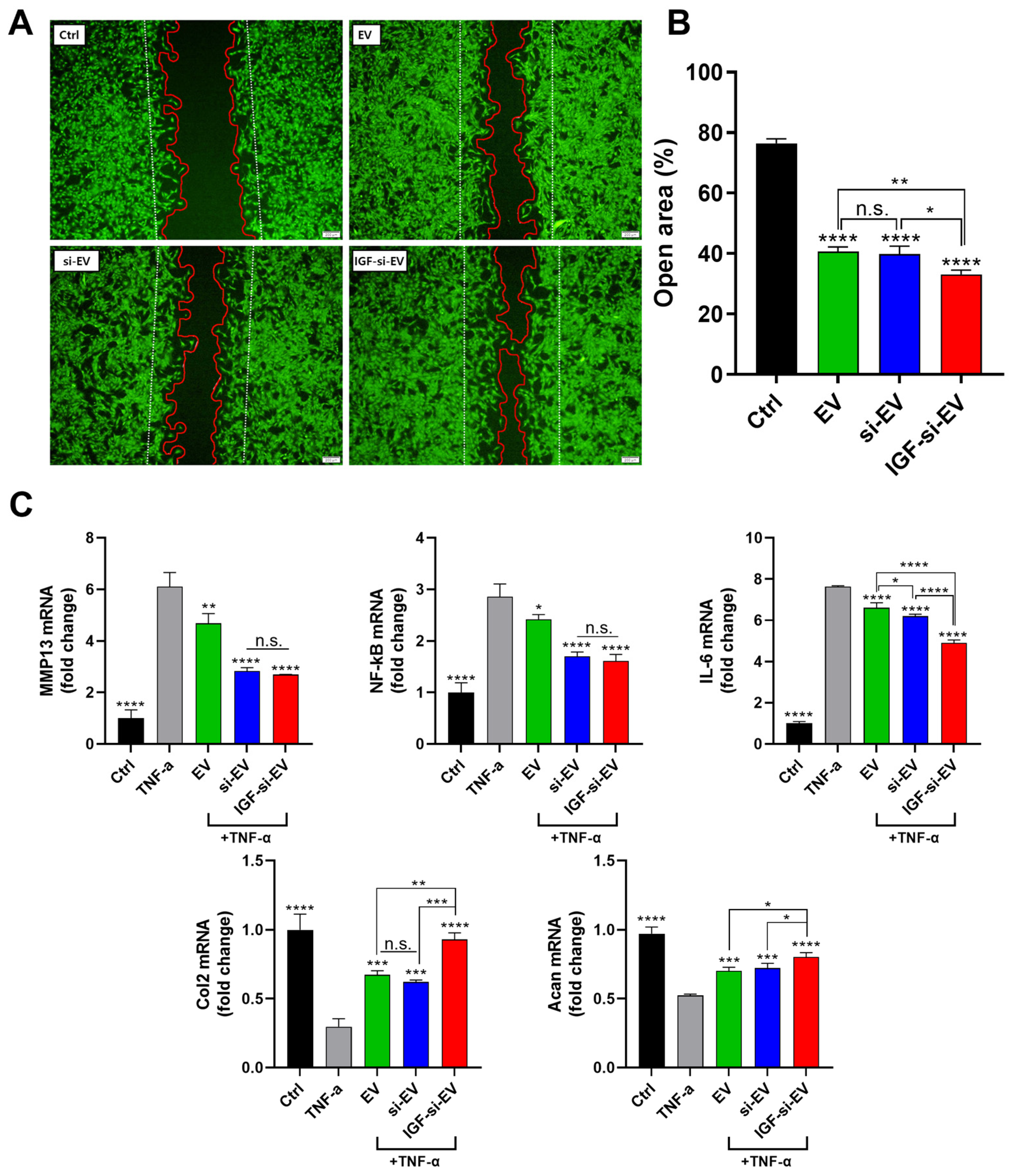
2.4. Evaluation of Wound-Healing Effects of EVs
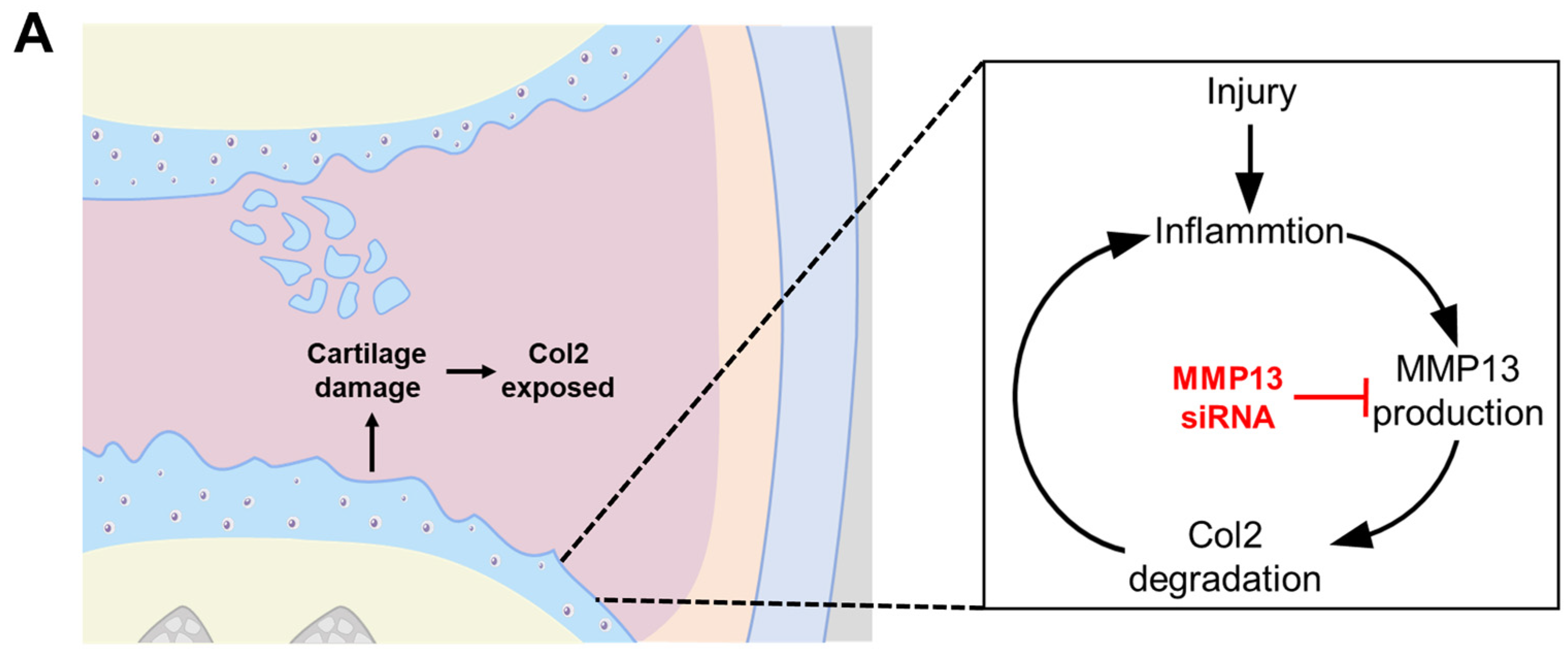
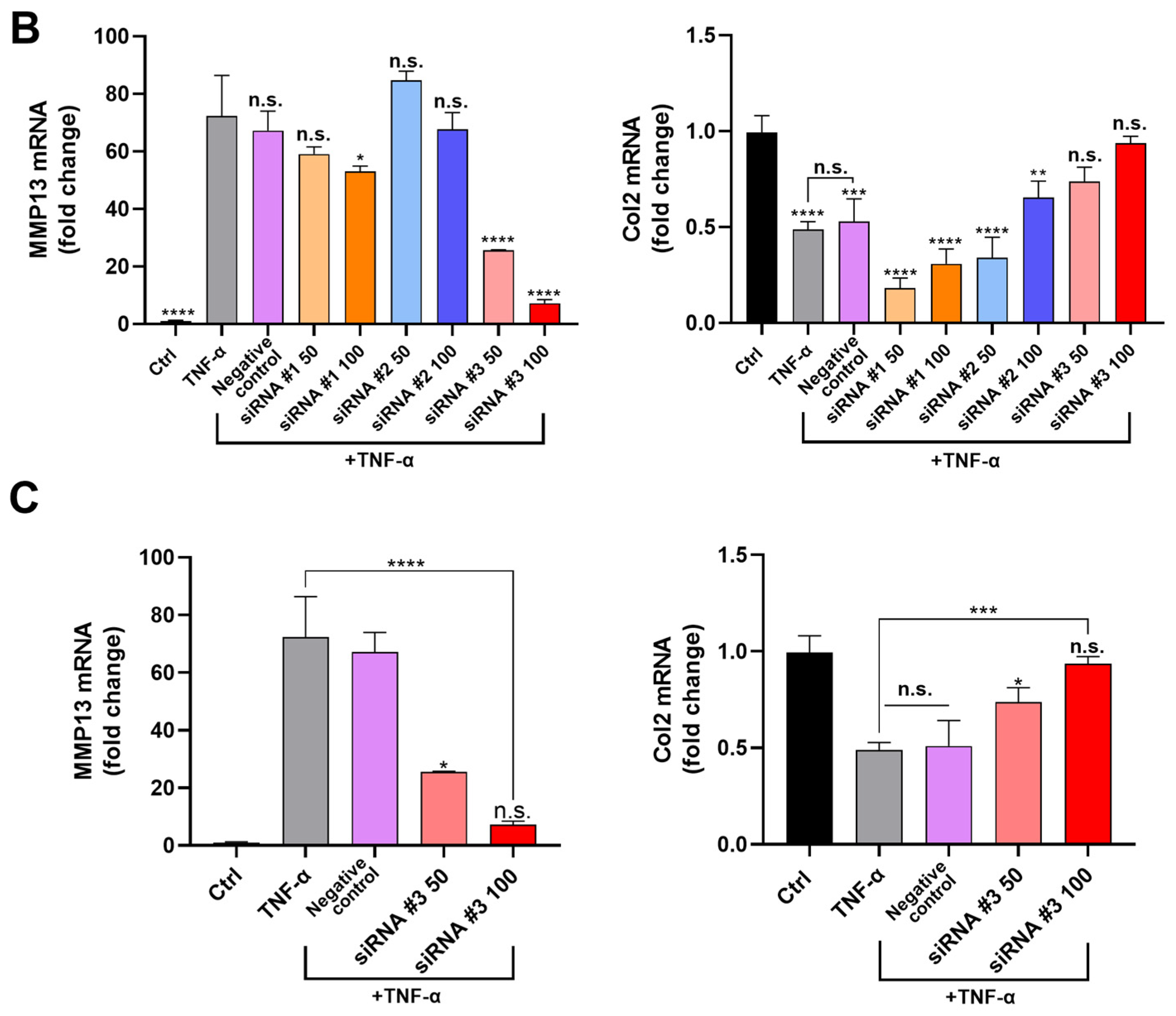
2.5. Confirming Optimum siRNA for MMP13 Inhibition
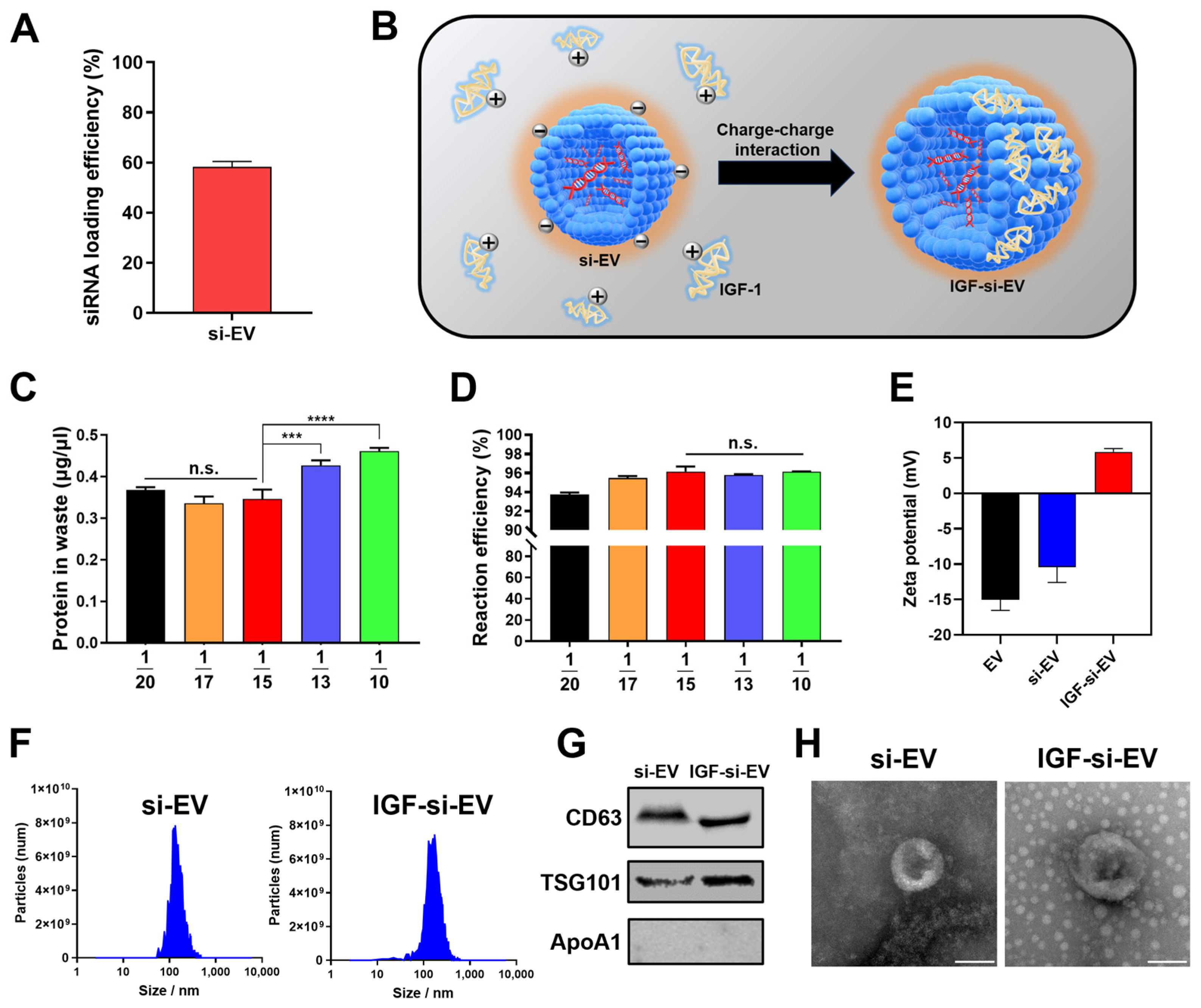
2.6. Fabrication and Characterization of si-EV and IGF-si-EV
2.7. Regenerative Activities of Nanovesicle
2.8. Affinity to Femoral Condyle of IGF-si-EV
3. Materials and Methods
3.1. Cell Culture
3.2. Cell Viability Assay
3.3. Extracellular Vesicles (EVs) Isolation
3.4. Characterization of EV and Particles
3.5. Western Blot Analysis
3.6. Antibody Array
3.7. Scratch Wound Healing Assay
3.8. Selecting the Optimum MMP13 siRNA
3.9. Synthesis of siRNA Loaded EVs (si-EVs) and IGF-si-EVs
3.10. Confirming Modification of Osteoarthritis Related Factors
3.11. Ex Vivo Nanovesicle Affinity Study
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Son, Y.O.; Park, S.; Kwak, J.S.; Won, Y.; Choi, W.S.; Rhee, J.; Chun, C.H.; Ryu, J.H.; Kim, D.K.; Choi, H.S.; et al. Estrogen-Related Receptor γ Causes Osteoarthritis by Upregulating Extracellular Matrix-Degrading Enzymes. Nat. Commun. 2017, 8, 2133. [Google Scholar] [CrossRef] [PubMed]
- Bedingfield, S.K.; Colazo, J.M.; Yu, F.; Liu, D.D.; Jackson, M.A.; Himmel, L.E.; Cho, H.; Crofford, L.J.; Hasty, K.A.; Duvall, C.L. Amelioration of Post-Traumatic Osteoarthritis via Nanoparticle Depots Delivering Small Interfering RNA to Damaged Cartilage. Nat. Biomed. Eng. 2021, 5, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Romero, E.A.; Fernández-Carnero, J.; Calvo-Lobo, C.; Ochoa Sáez, V.; Burgos Caballero, V.; Pecos-Martín, D. Is a Combination of Exercise and Dry Needling Effective for Knee OA? Pain Med. 2020, 21, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.A.S.; González-Zamorano, Y.; Arribas-Romano, A.; Martínez-Pozas, O.; Espinar, E.F.; Pedersini, P.; Villafañe, J.H.; Pérez, J.L.A.; Fernández-Carnero, J. Efficacy of Manual Therapy on Facilitatory Nociception and Endogenous Pain Modulation in Older Adults with Knee Osteoarthritis: A Case Series. Appl. Sci. 2021, 11, 1895. [Google Scholar] [CrossRef]
- Sinatti, P.; Sánchez Romero, E.A.; Martínez-Pozas, O.; Villafañe, J.H. Effects of Patient Education on Pain and Function and Its Impact on Conservative Treatment in Elderly Patients with Pain Related to Hip and Knee Osteoarthritis: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 6194. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Romero, E.A.; Battaglino, A.; Campanella, W.; Turroni, S.; Bishop, M.D.; Villafañe, J.H. Impact on Blood Tests of Lower Limb Joint Replacement for the Treatment of Osteoarthritis. Top. Geriatr. Rehabil. 2021, 37, 227–229. [Google Scholar] [CrossRef]
