Porous Hydrogels for Immunomodulatory Applications
Abstract
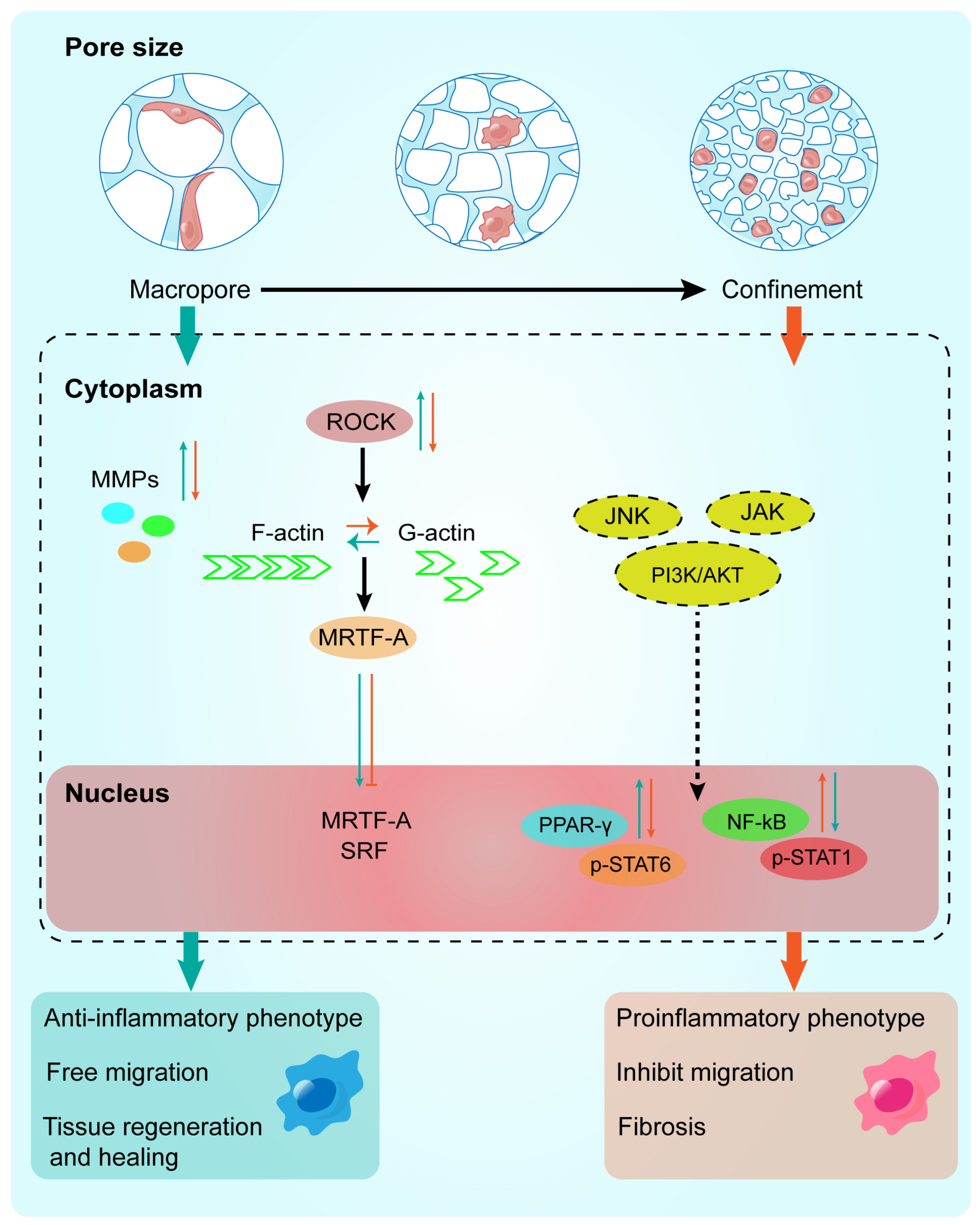
1. Introduction
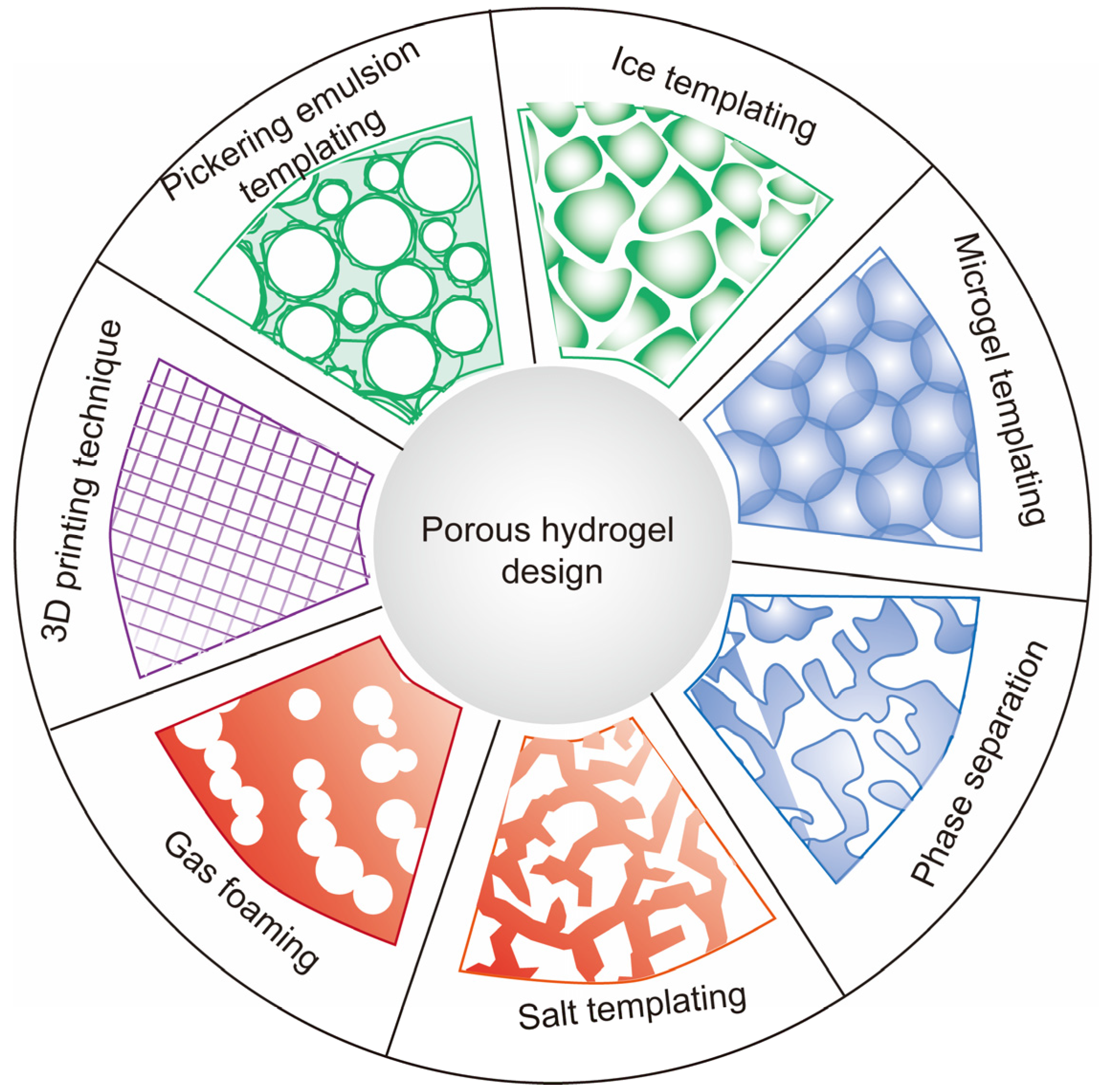
2. Strategies to Develop Porous Hydrogels
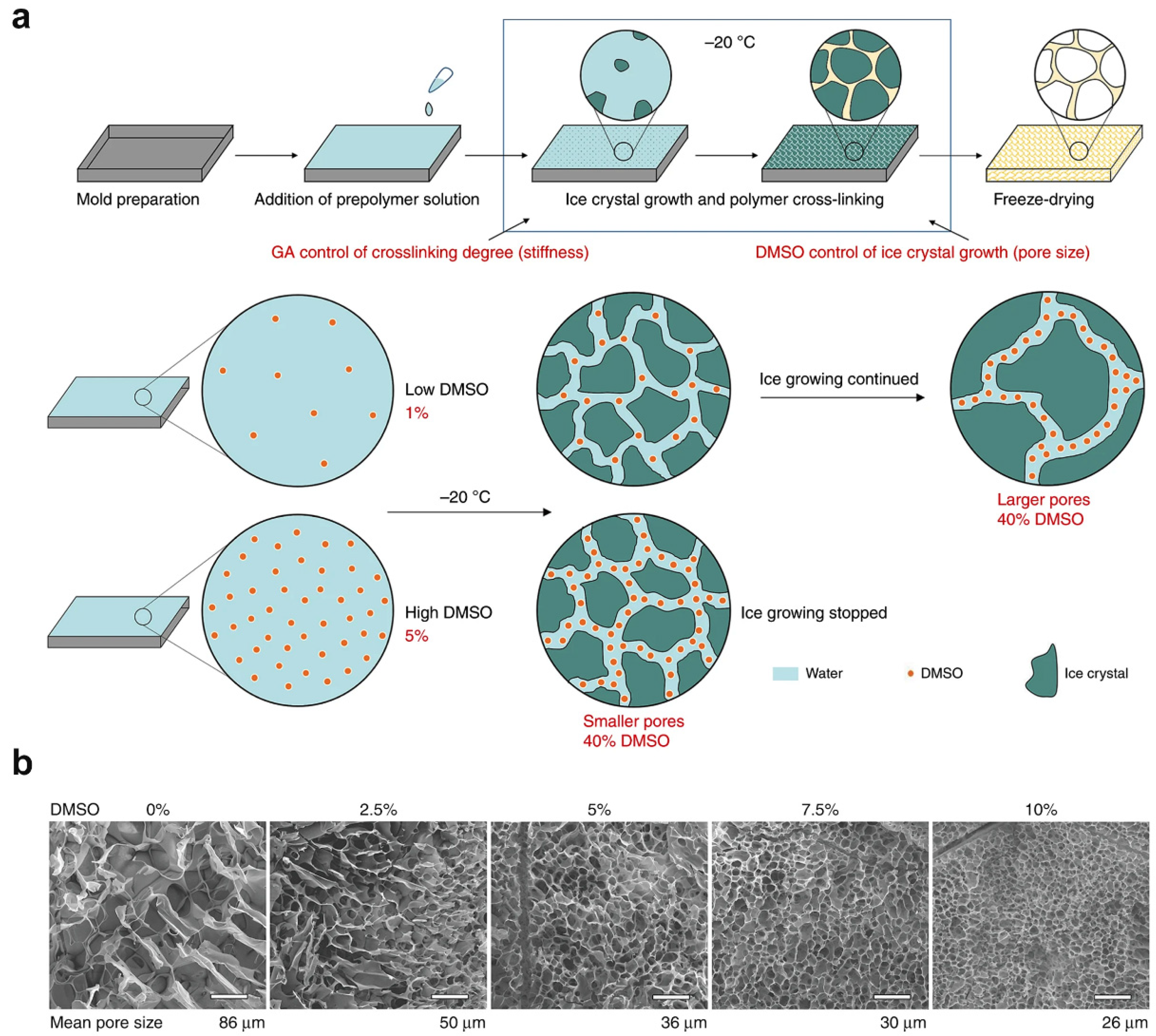
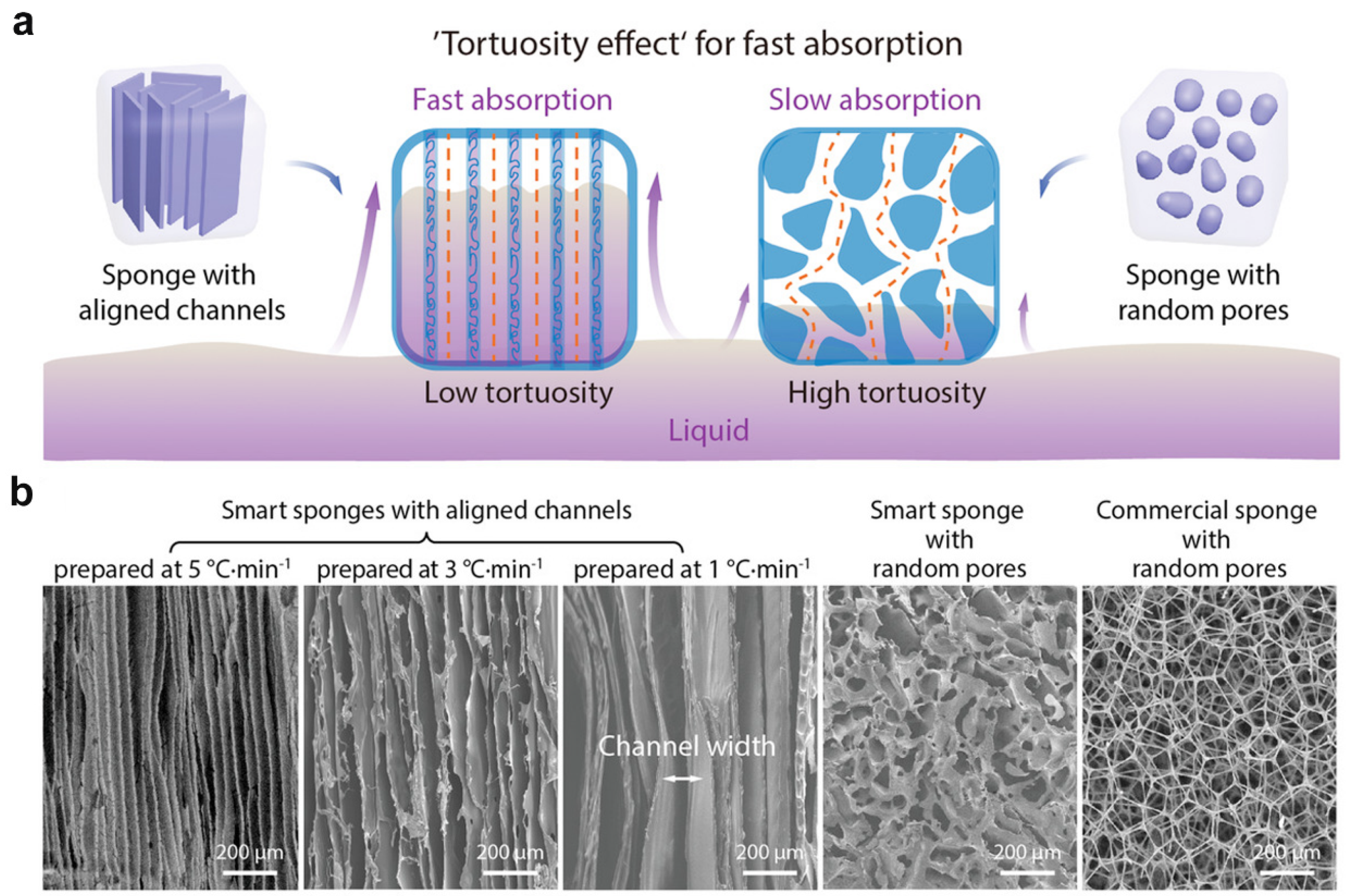
2.1. Ice Templating
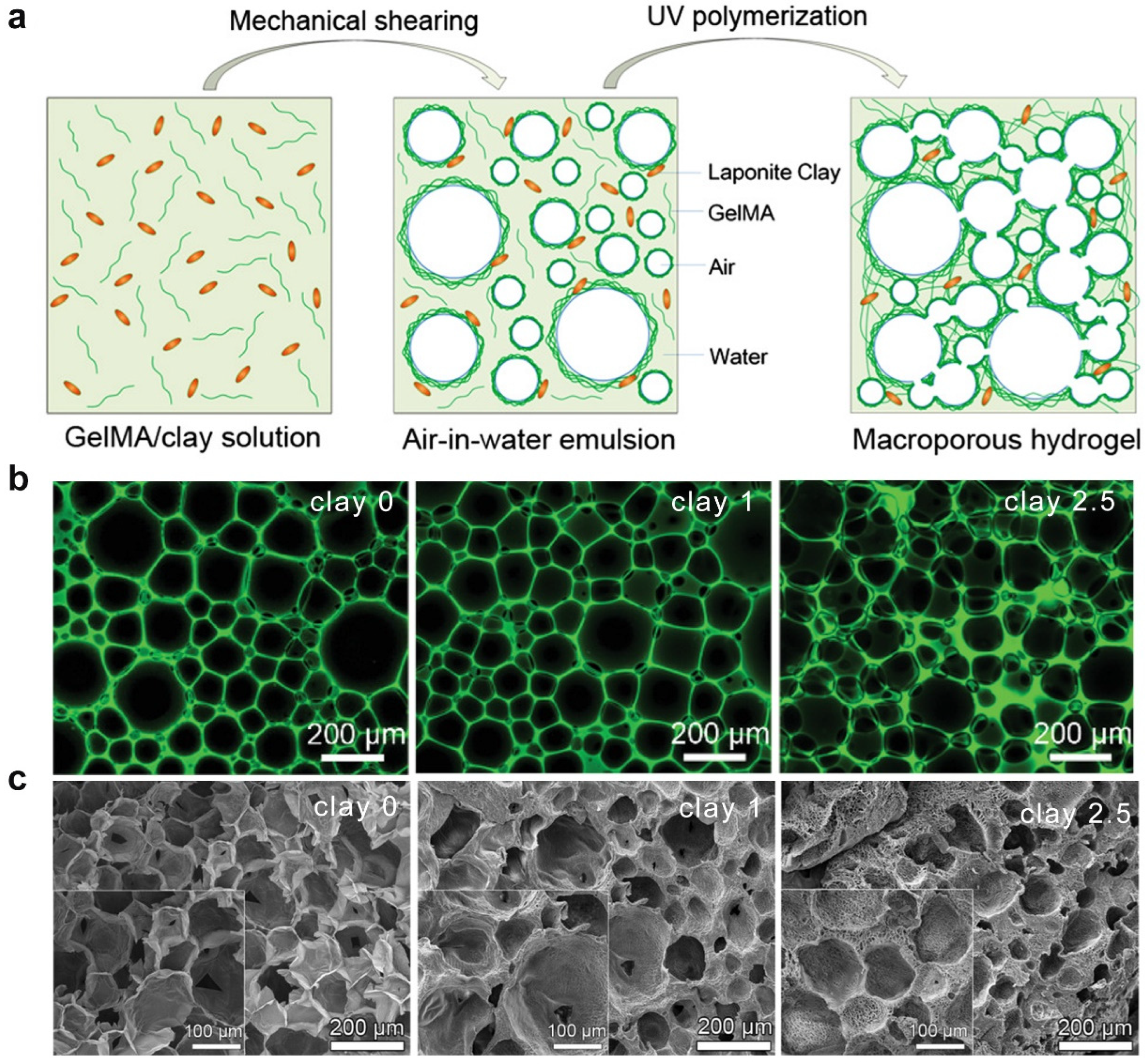
2.2. Pickering Emulsion Templating
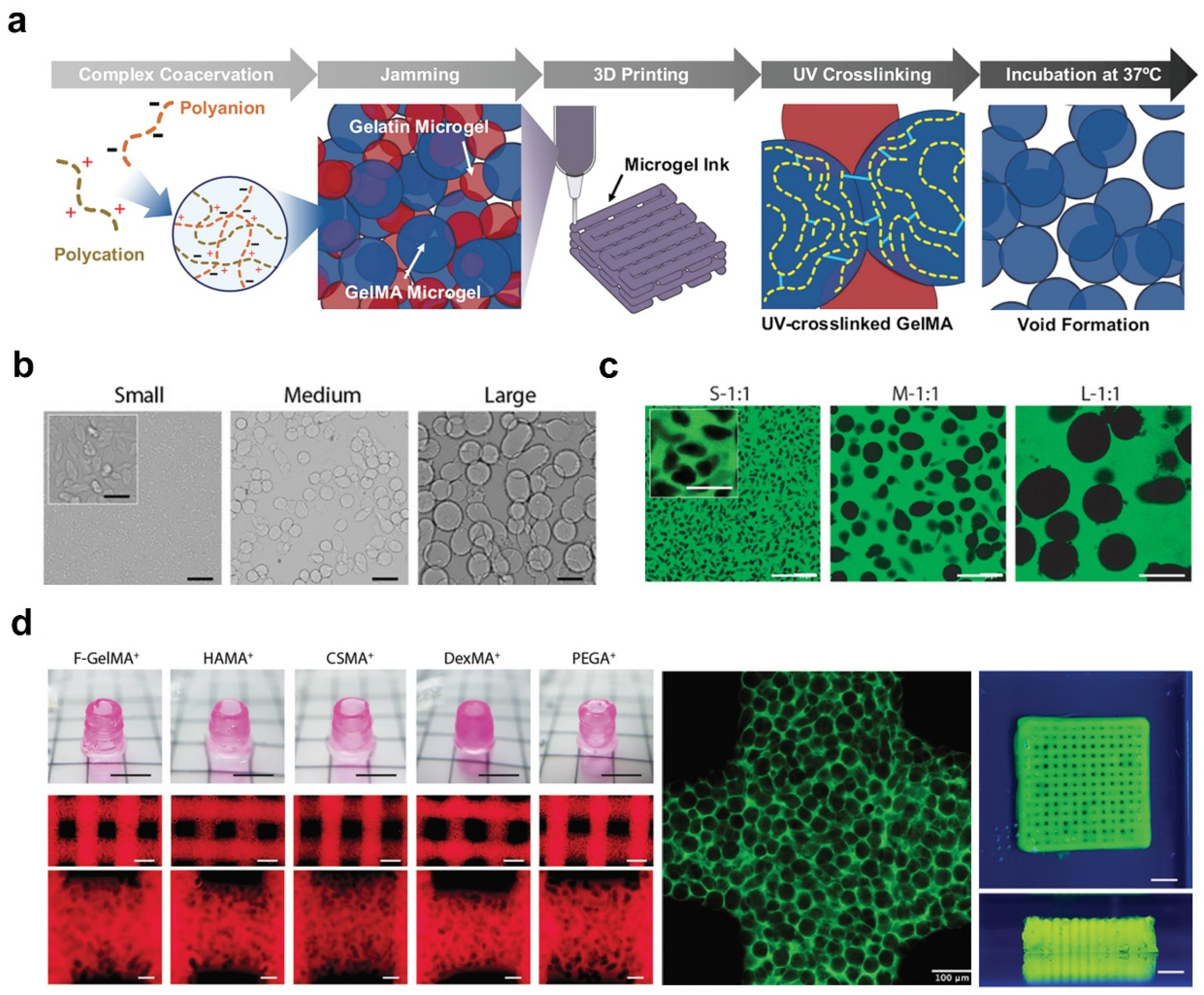
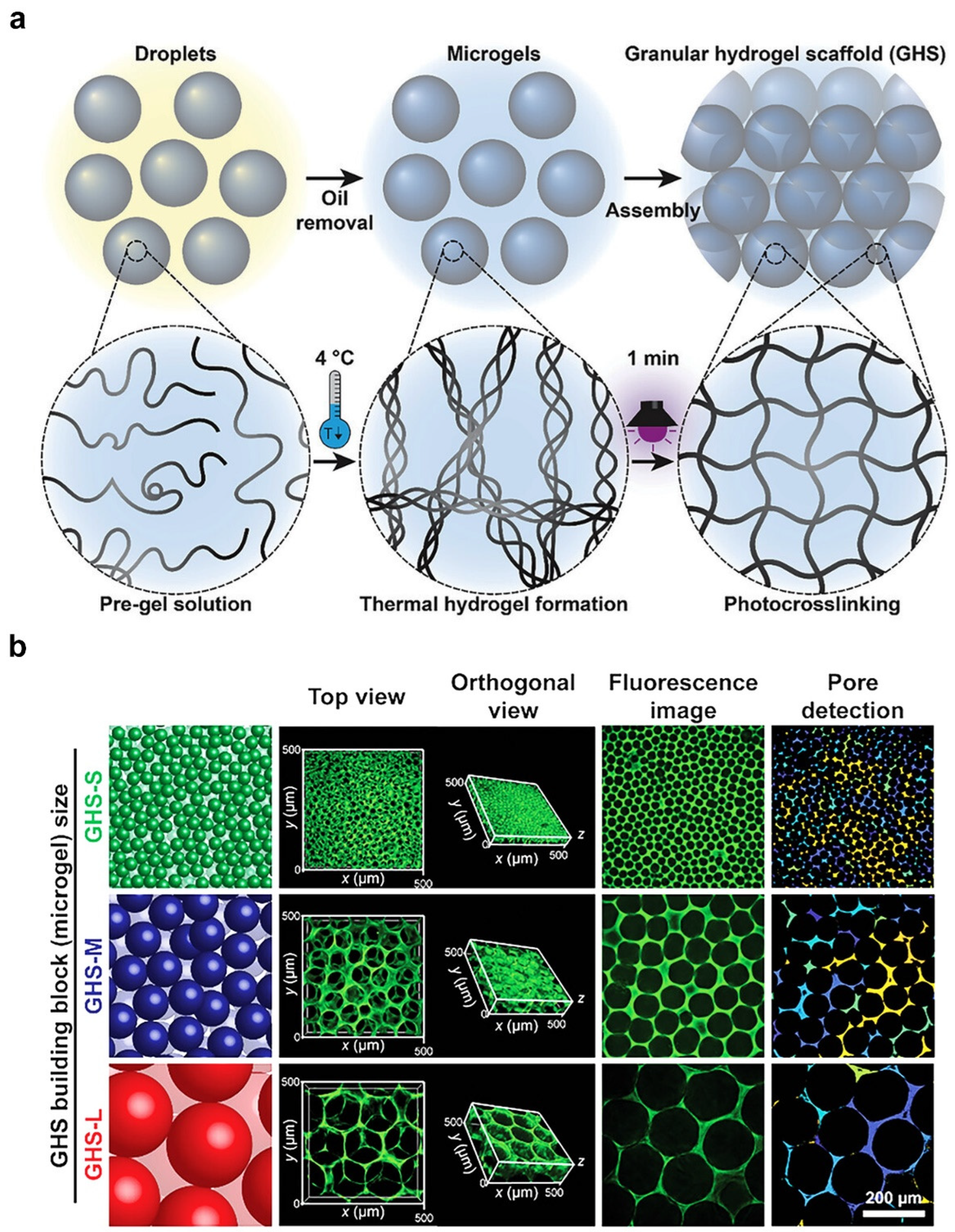
2.3. Microgel Templating
2.4. Phase Separation
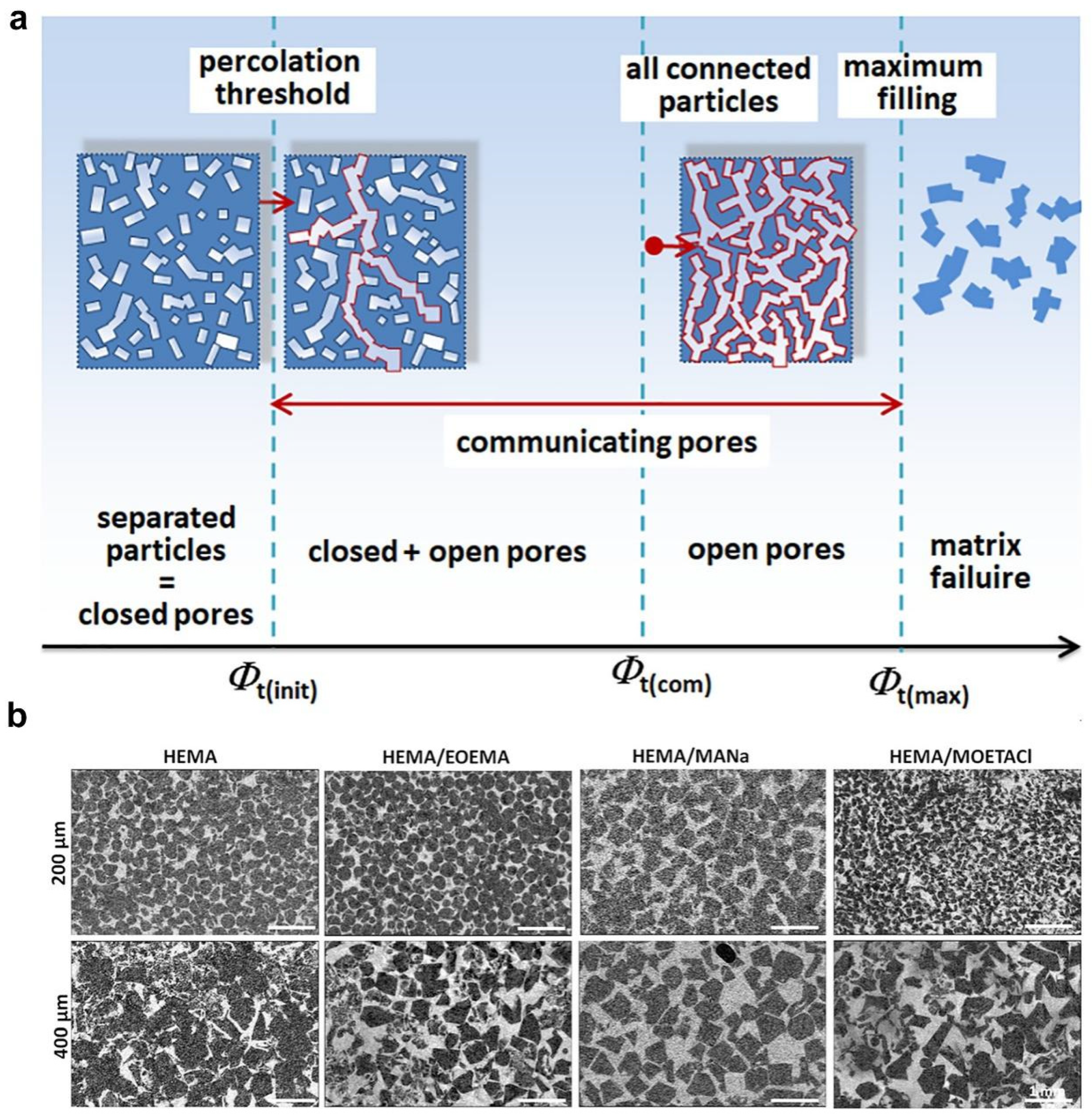
2.5. Salt Templating
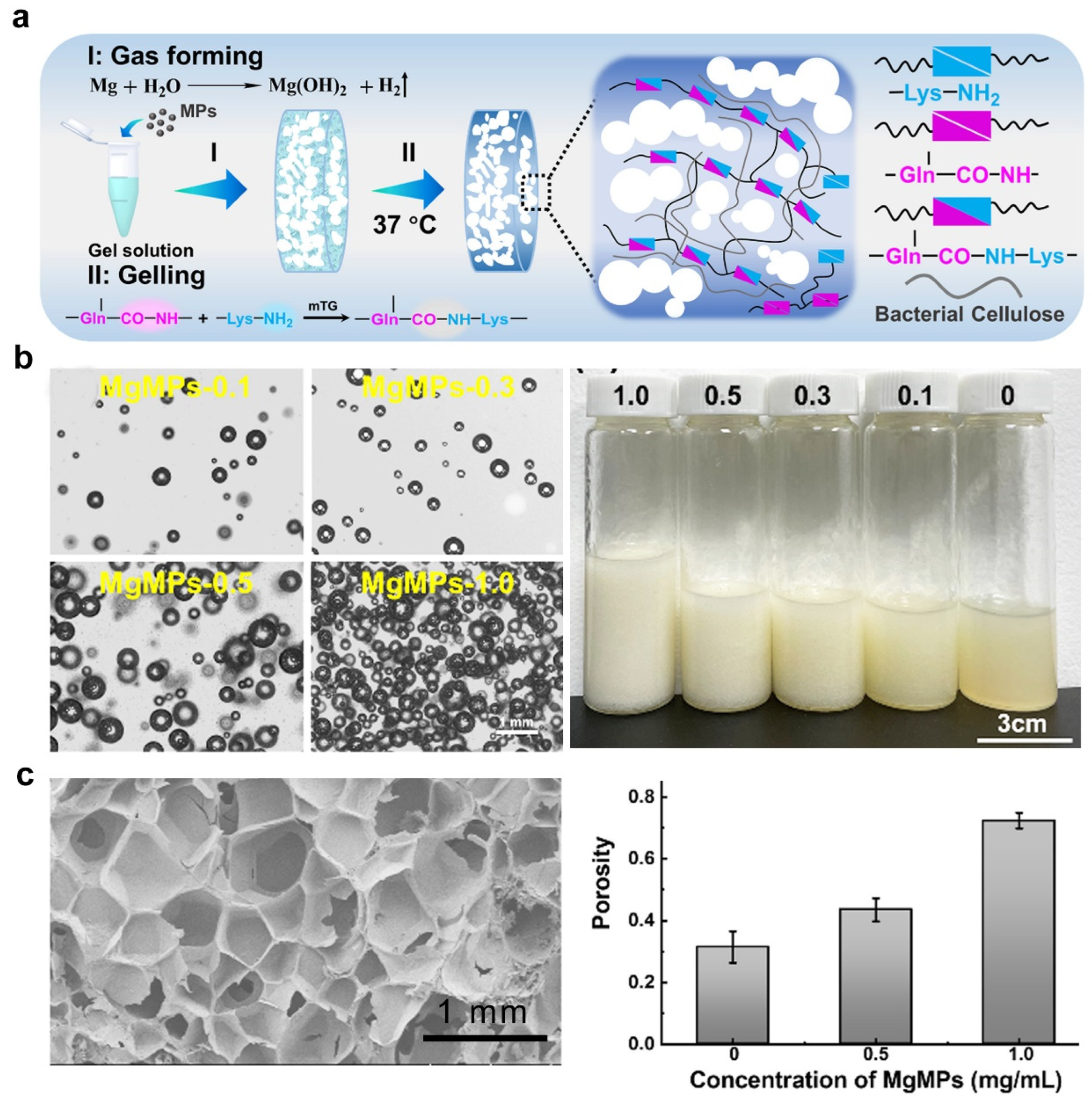
2.6. Gas Foaming
2.7. Three-Dimensional Printing Technique
3. Porous Hydrogel-Assisted Immunomodulation for Cancer Therapy
4. Porous Hydrogel-Assisted Immunomodulation for Tissue Regeneration
5. Concluding Remarks of Porous Hydrogel for Immunomodulatory Applications
6. Future Developments of Porous Hydrogel for Immunomodulatory Applications
Author Contributions
Funding
Conflicts of Interest
References
- Shields, C.W., IV; Wang, L.L.W.; Evans, M.A.; Mitragotri, S. Materials for immunotherapy. Adv. Mater. 2020, 32, 1901633. [Google Scholar] [CrossRef]
- Leach, D.G.; Young, S.; Hartgerink, J.D. Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomater. 2019, 88, 15–31. [Google Scholar] [CrossRef]
- Xue, Y.; Che, J.; Ji, X.; Li, Y.; Xie, J.; Chen, X. Recent advances in biomaterial-boosted adoptive cell therapy. Chem. Soc. Rev. 2022, 51, 1766–1794. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with oncolytic viruses: Progress and challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef]
- Ji, P.; Sun, W.; Zhang, S.; Xing, Y.; Wang, C.; Wei, M.; Li, Q.; Ji, G.; Yang, G. Modular hydrogel vaccine for programmable and coordinate elicitation of cancer immunotherapy. Adv. Sci. 2023, 10, 2301789. [Google Scholar] [CrossRef] [PubMed]
- Zarubova, J.; Hasani-Sadrabadi, M.M.; Ardehali, R.; Li, S. Immunoengineering strategies to enhance vascularization and tissue regeneration. Adv. Drug Deliv. Rev. 2022, 184, 114233. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv. Mater. 2021, 33, 2100176. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Mooney, D.J. Chemical strategies to engineer hydrogels for cell culture. Nat. Rev. Chem. 2022, 6, 726–744. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Biswal, A.K.; Chang, Y.-H.; Misra, P.K.; Yang, J.M. Synthesis and characterization of modified poly (vinyl alcohol) membrane and study of its enhanced water-induced shape-memory behavior. J. Polym. Environ. 2022, 30, 3409–3419. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Chen, X. Designing hydrogels for immunomodulation in cancer therapy and regenerative medicine. Adv. Mater. 2023, 36, 2308894. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Wu, Y.; Ghaemmaghami, A.M.; Sun, H.; Mata, A. Rational design of hydrogels for immunomodulation. Regen. Biomater. 2022, 9, rbac009. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C. Self-healing injectable hydrogels for tissue regeneration. Chem. Rev. 2022, 123, 834–873. [Google Scholar] [CrossRef]
