NR4A2 as a Novel Target Gene for Developmental and Epileptic Encephalopathy: A Systematic Review of Related Disorders and Therapeutic Strategies
Abstract
1. Introduction
2. Methods
3. Results
3.1. Case Reports—Clinical Findings
3.2. NR4A2 and Neurodegenerative Disease
3.3. Treatments
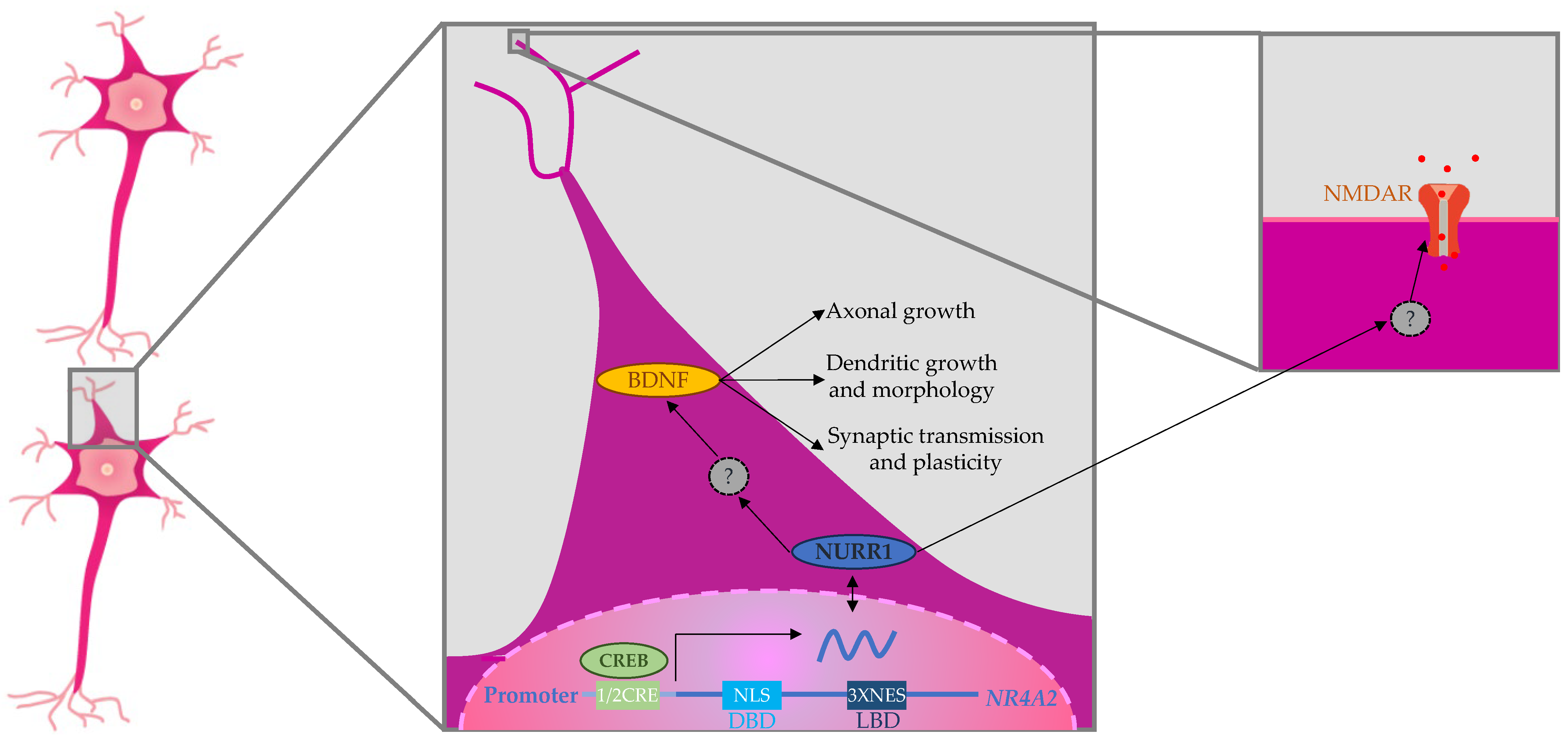
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.; Benoit, G.; Liu, J.; Prasad, S.; Aarnisalo, P.; Liu, X.; Xu, H.; Walker, N.P.C.; Perlmann, T. Structure and Function of Nurr1 Identifies a Class of Ligand-Independent Nuclear Receptors. Nature 2003, 423, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Flaig, R.; Greschik, H.; Peluso-Iltis, C.; Moras, D. Structural Basis for the Cell-Specific Activities of the NGFI-B and the Nurr1 Ligand-Binding Domain. J. Biol. Chem. 2005, 280, 19250–19258. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Han, B.-S.; Moon, J.; Kim, D.-J.; Shin, J.; Rajan, S.; Nguyen, Q.T.; Sohn, M.; Kim, W.-G.; Han, M.; et al. Nuclear Receptor Nurr1 Agonists Enhance Its Dual Functions and Improve Behavioral Deficits in an Animal Model of Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2015, 112, 8756–8761. [Google Scholar] [CrossRef]
- de Vera, I.M.S.; Munoz-Tello, P.; Zheng, J.; Dharmarajan, V.; Marciano, D.P.; Matta-Camacho, E.; Giri, P.K.; Shang, J.; Hughes, T.S.; Rance, M.; et al. Defining a Canonical Ligand-Binding Pocket in the Orphan Nuclear Receptor Nurr1. Structure 2019, 27, 66–77.e5. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Rechavi, M.; Escriva Garcia, H.; Laudet, V. The Nuclear Receptor Superfamily. J. Cell Sci. 2003, 116, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Brelivet, Y.; Kammerer, S.; Rochel, N.; Poch, O.; Moras, D. Signature of the Oligomeric Behaviour of Nuclear Receptors at the Sequence and Structural Level. EMBO Rep. 2004, 5, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Martinat, C.; Bacci, J.-J.; Leete, T.; Kim, J.; Vanti, W.B.; Newman, A.H.; Cha, J.H.; Gether, U.; Wang, H.; Abeliovich, A. Cooperative Transcription Activation by Nurr1 and Pitx3 Induces Embryonic Stem Cell Maturation to the Midbrain Dopamine Neuron Phenotype. Proc. Natl. Acad. Sci. USA 2006, 103, 2874–2879. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodaei, B.; Ito, T.; Joodmardi, E.; Mattsson, B.; Rouillard, C.; Carta, M.; Muramatsu, S.; Sumi-Ichinose, C.; Nomura, T.; Metzger, D.; et al. Nurr1 Is Required for Maintenance of Maturing and Adult Midbrain Dopamine Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 15923–15932. [Google Scholar] [CrossRef] [PubMed]
- Messmer, K.; Remington, M.P.; Skidmore, F.; Fishman, P.S. Induction of Tyrosine Hydroxylase Expression by the Transcription Factor Pitx3. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2007, 25, 29–37. [Google Scholar] [CrossRef]
- Saijo, K.; Winner, B.; Carson, C.T.; Collier, J.G.; Boyer, L.; Rosenfeld, M.G.; Gage, F.H.; Glass, C.K. A Nurr1/CoREST Pathway in Microglia and Astrocytes Protects Dopaminergic Neurons from Inflammation-Induced Death. Cell 2009, 137, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Buervenich, S.; Carmine, A.; Arvidsson, M.; Xiang, F.; Zhang, Z.; Sydow, O.; Jönsson, E.G.; Sedvall, G.C.; Leonard, S.; Ross, R.G.; et al. NURR1 Mutations in Cases of Schizophrenia and Manic-Depressive Disorder. Am. J. Med. Genet. 2000, 96, 808–813. [Google Scholar] [CrossRef]