- Guermazi, A.; Neogi, T.; Katz, J.N.; Kwoh, C.K.; Conaghan, P.G.; Felson, D.T.; Roemer, F.W. Intra-Articular Corticosteroid Injections for the Treatment of Hip and Knee Osteoarthritis-Related Pain: Considerations and Controversies with a Focus on Imaging-Radiology Scientific Expert Panel. Radiology 2020, 297, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Kim, K.-I.; Park, K.B.; Park, Y.G.; Bae, J.H.; Seo, Y.J.; Seon, J.K.; Shon, O.J.; Ahn, J.H.; Wang, L.; et al. Safety and Effectiveness of Intra-Articular Injection of a Highly Cross-Linked Hyaluronic Acid, LBSA0103 (Synovian): Results from a Post-Marketing Surveillance Study in South Korea. PLoS ONE 2023, 18, e0287222. [Google Scholar] [CrossRef]
- Kim, T.W.; Chang, M.J.; Shin, C.Y.; Chang, C.B.; Kang, S.B. A Randomized Controlled Trial for Comparing Efficacy and Safety between Intraarticular Polynucleotide and Hyaluronic Acid for Knee Osteoarthritis Treatment. Sci. Rep. 2023, 13, 9419. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.N.; Lee, Y.J.; Sim, S.E.; Ko, Y.R.; Shim, J.W.; Lee, K.S.; Joo, M.; Park, H.J. Pilot Study to Evaluate the Efficacy of Polynucleotide Sodium Compared to Sodium Hyaluronate and Crosslinked Sodium Hyaluronate in Patients with Knee Osteoarthritis. J. Clin. Med. 2021, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xi, L.; Yu, M.; Fan, Z.; Wang, W.; Ju, A.; Liang, Z.; Zhou, G.; Ren, W. Regeneration of Articular Cartilage Defects: Therapeutic Strategies and Perspectives. J. Tissue Eng. 2023, 14, 1–27. [Google Scholar]
- Lee, H.-I.; Rhim, W.K.; Kang, E.Y.; Choi, B.; Kim, J.H.; Han, D.K. A Multilayer Functionalized Drug-Eluting Balloon for Treatment of Coronary Artery Disease. Pharmaceutics 2021, 13, 614. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Rhim, W.K.; Baek, S.W.; Kim, J.Y.; Kim, D.S.; Han, D.K. Endogenous Stimulus-Responsive Nitric Oxide Releasing Bioactive Liposome for a Multilayered Drug-Eluting Balloon. Biomater. Sci. 2022, 11, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, Y.; Guo, Z.; Zhu, H.; Liao, H.; Niu, X.; Zhou, L.; Fu, S.; Li, Y.; Li, S.; et al. A New Cell-Free Therapeutic Strategy for Liver Regeneration: Human Placental Mesenchymal Stem Cell-Derived Extracellular Vesicles. J. Tissue Eng. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Citro, V.; Clerici, M.; Boccaccini, A.R.; Della Porta, G.; Maffulli, N.; Forsyth, N.R. Tendon Tissue Engineering: An Overview of Biologics to Promote Tendon Healing and Repair. J. Tissue Eng. 2023, 14, 1–24. [Google Scholar]