- Xiong, Y.; Feng, Q.; Lu, L.; Zha, K.; Yu, T.; Lin, Z.; Hu, Y.; Panayi, A.C.; Nosrati Ziahmagi, V.; Chu, X. Immunomodulatory hydrogels: Advanced regenerative tools for diabetic foot ulcer. Adv. Funct. Mater. 2023, 33, 2213066. [Google Scholar] [CrossRef]
- Eggermont, L.J.; Rogers, Z.J.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable cryogels for biomedical applications. Trends Biotechnol. 2020, 38, 418–431. [Google Scholar] [CrossRef]
- Djemaa, I.B.; Auguste, S.; Drenckhan-Andreatta, W.; Andrieux, S. Hydrogel foams from liquid foam templates: Properties and optimisation. Adv. Colloid. Interface Sci. 2021, 294, 102478. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Su, T.; Lu, F.; Chang, Q.; Gao, J. Recent advances in macroporous hydrogels for cell behavior and tissue engineering. Gels 2022, 8, 606. [Google Scholar] [CrossRef]
- Najibi, A.J.; Shih, T.-Y.; Mooney, D.J. Cryogel vaccines effectively induce immune responses independent of proximity to the draining lymph nodes. Biomaterials 2022, 281, 121329. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Shokrollahi Barough, M.; Rahmanian, M.; Mojtabavi, N.; Sarrami Forooshani, R.; Seyfoori, A.; Akbari, M. Cancer immunotherapy using bioengineered micro/nano structured hydrogels. Adv. Healthc. Mater. 2023, 12, 2301174. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Wang, C.; Huang, Y.; Han, Y.; Guo, B. Injectable conductive micro-cryogel as a muscle stem cell carrier improves myogenic proliferation, differentiation and in situ skeletal muscle regeneration. Acta Biomater. 2022, 151, 197–209. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous hydrogels: Present challenges and future opportunities. Langmuir. 2023, 39, 2092–2111. [Google Scholar] [CrossRef] [PubMed]
- Nicol, E. Photopolymerized porous hydrogels. Biomacromolecules 2021, 22, 1325–1345. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, B.; Yang, C.; Wu, J.; Zhao, Q. Macroporous hydrogels prepared by ice templating: Developments and challenges. Adv. Funct. Mater. 2023, 41, 3082–3096. [Google Scholar] [CrossRef]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion templating: Porous polymers and beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef]
- Pentzer, E.; Cruz Barrios, E.; Starvaggi, N. Pickering emulsions as templates for architecting composite structures. Acc. Mater. Res. 2023, 4, 641–647. [Google Scholar] [CrossRef]
- Caldwell, A.S.; Rao, V.V.; Golden, A.C.; Anseth, K.S. Porous bio-click microgel scaffolds control hMSC interactions and promote their secretory properties. Biomaterials 2020, 232, 119725. [Google Scholar] [CrossRef]
- Lu, M.; Liu, F.; Tan, R.; Xiao, Z.; Dong, X.-h.; Wang, H.; Tang, L.; Chen, T.; Wu, Z.L.; Hong, W. Phase-separation-induced porous hydrogels from amphiphilic triblock copolymer with high permeability and mechanical strength. Chem. Mater. 2022, 34, 10995–11006. [Google Scholar] [CrossRef]