- Le, W.-D.; Xu, P.; Jankovic, J.; Jiang, H.; Appel, S.H.; Smith, R.G.; Vassilatis, D.K. Mutations in NR4A2 Associated with Familial Parkinson Disease. Nat. Genet. 2003, 33, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.P.; McEvoy, A.; Conneely, O.M.; Bresnihan, B.; FitzGerald, O. Involvement of the Nuclear Orphan Receptor NURR1 in the Regulation of Corticotropin-Releasing Hormone Expression and Actions in Human Inflammatory Arthritis. Arthritis Rheum. 2001, 44, 782–793. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, M.; Markham, T.; McEvoy, A.N.; Fearon, U.; Veale, D.J.; FitzGerald, O.; Kirby, B.; Murphy, E.P. Increased Expression of the Orphan Nuclear Receptor NURR1 in Psoriasis and Modulation Following TNF-Alpha Inhibition. J. Invest. Dermatol. 2008, 128, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Grotto, I.; Balicer, R.; Magalashvili, D.; Feldman, A.; Gurevich, M. Microarray Analysis Identifies Altered Regulation of Nuclear Receptor Family Members in the Pre-Disease State of Multiple Sclerosis. Neurobiol. Dis. 2010, 38, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.L.P.; Monteiro, F.P.; Sampaio, L.P.B.; Costa, L.A.; Ribeiro, M.D.O.; Freitas, E.L.; Kitajima, J.P.; Kok, F. Heterozygous Loss of Function of NR4A2 Is Associated with Intellectual Deficiency, Rolandic Epilepsy, and Language Impairment. Clin. Case Rep. 2019, 7, 1582–1584. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Mariani, L.L.; Bergant, G.; Baulac, M.; Habert, M.-O.; Drouot, N.; Ollivier, E.; Hodžić, A.; Rudolf, G.; Nitschke, P.; et al. Loss-of-Function Mutations in NR4A2 Cause Dopa-Responsive Dystonia Parkinsonism. Mov. Disord. 2020, 35, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, A.; Zech, M.; Sigafoos, A.N.; Clark, K.J.; Dincer, Y.; Wagner, M.; Humberson, J.B.; Green, S.; van Gassen, K.; et al. De Novo Variants of NR4A2 Are Associated with Neurodevelopmental Disorder and Epilepsy. Genet. Med. Off. J. Am. Coll. Med. Genet. 2020, 22, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Jesús, S.; Hinarejos, I.; Carrillo, F.; Martínez-Rubio, D.; Macías-García, D.; Sánchez-Monteagudo, A.; Adarmes, A.; Lupo, V.; Pérez-Dueñas, B.; Mir, P.; et al. NR4A2 Mutations Can Cause Intellectual Disability and Language Impairment with Persistent Dystonia-Parkinsonism. Neurol. Genet. 2021, 7, e543. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Lévy, J.; Grotto, S.; Mignot, C.; Maruani, A.; Delahaye-Duriez, A.; Benzacken, B.; Keren, B.; Haye, D.; Xavier, J.; Heulin, M.; et al. NR4A2 Haploinsufficiency Is Associated with Intellectual Disability and Autism Spectrum Disorder. Clin. Genet. 2018, 94, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Duyzend, M.H.; Coe, B.P.; Baker, C.; Hoekzema, K.; Gerdts, J.; Turner, T.N.; Zody, M.C.; Beighley, J.S.; Murali, S.C.; et al. Genome Sequencing Identifies Multiple Deleterious Variants in Autism Patients with More Severe Phenotypes. Genet. Med. Off. J. Am. Coll. Med. Genet. 2019, 21, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xu, W.; Xiao, M.; Lu, Y.; Lan, X.; Tang, X.; Xu, N.; Yu, G.; Zhang, H.; Wu, S. Two Novel Heterozygous Truncating Variants in NR4A2 Identified in Patients with Neurodevelopmental Disorder and Brief Literature Review. Front. Neurosci. 2022, 16, 956429. [Google Scholar] [CrossRef] [PubMed]