- Shao, Z.; Xu, J.; Xu, X.; Wang, X.; Zhou, Y.; Li, Y.; Li, K. Exosomes Derived from Human Adipose Mesenchymal Stem Cells Inhibits Fibrosis and Treats Oral Submucous Fibrosis via the MiR-181a-5p/Smad2 Axis. Tissue Eng. Regen. Med. 2023, 21, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular Vesicles through the Blood–Brain Barrier: A Review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef]
- Koh, H.B.; Kim, H.J.; Kang, S.W.; Yoo, T.H. Exosome-Based Drug Delivery: Translation from Bench to Clinic. Pharmaceutics 2023, 15, 2042. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.Y.; Rhim, W.K.; Cimaglia, G.; Want, A.; Morgan, J.E.; Williams, P.A.; Park, C.G.; Han, D.K.; Rho, S. Extracellular Vesicle Encapsulated Nicotinamide Delivered via A Trans-Scleral Route Provides Retinal Ganglion Cell Neuroprotection. Acta Neuropathol. Commun. 2024, 12, 65. [Google Scholar] [CrossRef]
- Liu, W.; Liu, A.; Li, X.; Sun, Z.; Sun, Z.; Liu, Y.; Wang, G.; Huang, D.; Xiong, H.; Yu, S.; et al. Dual-Engineered Cartilage-Targeting Extracellular Vesicles Derived from Mesenchymal Stem Cells Enhance Osteoarthritis Treatment via MiR-223/NLRP3/Pyroptosis Axis: Toward a Precision Therapy. Bioact. Mater. 2023, 30, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, X.; Xu, L.; Iqbal, Z.; Ouyang, K.; Zhang, H.; Wen, C.; Duan, L.; Xia, J. Chondrocyte-Specific Genomic Editing Enabled by Hybrid Exosomes for Osteoarthritis Treatment. Theranostics 2022, 12, 4866–4878. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, M.; Su, J.; Cai, B.; Li, J.; Zhang, K. New Insights into Balancing Wound Healing and Scarless Skin Repair. J. Tissue Eng. 2023, 14, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, T.; Sun, T.; Yu, C.; Yan, D.; Zhu, L. Erythrocyte Membrane Camouflaged SiRNA/Chemodrug Nanoassemblies for Cancer Combination Therapy. Biomater. Sci. 2022, 10, 6601–6613. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Zhang, X.; Clausen, V.; Tran, C.; Arciprete, M.; Wang, Q.; Rocca, C.; Guan, L.H.; Zhang, G.; et al. Nonclinical Pharmacokinetics and Absorption, Distribution, Metabolism, and Excretion of Givosiran, the First Approved n-Acetylgalactosamine-Conjugated Rna Interference Therapeutic. Drug Metab. Dispos. 2021, 49, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Rhim, W.K.; Kim, J.Y.; Lee, S.Y.; Cha, S.G.; Park, J.M.; Park, H.J.; Park, C.G.; Han, D.K. Recent Advances in Extracellular Vesicle Engineering and Its Applications to Regenerative Medicine. Biomater. Res. 2023, 27, 130. [Google Scholar] [CrossRef] [PubMed]