- Coogan, K.R.; Stone, P.T.; Sempertegui, N.D.; Rao, S.S. Fabrication of micro-porous hyaluronic acid hydrogels through salt leaching. Eur. Polym, J. 2020, 135, 109870. [Google Scholar] [CrossRef]
- Dehghani, F.; Annabi, N. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666. [Google Scholar] [CrossRef]
- Puza, F.; Lienkamp, K. 3D printing of polymer hydrogels—From basic techniques to programmable actuation. Adv. Funct. Mater. 2022, 32, 2205345. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Joukhdar, H.; Seifert, A.; Jüngst, T.; Groll, J.; Lord, M.S.; Rnjak-Kovacina, J. Ice templating soft matter: Fundamental principles and fabrication approaches to tailor pore structure and morphology and their biomedical applications. Adv. Mater. 2021, 33, 2100091. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Xia, K.; Qian, T.; Hu, Z.; Hong, L.; Liao, Y.; Peng, G.; Yuan, Z.; Chen, Y.; Zeng, Z. Shape-recoverable macroporous nanocomposite hydrogels created via ice templating polymerization for noncompressible wound hemorrhage. ACS Biomater. Sci. Eng. 2022, 8, 2076–2087. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, G. Ice inhibition for cryopreservation: Materials, strategies, and challenges. Adv. Sci. 2021, 8, 2002425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, C.; Chen, L.; Dai, B. Control of ice crystal growth and its effect on porous structure of chitosan cryogels. Chem. Eng. Sci. 2019, 201, 50–57. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.S.; Bae, S.; Lee, H.; Hwang, N.S. Heparin functionalized injectable cryogel with rapid shape-recovery property for neovascularization. Biomacromolecules 2018, 19, 2257–2269. [Google Scholar] [CrossRef]
- Murray, K.A.; Gibson, M.I. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022, 6, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lyu, C.; Zhao, P.; Li, W.; Kong, W.; Huang, C.; Genin, G.M.; Du, Y. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat. Commun. 2019, 10, 3491. [Google Scholar] [CrossRef]
- Tam, R.Y.; Fisher, S.A.; Baker, A.E.; Shoichet, M.S. Transparent porous polysaccharide cryogels provide biochemically defined, biomimetic matrices for tunable 3D cell culture. Chem. Mater. 2016, 28, 3762–3770. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Shao, Z.; Mao, A.; Gao, W.; Bai, H. Smart sponge for fast liquid absorption and thermal responsive self-squeezing. Adv. Mater. 2020, 32, 1908249. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Agresti, F.; Fedele, L.; Barison, S.; Hermida-Merino, C.; Losada-Barreiro, S.; Bobbo, S.; Piñeiro, M. Review on phase change material emulsions for advanced thermal management: Design, characterization and thermal performance. Renew. Sustain. Energy Rev. 2022, 159, 112238. [Google Scholar] [CrossRef]
- Dai, H.; Luo, Y.; Huang, Y.; Ma, L.; Chen, H.; Fu, Y.; Yu, Y.; Zhu, H.; Wang, H.; Zhang, Y. Recent advances in protein-based emulsions: The key role of cellulose. Food Hydrocolloids 2023, 136, 108260. [Google Scholar] [CrossRef]