- Krgovic, D.; Gorenjak, M.; Rihar, N.; Opalic, I.; Stangler Herodez, S.; Gregoric Kumperscak, H.; Dovc, P.; Kokalj Vokac, N. Impaired Neurodevelopmental Genes in Slovenian Autistic Children Elucidate the Comorbidity of Autism with Other Developmental Disorders. Front. Mol. Neurosci. 2022, 15, 912671. [Google Scholar] [CrossRef] [PubMed]
- Winter, B.; Krämer, J.; Meinhardt, T.; Berner, D.; Alt, K.; Wenzel, M.; Winkelmann, J.; Zech, M. NR4A2 and Dystonia with Dopa Responsiveness. Mov. Disord. 2021, 36, 2203–2204. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.S.; Krumbiegel, M.; Schlüter, G.; Ekici, A.B.; Reis, A.; Zweier, C. Haploinsufficiency of NR4A2 Is Associated with a Neurodevelopmental Phenotype with Prominent Language Impairment. Am. J. Med. Genet. A 2017, 173, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Feliciano, P.; Shu, C.; Wang, T.; Astrovskaya, I.; Hall, J.B.; Obiajulu, J.U.; Wright, J.R.; Murali, S.C.; Xu, S.X.; et al. Integrating de Novo and Inherited Variants in 42,607 Autism Cases Identifies Mutations in New Moderate-Risk Genes. Nat. Genet. 2022, 54, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Kaplanis, J.; Samocha, K.E.; Wiel, L.; Zhang, Z.; Arvai, K.J.; Eberhardt, R.Y.; Gallone, G.; Lelieveld, S.H.; Martin, H.C.; McRae, J.F.; et al. Evidence for 28 Genetic Disorders Discovered by Combining Healthcare and Research Data. Nature 2020, 586, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, P.; Zhou, X.; Astrovskaya, I.; Turner, T.N.; Wang, T.; Brueggeman, L.; Barnard, R.; Hsieh, A.; Snyder, L.G.; Muzny, D.M.; et al. Exome Sequencing of 457 Autism Families Recruited Online Provides Evidence for Autism Risk Genes. NPJ Genom. Med. 2019, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Vissers, L.E.L.M.; van Nimwegen, K.J.M.; Schieving, J.H.; Kamsteeg, E.-J.; Kleefstra, T.; Yntema, H.G.; Pfundt, R.; van der Wilt, G.J.; Krabbenborg, L.; Brunner, H.G.; et al. A Clinical Utility Study of Exome Sequencing versus Conventional Genetic Testing in Pediatric Neurology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017, 19, 1055–1063. [Google Scholar] [CrossRef]
- Sleiman, P.M.A.; Healy, D.G.; Muqit, M.M.K.; Yang, Y.X.; Van Der Brug, M.; Holton, J.L.; Revesz, T.; Quinn, N.P.; Bhatia, K.; Diss, J.K.J.; et al. Characterisation of a Novel NR4A2 Mutation in Parkinson’s Disease Brain. Neurosci. Lett. 2009, 457, 75–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grimes, D.A.; Han, F.; Panisset, M.; Racacho, L.; Xiao, F.; Zou, R.; Westaff, K.; Bulman, D.E. Translated Mutation in the Nurr1 Gene as a Cause for Parkinson’s Disease. Mov. Disord. 