- Oggu, G.S.; Sasikumar, S.; Reddy, N.; Ella, K.K.R.; Rao, C.M.; Bokara, K.K. Gene Delivery Approaches for Mesenchymal Stem Cell Therapy: Strategies to Increase Efficiency and Specificity. Stem Cell Rev. Reports 2017, 13, 725–740. [Google Scholar] [CrossRef]
- Mullen, L.M.; Best, S.M.; Brooks, R.A.; Ghose, S.; Gwynne, J.H.; Wardale, J.; Rushton, N.; Cameron, R.E. Binding and Release Characteristics of Insulin-like Growth Factor-1 from a Collagen-Glycosaminoglycan Scaffold. Tissue Eng.—Part C Methods 2010, 16, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhang, J.; Zhou, Z.; Pan, D.; Zhao, D.; Dong, H.; Yao, B. Novel Insights into the Effect of Deer IGF-1 on Chondrocyte Viability and IL-1β-Induced Inflammation Response. J. Biochem. Mol. Toxicol. 2023, 37, e23227. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rhim, W.K.; Cha, S.G.; Woo, J.; Lee, J.Y.; Park, C.G.; Han, D.K. Bolstering the Secretion and Bioactivities of Umbilical Cord MSC-Derived Extracellular Vesicles with 3D Culture and Priming in Chemically Defined Media. Nano Converg. 2022, 9, 57. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rhim, W.K.; Yoo, Y.I.; Kim, D.S.; Ko, K.W.; Heo, Y.; Park, C.G.; Han, D.K. Defined MSC Exosome with High Yield and Purity to Improve Regenerative Activity. J. Tissue Eng. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Hyo, W.R.; Seo, J.; Youn, J.; Park, C.G.; Han, D.K. Comparative Analysis of MSC-Derived Exosomes Depending on Cell Culture Media for Regenerative Bioactivity. Tissue Eng. Regen. Med. 2021, 18, 355–367. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rhim, W.; Woo, J.; Cha, S.; Park, C.G.; Han, D.K. The Upregulation of Regenerative Activity for Extracellular Vesicles with Melatonin Modulation in Chemically Defined Media. Int. J. Mol. Sci. 2022, 23, 15089. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Funck-Brentano, T.; Bouaziz, W.; Marty, C.; Geoffroy, V.; Hay, E.; Cohen-Solal, M. Dkk-1-Mediated Inhibition of Wnt Signaling in Bone Ameliorates Osteoarthritis in Mice. Arthritis Rheumatol. 2014, 66, 3028–3039. [Google Scholar] [CrossRef]