- Rodriguez, A.M.B.; Binks, B.P. High internal phase pickering emulsions. Curr. Opin. Colloid. Interface Sci. 2022, 57, 101556. [Google Scholar] [CrossRef]
- Jie, Y.; Chen, F.; Zhu, T.; Lv, D. High internal phase emulsions stabilized solely by carboxymethyl chitosan. Food Hydrocolloids 2022, 127, 107554. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, S.; Zheng, W.; Chen, M.; Zhou, S.; Liao, M.; Huang, W.; Hu, Y.; Zhou, W. Formation of poly (ε-caprolactone)-embedded bioactive nanoparticles/collagen hierarchical scaffolds with the designed and customized porous structures. J. Appl. Polym. Sci. 2022, 139, e52749. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering emulsions: Versatility of colloidal particles and recent applications. Curr. Opin. Colloid. Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.; Chen, Y.; Gao, H.; Shi, X.; Cheng, W.; Ren, L.; Wang, Y. High internal phase emulsions stabilised by supramolecular cellulose nanocrystals and their application as cell-adhesive macroporous hydrogel monoliths. J. Mater. Chem. 2017, 5, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.; Chen, Y.; Teng, L.; Qi, D.; Ren, L. Air-in-water emulsion solely stabilized by gelatin methacryloyl and templating for macroporous nanocomposite hydrogels. Macromol. Chem. Phys. 2019, 220, 1800500. [Google Scholar] [CrossRef]
- Xu, M.; Li, Q.; Fang, Z.; Jin, M.; Zeng, Q.; Huang, G.; Jia, Y.-G.; Wang, L.; Chen, Y. Conductive and antimicrobial macroporous nanocomposite hydrogels generated from air-in-water pickering emulsions for neural stem cell differentiation and skin wound healing. Biomater. Sci. 2020, 8, 6957–6968. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zheng, Z.; Pu, S.; Jia, Y.G.; Wang, L.; Chen, Y. Macroporous adhesive nano-enabled hydrogels generated from air-in-water emulsions. Macromol. Biosci. 2022, 22, 2100491. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, X.; Luo, J.; Yang, Q.; Chen, Y.; Wang, Y. Double-network DNA macroporous hydrogel enables aptamer-directed cell recruitment to accelerate bone healing. Adv. Sci. 2023, 11, 2303637. [Google Scholar] [CrossRef]
- Feng, Q.; Li, D.; Li, Q.; Cao, X.; Dong, H. Microgel assembly: Fabrication, characteristics and application in tissue engineering and regenerative medicine. Bioact. Mater. 2022, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lin, F.-Y.; Jiang, K.; Nguyen, H.; Chang, C.-Y.; Lin, C.-C. Dissolvable microgel-templated macroporous hydrogels for controlled cell assembly. Biomater. Adv. 2022, 134, 112712. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Wojciechowski, J.P.; Tang, J.; Guo, Y.; Stevens, M.M. Tunable microgel-templated porogel (MTP) bioink for 3D bioprinting applications. Adv. Healthc. Mater. 2022, 11, 2200027. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Seymour, A.J.; Shin, S.; Heilshorn, S.C. 3D printing of microgel scaffolds with tunable void fraction to promote cell infiltration. Adv. Healthc. Mater. 2021, 10, 2100644. [Google Scholar] [CrossRef] [PubMed]