2006, 21, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Tsai, M.T.; Shaw, C.K.; Chen, C.H. Mutation Analysis of the Human NR4A2 Gene, an Essential Gene for Midbrain Dopaminergic Neurogenesis, in Schizophrenic Patients. Am. J. Med. Genet. 2001, 105, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Rossen, J.L.; Bohnsack, B.L.; Zhang, K.X.; Ing, A.; Drackley, A.; Castelluccio, V.; Ralay-Ranaivo, H. Evaluation of Genetic Testing in a Cohort of Diverse Pediatric Patients in the United States with Congenital Cataracts. Genes 2023, 14, 608. [Google Scholar] [CrossRef] [PubMed]
- Dzinovic, I.; Boesch, S.; Skorvanek, M.; Necpal, J.; Svantnerova, J.; Pavelekova, P.; Havrankova, P.; Tsoma, E.; Indelicato, E.; Runkel, E.; et al. Genetic Overlap between Dystonia and Other Neurologic Disorders: A Study of 1,100 Exomes. Park. Relat. Disord. 2022, 102, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Klee, E.W.; Cousin, M.A.; e Vairo, F.P.; Morales-Rosado, J.A.; Macke, E.L.; Jenkinson, W.G.; Ferrer, A.; Schultz-Rogers, L.E.; Olson, R.J.; Oliver, G.R.; et al. Impact of Integrated Translational Research on Clinical Exome Sequencing. Genet. Med. Off. J. Am. Coll. Med. Genet. 2021, 23, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Zech, M.; Jech, R.; Boesch, S.; Škorvánek, M.; Weber, S.; Wagner, M.; Zhao, C.; Jochim, A.; Necpál, J.; Dincer, Y.; et al. Monogenic Variants in Dystonia: An Exome-Wide Sequencing Study. Lancet. Neurol. 2020, 19, 908–918. [Google Scholar] [CrossRef]
- Barge-Schaapveld, D.Q.C.M.; Ofman, R.; Knegt, A.C.; Alders, M.; Höhne, W.; Kemp, S.; Hennekam, R.C.M. Intellectual Disability and Hemizygous GPD2 Mutation. Am. J. Med. Genet. A 2013, 161A, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, K.; Okamoto, N.; Yamamoto, T. Possible Genes Responsible for Developmental Delay Observed in Patients with Rare 2q23q24 Microdeletion Syndrome: Literature Review and Description of an Additional Patient. Congenit. Anom. Kyoto 2017, 57, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Gabaldon-Albero, A.; Baviera, R.; Hernandez-Muela, S.; Garces, M.; Villanueva, V.; Martínez, F. Translational Research Group in Genetics, Instituto de Investigación Sanitaria La Fe, Valencia, Spain. 2024; manuscript in preparation. [Google Scholar]
- Jankovic, J.; Chen, S.; Le, W.D. The Role of Nurr1 in the Development of Dopaminergic Neurons and Parkinson’s Disease. Prog. Neurobiol. 2005, 77, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Jeong, I.; Kim, C.-H.; Kim, J.; Lee, P.K.J.; Mook-Jung, I.; Leblanc, P.; Kim, K.-S. Correlation between Orphan Nuclear Receptor Nurr1 Expression and Amyloid Deposition in 5XFAD Mice, an Animal Model of Alzheimer’s Disease. J. Neurochem. 2015, 132, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodaei, B.; Alvarsson, A.; Schintu, N.; Ramsköld, D.; Volakakis, N.; Joodmardi, E.; Yoshitake, T.; Kehr, J.; Decressac, M.; Björklund, A.; et al. Transcription Factor Nurr1 Maintains Fiber Integrity and Nuclear-Encoded Mitochondrial Gene Expression in Dopamine Neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 2360–2365. [Google Scholar] [CrossRef] [PubMed]
- Al-Nusaif, M.; Lin, Y.; Li, T.; Cheng, C.; Le, W. Advances in NURR1-Regulated Neuroinflammation Associated with Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 16184. [Google Scholar] [CrossRef] [PubMed]