- Adamopoulos, I.; Athanasou, N. Hepatocyte Growth Factor in Normal and Diseased Bone and Joint Tissues. Curr. Rheumatol. Rev. 2006, 2, 1–7. [Google Scholar]
- Maumus, M.; Manferdini, C.; Toupet, K.; Chuchana, P.; Casteilla, L.; Gachet, M.; Jorgensen, C.; Lisignoli, G.; Noël, D. Thrombospondin-1 Partly Mediates the Cartilage Protective Effect of Adipose-Derived Mesenchymal Stem Cells in Osteoarthritis. Front. Immunol. 2017, 8, 1638. [Google Scholar] [CrossRef]
- Han, C.K.; Lee, W.F.; Hsu, C.J.; Huang, Y.L.; Lin, C.Y.; Tsai, C.H.; Huang, C.C.; Fong, Y.C.; Wu, M.H.; Liu, J.F.; et al. DPP4 Reduces Proinflammatory Cytokine Production in Human Rheumatoid Arthritis Synovial Fibroblasts. J. Cell. Physiol. 2021, 236, 8060–8069. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.M.; Chen, Y.H.; Sun, H.S.; Tsai, S.J. Fibroblast Growth Factors: Potential Novel Targets for Regenerative Therapy of Osteoarthritis. Chin. J. Physiol. 2019, 62, 2–10. [Google Scholar] [PubMed]
- Zaripova, L.; Pallav, M.; Tazhibaeva, D.; Kabdualieva, N.; Aitbayeva, Z.; Beglarova, G.; Yermentayeva, L.; Niyazbekova, K. Biological Therapy for Osteoarthritis, Efficacy and Safety: Focus on Monoclonal Antibodies against Nerve Growth Factor and Fibroblast Growth Factor-18. Open Access Maced. J. Med. Sci. 2022, 10, 697–704. [Google Scholar] [CrossRef]
- De Liyis, B.G.; Nolan, J.; Maharjana, M.A. Fibroblast Growth Factor Receptor 1-Bound Extracellular Vesicle as Novel Therapy for Osteoarthritis. Biomed. 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pustjens, M.F.; Mastbergen, S.C.; Eijkelkamp, N.; Lafeber, F.P. Expression of GM-CSF and Its Receptor CD116 in the Synovium of OA Patients Is Negatively Correlated with Pain. Osteoarthr. Cartil. 2016, 24, S454–S455. [Google Scholar] [CrossRef]
- Saleh, R.; Lee, M.-C.; Khiew, S.H.; Louis, C.; Fleetwood, A.J.; Achuthan, A.; Förster, I.; Cook, A.D.; Hamilton, J.A. CSF-1 in Inflammatory and Arthritic Pain Development. J. Immunol. 2018, 201, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, Z.; Sun, G.; Chen, L.; Qi, D.; Zhang, H.; Xiong, J.; Furey, A.; Rahman, P.; Lei, G.; et al. Macrophage Migration Inhibitory Factor May Play a Protective Role in Osteoarthritis. Arthritis Res. Ther. 2021, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in Various Inflammatory and Cardiovascular Disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Lichanska, A.M.; Waters, M.J. New Insights into Growth Hormone Receptor Function and Clinical Implications. Horm. Res. 2008, 69, 138–145. [Google Scholar] [CrossRef]