- Ataie, Z.; Horchler, S.; Jaberi, A.; Koduru, S.V.; El-Mallah, J.C.; Sun, M.; Kheirabadi, S.; Kedzierski, A.; Risbud, A.; Silva, A.R.A.E. Accelerating patterned vascularization using granular hydrogel scaffolds and surgical micropuncture. Small 2023, 20, 2307928. [Google Scholar] [CrossRef] [PubMed]
- Kittel, Y.; Kuehne, A.J.; De Laporte, L. Translating therapeutic microgels into clinical applications. Adv. Healthc. Mater. 2022, 11, 2101989. [Google Scholar] [CrossRef] [PubMed]
- Mittag, T.; Pappu, R.V. A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell 2022, 82, 2201–2214. [Google Scholar] [CrossRef]
- Xiao, Q.; McAtee, C.K.; Su, X. Phase separation in immune signalling. Nat. Rev. Immunol. 2022, 22, 188–199. [Google Scholar] [CrossRef]
- Ryu, J.K.; Hwang, D.E.; Choi, J.M. Current understanding of molecular phase separation in chromosomes. Int. J. Mol. Sci. 2021, 22, 10736. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Zhang, J. Liquid–liquid phase separation drives cellular function and dysfunction in cancer. Nat. Rev. Cancer 2022, 22, 239–252. [Google Scholar] [CrossRef]
- Broguiere, N.; Husch, A.; Palazzolo, G.; Bradke, F.; Madduri, S.; Zenobi-Wong, M. Macroporous hydrogels derived from aqueous dynamic phase separation. Biomaterials 2019, 200, 56–65. [Google Scholar] [CrossRef]
- Musacchio, A. On the role of phase separation in the biogenesis of membraneless compartments. EMBO J. 2022, 41, e109952. [Google Scholar] [CrossRef]
- Cao, H.; Li, H.; Liu, L.; Xue, K.; Niu, X.; Hou, J.; Chen, L. Salt-templated nanoarchitectonics of CoSe(2)-NC nanosheets as an efficient bifunctional oxygen electrocatalyst for water splitting. Int. J. Mol. Sci. 2022, 23, 5239. [Google Scholar] [CrossRef]
- Dušková-Smrčková, M.; Zavřel, J.; Bartoš, M.; Kaberova, Z.; Filová, E.; Zárubová, J.; Šlouf, M.; Michálek, J.; Vampola, T.; Kubies, D. Communicating macropores in PHEMA-based hydrogels for cell seeding: Probabilistic open pore simulation and direct micro-CT proof. Mater. Des. 2021, 198, 109312. [Google Scholar] [CrossRef]
- De France, K.J.; Xu, F.; Hoare, T. Structured macroporous hydrogels: Progress, challenges, and opportunities. Adv. Healthc. Mater. 2018, 7, 1700927. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, S.; Yin, S.; Jiang, F.; Zhou, M.; Yang, G.; Sun, N.; Zhang, W.; Jiang, X. In situ gas foaming based on magnesium particle degradation: A novel approach to fabricate injectable macroporous hydrogels. Biomaterials 2020, 232, 119727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, M.; Liao, S.; Yuan, B.; Shi, R.; Hu, X.; Wang, Y. An injectable macroporous hydrogel templated by gasification reaction for enhanced tissue regeneration. Supramol. Mater. 2023, 2, 100037. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D bioprinting of human tissues: Biofabrication, bioinks, and bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yue, Z.; Lucarelli, E.; Wallace, G.G. Hybrid printing using cellulose nanocrystals reinforced GelMA/HAMA hydrogels for improved structural integration. Adv. Healthc. Mater. 2020, 9, 2001410. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. State-of-the-art of 3D printing technology of alginate-based hydrogels—An emerging technique for industrial applications. Adv. Colloid. Interface Sci. 2021, 293, 102436. [Google Scholar] [CrossRef]