- Wellenbrock, C.; Hedrich, K.; Schäfer, N.; Kasten, M.; Jacobs, H.; Schwinger, E.; Hagenah, J.; Pramstaller, P.P.; Vieregge, P.; Klein, C. NR4A2 Mutations Are Rare among European Patients with Familial Parkinson’s Disease. Ann. Neurol. 2003, 54, 415. [Google Scholar] [CrossRef]
- Zimprich, A.; Asmus, F.; Leitner, P.; Castro, M.; Bereznai, B.; Homann, N.; Ott, E.; Rutgers, A.W.F.; Wieditz, G.; Trenkwalder, C.; et al. Point Mutations in Exon 1 of the NR4A2 Gene Are Not a Major Cause of Familial Parkinson’s Disease. Neurogenetics 2003, 4, 219–220. [Google Scholar]
- Hering, R.; Petrovic, S.; Mietz, E.-M.; Holzmann, C.; Berg, D.; Bauer, P.; Woitalla, D.; Müller, T.; Berger, K.; Krüger, R.; et al. Extended Mutation Analysis and Association Studies of Nurr1 (NR4A2) in Parkinson Disease. Neurology 2004, 62, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, P.; Lohmann, E.; Pollak, P.; Durif, F.; Tranchant, C.; Agid, Y.; Dürr, A.; Brice, A. Absence of NR4A2 Exon 1 Mutations in 108 Families with Autosomal Dominant Parkinson Disease. Neurology 2004, 62, 2133–2134. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Li, T.; Cui, J.; Fu, Y.; Ren, J.; Sun, X.; Jiang, P.; Yu, S.; Li, C. NR4A2 Genetic Variation and Parkinson’s Disease: Evidence from a Systematic Review and Meta-Analysis. Neurosci. Lett. 2017, 650, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hawk, J.D.; Bookout, A.L.; Poplawski, S.G.; Bridi, M.; Rao, A.J.; Sulewski, M.E.; Kroener, B.T.; Manglesdorf, D.J.; Abel, T. NR4A Nuclear Receptors Support Memory Enhancement by Histone Deacetylase Inhibitors. J. Clin. Invest. 2012, 122, 3593–3602. [Google Scholar] [CrossRef]
- Català-Solsona, J.; Miñano-Molina, A.J.; Rodríguez-Álvarez, J. Nr4a2 Transcription Factor in Hippocampal Synaptic Plasticity, Memory and Cognitive Dysfunction: A Perspective Review. Front. Mol. Neurosci. 2021, 14, 786226. [Google Scholar] [CrossRef]
- Terzioglu-Usak, S.; Negis, Y.; Karabulut, D.S.; Zaim, M.; Isik, S. Cellular Model of Alzheimer’s Disease: Aβ1-42 Peptide Induces Amyloid Deposition and a Decrease in Topo Isomerase IIβ and Nurr1 Expression. Curr. Alzheimer Res. 2017, 14, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Parra-Damas, A.; Valero, J.; Chen, M.; España, J.; Martín, E.; Ferrer, I.; Rodríguez-Alvarez, J.; Saura, C.A. Crtc1 Activates a Transcriptional Program Deregulated at Early Alzheimer’s Disease-Related Stages. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 5776–5787. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Jung, E.S.; Jeon, S.G.; Cha, M.-Y.; Jang, Y.; Kim, W.; Lopes, C.; Mook-Jung, I.; Kim, K.-S. Nurr1 (NR4A2) Regulates Alzheimer’s Disease-Related Pathogenesis and Cognitive Function in the 5XFAD Mouse Model. Aging Cell 2019, 18, e12866. [Google Scholar] [CrossRef] [PubMed]