- Vyas, S.P.; Singh, A.; Sihorkar, V. Ligand-Receptor-Mediated Drug Delivery: An Emerging Paradigm in Cellular Drug Targeting. Crit. Rev. Ther. Drug Carr. Syst. 2001, 18, 139–148. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Kitaeva, K.V.; Green, A.R.; Rizvanov, A.A.; Solovyeva, V.V. Molecular Aspects and Future Perspectives of Cytokine-Based Anti-Cancer Immunotherapy. Front. Cell Dev. Biol. 2020, 8, 402. [Google Scholar] [CrossRef]
- Leonard, W.J.; Spolski, R. Interleukin-21: A Modulator of Lymphoid Proliferation, Apoptosis and Differentiation. Nat. Rev. Immunol. 2005, 5, 688–698. [Google Scholar] [CrossRef]
- Coleman, W.B.; Tsongalis, G.J. Positive Mediators of Cell Proliferation in Neoplasia: Growth Factors and Receptors. In The Molecular Basis of Human Cancer; Humana Press: New York, NY, USA, 2016; pp. 159–182. [Google Scholar]
- Theodorus, E.; Valentina, D.C.D.; Agverianti, T.; Kusumaningrum, R. Terapi Mesenchymal Stem Cell Sebagai Modalitas Tatalaksana Osteoartritis. Essent. Essence Sci. Med. J. 2022, 19, 1–4. [Google Scholar] [CrossRef]
- Rhim, W.; Woo, J.; Yong, J.; Hye, E.; Cha, S.; Kim, D.; Baek, S.; Gwon, C.; Soo, B.; Gyun, T.; et al. Multiplexed PLGA Scaffolds with Nitric Oxide-Releasing Zinc Oxide and Melatonin-Modulated Extracellular Vesicles for Severe Chronic Kidney Disease. J. Adv. Res. 2024, 59, 1–15. [Google Scholar] [CrossRef]
- Cha, S.G.; Rhim, W.K.; Kim, J.Y.; Lee, E.H.; Lee, S.Y.; Park, J.M.; Lee, J.E.; Yoon, H.; Park, C.G.; Kim, B.S.; et al. Kidney Tissue Regeneration Using Bioactive Scaffolds Incorporated with Differentiating Extracellular Vesicles and Intermediate Mesoderm Cells. Biomater. Res. 2023, 27, 126. [Google Scholar] [CrossRef]
- Song, X.; Bai, S.; He, N.; Wang, R.; Xing, Y.; Lv, C.; Yu, F. Real-Time Evaluation of Hydrogen Peroxide Injuries in Pulmonary Fibrosis Mice Models with a Mitochondria-Targeted Near-Infrared Fluorescent Probe. ACS Sensors 2021, 6, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.K.; Kamalakaran, S. Pro-Apoptotic Role of NF-ΚB: Implications for Cancer Therapy. Biochim. Biophys. Acta—Rev. Cancer 2006, 1766, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, G.; Lee, J.; Lee, Y.; Kim, J.H. Secretome of Stem Cells: Roles of Extracellular Vesicles in Diseases, Stemness, Differentiation, and Reprogramming. Tissue Eng. Regen. Med. 2022, 19, 19–33. [Google Scholar] [CrossRef]
- Buckley, C.D.; Simmons, D.L. Cell Adhesion: A New Target for Therapy. Mol. Med. Today 1997, 3, 449–456. [Google Scholar] [CrossRef]