- Li, Q.; Xu, S.; Feng, Q.; Dai, Q.; Yao, L.; Zhang, Y.; Gao, H.; Dong, H.; Chen, D.; Cao, X. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact. Mater. 2021, 6, 3396–3410. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ding, X.; Yu, X.; Chen, X.; Zhang, X.; Cui, S.; Shi, J.; Chen, J.; Yu, L.; Chen, S. Cell-free bilayered porous scaffolds for osteochondral regeneration fabricated by continuous 3D-printing using nascent physical hydrogel as ink. Adv. Healthc. Mater. 2021, 10, 2001404. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.; Ahadian, S.; Davenport Huyer, L.; Lo Rito, M.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017, 16, 1038–1046. [Google Scholar] [CrossRef]
- Wales, D.J.; Keshavarz, M.; Howe, C.; Yeatman, E. 3D Printability assessment of poly (octamethylene maleate (anhydride) citrate) and poly (ethylene glycol) diacrylate copolymers for biomedical applications. ACS Appl. Polym. Mater. 2022, 4, 5457–5470. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.; Papneja, N.; Miller, W. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Taefehshokr, S.; Parhizkar, A.; Hayati, S.; Mousapour, M.; Mahmoudpour, A.; Eleid, L.; Rahmanpour, D.; Fattahi, S.; Shabani, H.; Taefehshokr, N. Cancer immunotherapy: Challenges and limitations. Pathol. Res. Pract. 2022, 229, 153723. [Google Scholar] [CrossRef]
- Erfani, A.; Diaz, A.E.; Doyle, P.S. Hydrogel-enabled, local administration and combinatorial delivery of immunotherapies for cancer treatment. Mater. Today 2023, 65, 227–243. [Google Scholar] [CrossRef]
- Iudin, D.; Vasilieva, M.; Knyazeva, E.; Korzhikov-Vlakh, V.; Demyanova, E.; Lavrentieva, A.; Skorik, Y.; Korzhikova-Vlakh, E. Hybrid nanoparticles and composite hydrogel systems for delivery of peptide antibiotics. Int. J. Mol. Sci. 2022, 23, 2771. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Warren Sands, R.; Ali, O.A.; Li, W.A.; Lewin, S.A.; Braschler, T.M.; Shih, T.-Y.; Verbeke, C.S.; Bhatta, D.; Dranoff, G. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 2015, 6, 7556. [Google Scholar] [CrossRef] [PubMed]
- Bauleth-Ramos, T.; Shih, T.Y.; Shahbazi, M.A.; Najibi, A.J.; Mao, A.S.; Liu, D.; Granja, P.; Santos, H.A.; Sarmento, B.; Mooney, D.J. Acetalated dextran nanoparticles loaded into an injectable alginate cryogel for combined chemotherapy and cancer vaccination. Adv. Funct. Mater. 2019, 29, 1903686. [Google Scholar] [CrossRef]