- Stiller, T.; Merk, D. Exploring Fatty Acid Mimetics as NR4A Ligands. J. Med. Chem. 2023, 66, 15362–15369. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Tao, S.; Rojo de la Vega, M.; Park, S.L.; Vonderfecht, A.A.; Jacobs, S.L.; Zhang, D.D.; Wondrak, G.T. The Antimalarial Amodiaquine Causes Autophagic-Lysosomal and Proliferative Blockade Sensitizing Human Melanoma Cells to Starvation- and Chemotherapy-Induced Cell Death. Autophagy 2013, 9, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Jeon, S.G.; Kim, K.A.; Kim, Y.J.; Song, E.J.; Choi, J.; Ahn, K.J.; Kim, C.-J.; Chung, H.Y.; Moon, M.; et al. The Pharmacological Stimulation of Nurr1 Improves Cognitive Functions via Enhancement of Adult Hippocampal Neurogenesis. Stem Cell Res. 2016, 17, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; Marschner, J.A.; Kilu, W.; Faudone, G.; Busch, R.; Duensing-Kropp, S.; Heering, J.; Merk, D. Nurr1 Modulation Mediates Neuroprotective Effects of Statins. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2022, 9, e2104640. [Google Scholar] [CrossRef] [PubMed]
- Vietor, J.; Gege, C.; Stiller, T.; Busch, R.; Schallmayer, E.; Kohlhof, H.; Höfner, G.; Pabel, J.; Marschner, J.A.; Merk, D. Development of a Potent Nurr1 Agonist Tool for In Vivo Applications. J. Med. Chem. 2023, 66, 6391–6402. [Google Scholar] [CrossRef] [PubMed]
- García-Yagüe, J.; Cuadrado, A. Mechanisms of NURR1 Regulation: Consequences for Its Biological Activity and Involvement in Pathology. Int. J. Mol. Sci. 2023, 24, 12280. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shang, J.; Kojetin, D.J. Molecular Basis of Ligand-Dependent Nurr1-RXRα Activation. eLife 2023, 12, e85039. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Fuertinger, S.; Zinn, J.C.; Sharan, A.D.; Hamzei-Sichani, F.; Simonyan, K. Dopamine drives left-hemispheric lateralization of neural networks during human speech. J. Comp. Neurol. 2018, 526, 920–931. [Google Scholar] [CrossRef] [PubMed]
- García-Yagüe, J.; Rada, P.; Rojo, A.I.; Lastres-Becker, I.; Cuadrado, A.; Tong, X.; Zhang, D.; Buelow, K.; Guha, A.; Arthurs, B.; et al. Nuclear import and export signals control the subcellular localization of Nurr1 protein in response to oxidative stress. J. Biol. Chem. 2013, 288, 5506–5517. [Google Scholar] [CrossRef] [PubMed]
- Volakakis, N.; Kadkhodaei, B.; Joodmardi, E.; Wallis, K.; Panman, L.; Silvaggi, J.; Spiegelman, B.M.; Perlmann, T. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc. Natl. Acad. Sci. USA 2010, 107, 12317–12322. [Google Scholar] [CrossRef] [PubMed]
- Barneda-Zahonero, B.; Servitja, J.-M.; Badiola, N.; Miñano-Molina, A.J.; Fadó, R.; Saura, C.A.; Rodríguez-Alvarez, J. Nurr1 protein is required for N-methyl-D-aspartic acid (NMDA) receptor-mediated neuronal survival. J. Biol. Chem. 2012, 287, 11351–11362. [Google Scholar] [CrossRef] [PubMed]
- AlRuwaili R, Al-Kuraishy HM, Al-Gareeb AI, Ali NH, Alexiou A, Papadakis M, Saad HM, Batiha GE. The Possible Role of Brain-derived Neurotrophic Factor in Epilepsy. Neurochem. Res. 2024, 49, 533–547. [Google Scholar] [CrossRef] [PubMed]


| Reference | Patient 1 [30] | Patient 2 [16] | Patient 3 [17] | Patient 4 [17] | Patient 5 [18] | Patient 6 [18] | Patient 7 [18] | Patient 8 [18] | Patient 9 [18] |
| Age (years)/Sex | 15/F | 9/M | 32/M | 57/F | 15/F | 12/M | 9/F | 3/F | 5/M |
| Motor milestones | Delayed | Normal | Normal | Clumsiness | Delayed | Normal | NA | Delayed | Delayed |