- Miao, S.; Zhou, J.; Liu, B.; Lei, X.; Wang, T.; Hao, X.; Cheng, P.; Wu, H.; Song, Y.; Pei, G.; et al. A 3D Bioprinted Nano-Laponite Hydrogel Construct Promotes Osteogenesis by Activating PI3K/AKT Signaling Pathway. Mater. Today Bio 2022, 16, 100342. [Google Scholar] [CrossRef] [PubMed]
- Boettner, B.; Van Aelst, L. Control of Cell Adhesion Dynamics by Rap1 Signaling. Curr. Opin. Cell Biol. 2009, 21, 684–693. [Google Scholar] [CrossRef]
- Xie, L.; Overbeek, P.A.; Reneker, L.W. Ras Signaling Is Essential for Lens Cell Proliferation and Lens Growth during Development. Dev. Biol. 2006, 298, 403–414. [Google Scholar] [CrossRef]
- Takanashi, M.; Oikawa, K.; Sudo, K.; Tanaka, M.; Fujita, K.; Ishikawa, A.; Nakae, S.; Kaspar, R.L.; Matsuzaki, M.; Kudo, M.; et al. Therapeutic Silencing of an Endogenous Gene by SiRNA Cream in an Arthritis Model Mouse. Gene Ther. 2009, 16, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic siRNA: State of the Art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Nayan, M.I.H.; Nandi, T. SiRNA: A Comprehensive Review of Marketed Products till August 2022. J. Adv. Med. Pharm. Sci. 2023, 25, 31–43. [Google Scholar] [CrossRef]
- Cao, C.; Shi, Y.; Zhang, X.; Li, Q.; Zhang, J.; Zhao, F.; Meng, Q.; Dai, W.; Liu, Z.; Yan, W.; et al. Cholesterol-Induced LRP3 Downregulation Promotes Cartilage Degeneration in Osteoarthritis by Targeting Syndecan-4. Nat. Commun. 2022, 13, 7139. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Schüter, R.; Endlich, N. Direct exosome transfection with fluorescently labeled small rnas is a useful tool for exosomal cargo trafficking and rnai in cultured podocytes. Nephrol. Dial. Transplant. 2023, 38, 3633. [Google Scholar] [CrossRef]
- Geiger, B.C.; Wang, S.; Padera, R.F.; Grodzinsky, A.J.; Hammond, P.T. Cartilage-Penetrating Nanocarriers Improve Delivery and Efficacy of Growth Factor Treatment of Osteoarthritis. Sci. Transl. Med. 2018, 10, eaat8800. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; He, T.; Zhang, C.; Amiji, S.M.; Hakim, B.; Bajpayee, A.G. Charge-Based Drug Delivery to Cartilage: Hydrophobic and Not Electrostatic Interactions Are the Dominant Cause of Competitive Binding of Cationic Carriers in Synovial Fluid. Acta Biomater. 