- Shah, N.J.; Najibi, A.J.; Shih, T.-Y.; Mao, A.S.; Sharda, A.; Scadden, D.T.; Mooney, D.J. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat. Biomed. Eng. 2020, 4, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Najibi, A.J.; Sobral, M.C.; Seo, B.R.; Lee, J.Y.; Wu, D.; Li, A.W.; Verbeke, C.S.; Mooney, D.J. Biomaterial-based scaffold for in situ chemo-immunotherapy to treat poorly immunogenic tumors. Nat. Commun. 2020, 11, 5696. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Y.; Chen, J.; Yu, P.; Tang, F.; Hu, Z.; Zhou, J.; Liu, L.; Qiu, W.; Ye, Y. Regulation of biomaterial implantation-induced fibrin deposition to immunological functions of dendritic cells. Materials Today Bio 2022, 14, 100224. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Gao, W.; Gong, Y.; Guo, P.; Li, W.; Shu, X.; Lü, S.; Zeng, Z.; Zhang, Y.; Long, M. Trail formation alleviates excessive adhesion and maintains efficient neutrophil migration. ACS Appl. Mater. Interfaces 2023, 15, 17577–17591. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, Y.; Si, X.; Yao, H.; Ma, S.; Xu, Y.; Zhao, J.; Ma, C.; He, C.; Tang, Z. Biopolymer immune implants’ sequential activation of innate and adaptive immunity for colorectal cancer postoperative immunotherapy. Adv. Mater. 2021, 33, 2004559. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.; Han, J.; Liu, Y.; Bo, Y.; Wang, H. T cell-responsive macroporous hydrogels for in situ T cell expansion and enhanced antitumor efficacy. Biomaterials 2023, 293, 121972. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-T.; Cho, S.M.; Youn, D.H.; Hong, E.P.; Park, C.H.; Lee, Y.; Jung, H.; Jeon, J.P. Therapeutic effect of a hydrogel-based neural stem cell delivery sheet for mild traumatic brain injury. Acta Biomater. 2023, 167, 335–347. [Google Scholar] [CrossRef]
- Pazhouhnia, Z.; Beheshtizadeh, N.; Namini, M.S.; Lotfibakhshaiesh, N. Portable hand-held bioprinters promote in situ tissue regeneration.Bioeng. Transl. Med. 2022, 7, e10307. [Google Scholar]
- Bakhshandeh, B.; Ranjbar, N.; Abbasi, A.; Amiri, E.; Abedi, A.; Mehrabi, M.R.; Dehghani, Z.; Pennisi, C.P. Recent progress in the manipulation of biochemical and biophysical cues for engineering functional tissues. Bioeng. Transl. Med. 2023, 8, e10383. [Google Scholar] [CrossRef]
- Yang, R.; Xue, W.; Ma, X.; Ren, Y.; Xu, L.; Kong, W.; Zhang, W.; Wang, P.; Tan, X.; Chi, B. Engineering the dynamics of biophysical cues in supramolecular hydrogels to facile control stem cell chondrogenesis for cartilage regeneration. Compos. Part. B 2023, 250, 110429. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Gao, Y.; Xu, Z.; Dai, C.; Li, G.; Sun, C.; Yang, Y.; Zhang, K. Conductive biocomposite hydrogels with multiple biophysical cues regulate schwann cell behaviors. J. Mater. Chem. B 2022, 10, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and assessment of biodegradable macroporous cryogels as advanced tissue engineering and drug carrying materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, F.; Tang, Y.; Wang, J.; Chen, X.; Li, X.; Zhang, X. Regulation of macrophage polarization and functional status by modulating hydroxyapatite ceramic micro/nano-topography. Mater. Des. 2022, 213, 110302. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Yang, Y.; Zhang, J.; Wang, Y.; Zhang, R.; Yang, L. Versatile hydrogel dressings that dynamically regulate the healing of infected deep burn wounds. Adv. Healthc. Mater. 2023, 12, 2301224. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Q.; Shi, C.; Chen, M.; Ma, K.; Wan, J.; Liu, R. Dealing with the foreign-body response to implanted biomaterials: Strategies and applications of new materials. Adv. Funct. Mater. 2021, 31, 2007226. [Google Scholar] [CrossRef]