| Hypotonia | NA | No | No | NA | NA | Yes | NA | Yes | Yes |
| ID severity | NA | Mild | Mild | Mild | Severe | Mild | Mild-moderate | Severe | Mild |
| Language | NA | Delayed | Delayed | Normal | NA | LD | NA | NA | LD |
| Psychiatric and behavioral | NA | No | No | No | ASD | Hyperactivity Anxiety | NA | No | Attachment disorder, ADHD |
| Epilepsy/Age at onset | No | Yes/5 years | Yes/26 years | No | Yes/6.5 years | Yes/10 years | No | Yes/5 months | No |
| Seizure type/Epilepsy classification | - | Rolandic epilepsy | Generalized tonic-clonic seizures | - | Focal motor | Rolandic epilepsy | - | Infantile spasms | - |
| Refractory epilepsy | No | No | NA | - | No | No | - | No | - |
| Movement disorder/Age at onset | - | No | Dystonia- Parkinsonism/ 29 years | Dystonia- Parkinsonism/ 30 years | No | No | Dystonia Choreo-athetosis Ataxic gait/NA | Dystonia/ NA | No |
| Dopa responsive | NA | - | Yes | Yes | - | - | NA | NA | - |
| Other signs | - | - | - | - | - | RWLD | - | Microcephaly CVI | Hyposensitivity CVI |
| MRI | NA | Normal | NA | Thinning of substantia nigra | Normal | Normal | Gliosis of thalamus and basal ganglia. | Cerebellar atrophy | NA |
| Variant (NM_006186.3) | c.920T > G | c.326dupA | c.326dupA | c.881dupA | c.839G > A | c.865-1_865delGCinsAAA | c.914G > A | c.1175A > G | c.1576G > T |
| Type | Missense | Frameshift | Frameshift | Frameshift | Missense | Splicing | Missense | Missense | Nonsense |
| de novo | Yes | Yes | Yes | NA | Yes | Yes | Yes | Yes | NA |
| Reference | Patient 10 [18] | Patient 11 [18] | Patient 12 [18] | Patient 13 [19] | Patient 14 [25] | Patient 15 [23] | Patient 16 [23] | Patient 17 | Patient 18 |
| Age (years)/Sex | 2/M | 4/F | 19/F | 30/M | 2.5/M | 11/M | 12/M | 11/F | 25/F |
| Motor milestones | Delayed | Delayed | Delayed | Clumsiness | Delayed | Normal | Delayed | Clumsiness | Normal |
| Hypotonia | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No |
| ID severity | Severe | Moderate | Severe | Mild | Mild-moderate | Mild | Mild | Mild | Moderate |
| Language | Normal | LD | LD | LD | Delay | LD | Delay | LD | LD |
| Psychiatric and behavioral problems | No | No | No | ADHD Trichotilomania | No | ADHD | ADHD | ADHD | Delusions, hallucinations, and mood swings |
| Epilepsy/Age at onset | Yes/6 months | No | No | No | NA | No | No | No | Yes/23 years |
| Seizure type/Epilepsy classification | Infantile spasms Lennox–Gastaut Syndrome | - | - | - | - | - | - | - | Focal (motor) |
| Refractory epilepsy | Yes | - | - | - | - | - | - | - | Yes |
| Movement disorder/Age at onset | No | No | No | Motor tics, cervical dystonia, dystonia-parkinsonism/16 years | Multifocal dystonia/22 months | No | No | No | Oculogyric crisis/ 24 years |
| Dopa responsive | - | - | - | NA | Yes | - | - | - | NA |
| Other signs/symptoms | Sensory and sleep disorder | - | - | - | - | RWLD | RWLD | RWLD | RWLD |
| MRI | Pontine hypoplasia Ventriculomegaly | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Ventriculomegaly Widened subarachnoid space |