2022, 151, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Yang, Y.J.; Chang, C.H.; Tang, A.C.L.; Liao, W.Y.; Cheng, F.Y.; Yeh, C.S.; Lai, J.J.; Stayton, P.S.; Hsieh, P.C.H. Functionalized Nanoparticles Provide Early Cardioprotection after Acute Myocardial Infarction. J. Control. Release 2013, 170, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, F.Y.K.; Muzzi, L.A.L.; Lacreta, A.C.C., Jr.; Raymundo, D.L.; Alves, E.G.L.; Guimarães E Silva, W.; Muzzi, R.A.L. Autogenous Osteochondral Graft Associated with IGF-1 in Induced Articular Cartilage Lesion in Rabbits [Enxerto Osteocondral Autógeno Associado Ao IGF-1 Em Lesão Induzida Na Cartilagem Articular de Coelhos]. Acta Sci. Vet. 2018, 46, 10. [Google Scholar] [CrossRef]
- Middleton, J.; Manthey, A.; Tyler, J. Insulin-like Growth Factor (IGF) Receptor, IGF-I, Interleukin-1β (IL-1β), and IL-6 MRNA Expression in Osteoarthritic and Normal Human Cartilage. J. Histochem. Cytochem. 1996, 44, 133–141. [Google Scholar] [CrossRef]
- Bedingfield, S.K.; Colazo, J.M.; Di Francesco, M.; Yu, F.; Liu, D.D.; Di Francesco, V.; Himmel, L.E.; Gupta, M.K.; Cho, H.; Hasty, K.A.; et al. Top-Down Fabricated MicroPlates for Prolonged, Intra-Articular Matrix Metalloproteinase 13 SiRNA Nanocarrier Delivery to Reduce Post-Traumatic Osteoarthritis. ACS Nano 2021, 15, 14475–14491. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Lee, S.Y.; Cha, S.-G.; Park, J.M.; Song, D.H.; Lee, S.-H.; Hwang, D.-Y.; Kim, B.J.; Rho, S.; Park, C.G.; et al. Combinatory Nanovesicle with siRNA-Loaded Extracellular Vesicle and IGF-1 for Osteoarthritis Treatments. Int. J. Mol. Sci. 2024, 25, 5242. https://doi.org/10.3390/ijms25105242
Kim JY, Lee SY, Cha S-G, Park JM, Song DH, Lee S-H, Hwang D-Y, Kim BJ, Rho S, Park CG, et al. Combinatory Nanovesicle with siRNA-Loaded Extracellular Vesicle and IGF-1 for Osteoarthritis Treatments. International Journal of Molecular Sciences. 2024; 25(10):5242. https://doi.org/10.3390/ijms25105242
Chicago/Turabian StyleKim, Jun Yong, Seung Yeon Lee, Seung-Gyu Cha, Jung Min Park, Duck Hyun Song, Sang-Hyuk Lee, Dong-Youn Hwang, Byoung Ju Kim, Seungsoo Rho, Chun Gwon Park, and et al. 2024. "Combinatory Nanovesicle with siRNA-Loaded Extracellular Vesicle and IGF-1 for Osteoarthritis Treatments" International Journal of Molecular Sciences 25, no. 10: 5242. https://doi.org/10.3390/ijms25105242
APA StyleKim, J. Y., Lee, S. Y., Cha, S.-G., Park, J. M., Song, D. H., Lee, S.-H., Hwang, D.-Y., Kim, B. J., Rho, S., Park, C. G., Rhim, W.-K., & Han, D. K. (2024). Combinatory Nanovesicle with siRNA-Loaded Extracellular Vesicle and IGF-1 for Osteoarthritis Treatments. International Journal of Molecular Sciences, 25(10), 5242. https://doi.org/10.3390/ijms25105242