| Variant (NM_006186.3) | c.325dupC | c.857T > C | c.968G > T | c.956G > A | c.863A > G | c.1541-2A > C | c.915C > A | c.844G > C | c.905delA |
| Type | Frameshift | Missense | Missense | Missense | Missense | Splicing | Nonsense | Missense | Frameshift |
| de novo | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Reference | Patient 19 | Patient 20 [38] | Patient 21 [26] | Patient 22 [21] | Patient 23 [21] | Patient 24 [21] | Patient 25 [39] | Patient 26 [18] | |
| Age (years)/Sex | 32/F | 25/F | 8/F | 17/M | 8/F | 9/M | 6/F | 43/M | |
| Motor milestones | Clumsiness | Normal | Delayed | Normal | Normal | Normal | Delayed | Delayed | |
| Hypotonia | No | ||||||||
| ID severity | Mild | Moderate | Mild | Mild-moderate | NA | NA | Severe | Severe | |
| Language | LD | Delay | LD | Delay | LD | LD | LD | NA | |
| Psychiatric and behavioral problems | Behavioral disorder | NA | No | ASD | Restlessness | ASD | NA | ADHD | |
| ADHD | Behavioral disorder | Behavioral disorder | |||||||
| Epilepsy/Age at onset | Yes/26 years | NA | NA | No | No | No | NA | Yes/13 years | |
| Seizure type/Epilepsy classification | Focal (motor) | - | - | - | - | - | - | Lennox–Gastaut Syndrome | |
| Refractory epilepsy | Yes | - | - | - | - | - | - | Yes | |
| Movement disorder/Age at onset | No | No | No | No | No | No | NA | Ataxia/adulthood | |
| Dopa responsive | - | - | - | - | - | - | - | NA | |
| Other signs/symptoms | RWLD | - | - | RWLD | - | - | - | - | |
| MRI | Bilateral operculo-insular polymicrogyria foci | Normal | NA | NA | NA | NA | Normal | Widened subarachnoid space | |
| Variant (NM_006186.3) | c.911dupA | 2q24.1(157161283-157459740)×1 | 2q24.1(157120975–157210126)×1 | 2q24.1(157094848_157216692)×1 | 2q24.1(157141281_157216692)×1 | 2q24.1(157141280_157315748)×1 | 2q23.3q24.1(152796289–157299545)×1 | 2q23.3q24.1(154790212_158488241)×1 | |
| Type/size | Frameshift | 298 kb | 89 kb | 122 kb | 75 kb | 174 kb | 4.5 Mb | >3.6 Mb | |
| de novo | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabaldon-Albero, A.; Mayo, S.; Martinez, F. NR4A2 as a Novel Target Gene for Developmental and Epileptic Encephalopathy: A Systematic Review of Related Disorders and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 5198. https://doi.org/10.3390/ijms25105198
Gabaldon-Albero A, Mayo S, Martinez F. NR4A2 as a Novel Target Gene for Developmental and Epileptic Encephalopathy: A Systematic Review of Related Disorders and Therapeutic Strategies. International Journal of Molecular Sciences. 2024; 25(10):5198. https://doi.org/10.3390/ijms25105198
Chicago/Turabian StyleGabaldon-Albero, Alba, Sonia Mayo, and Francisco Martinez. 2024. "NR4A2 as a Novel Target Gene for Developmental and Epileptic Encephalopathy: A Systematic Review of Related Disorders and Therapeutic Strategies" International Journal of Molecular Sciences 25, no. 10: 5198. https://doi.org/10.3390/ijms25105198
APA StyleGabaldon-Albero, A., Mayo, S., & Martinez, F. (2024). NR4A2 as a Novel Target Gene for Developmental and Epileptic Encephalopathy: A Systematic Review of Related Disorders and Therapeutic Strategies. International Journal of Molecular Sciences, 25(10), 5198. https://doi.org/10.3390/ijms25105198