- Yu, J.; Lin, Y.; Wang, G.; Song, J.; Hayat, U.; Liu, C.; Raza, A.; Huang, X.; Lin, H.; Wang, J.-Y. Zein-induced immune response and modulation by size, pore structure and drug-loading: Application for sciatic nerve regeneration. Acta Biomater. 2022, 140, 289–301. [Google Scholar] [CrossRef]
- Mass, E.; Nimmerjahn, F.; Kierdorf, K.; Schlitzer, A. Tissue-specific macrophages: How they develop and choreograph tissue biology. Nat. Rev. Immunol. 2023, 23, 563–579. [Google Scholar] [CrossRef]
- Park, M.D.; Silvin, A.; Ginhoux, F.; Merad, M. Macrophages in health and disease. Cell 2022, 185, 4259–4279. [Google Scholar] [CrossRef]
- Mao, J.; Chen, L.; Cai, Z.; Qian, S.; Liu, Z.; Zhao, B.; Zhang, Y.; Sun, X.; Cui, W. Advanced biomaterials for regulating polarization of macrophages in wound healing. Adv. Funct. Mater. 2022, 32, 2111003. [Google Scholar] [CrossRef]
- Yin, Y.; He, X.-T.; Wang, J.; Wu, R.-X.; Xu, X.-Y.; Hong, Y.-L.; Tian, B.-M.; Chen, F.-M. Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl. Mater. Today 2020, 18, 100466. [Google Scholar] [CrossRef]
- Li, W.; Dai, F.; Zhang, S.; Xu, F.; Xu, Z.; Liao, S.; Zeng, L.; Song, L.; Ai, F. Pore size of 3D-printed polycaprolactone/polyethylene glycol/hydroxyapatite scaffolds affects bone regeneration by modulating macrophage polarization and the foreign body response. ACS Appl. Mater. Interfaces 2022, 14, 20693–20707. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Iwaki, R.; Reinhardt, J.W.; Chang, Y.-C.; Miyamoto, S.; Kelly, J.; Zbinden, J.; Blum, K.; Mirhaidari, G.; Ulziibayar, A. The effect of pore diameter on neo-tissue formation in electrospun biodegradable tissue-engineered arterial grafts in a large animal model. Acta Biomater. 2020, 115, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Hao, D.; Barrera, J.; Henn, D.; Lin, S.; Moeinzadeh, S.; Kim, S.; Maloney, W.; Gurtner, G.; Wang, A. A bioactive compliant vascular graft modulates macrophage polarization and maintains patency with robust vascular remodeling. Bioact. Mater. 2023, 19, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Moeller, J.; Vogel, V. Mechanobiology of macrophages: How physical factors coregulate macrophage plasticity and phagocytosis. Annu. Rev. Biomed. Eng. 2019, 21, 267–297. [Google Scholar] [CrossRef]
- Liu, Y.; Suarez-Arnedo, A.; Riley, L.; Miley, T.; Xia, J.; Segura, T. Spatial confinement modulates macrophage response in microporous annealed particle (MAP) scaffolds. Adv. Healthc. Mater. 2023, 12, 2300823. [Google Scholar] [CrossRef]
- Jain, N.; Vogel, V. Spatial confinement downsizes the inflammatory response of macrophages. Nat. Mater. 2018, 17, 1134–1144. [Google Scholar] [CrossRef]



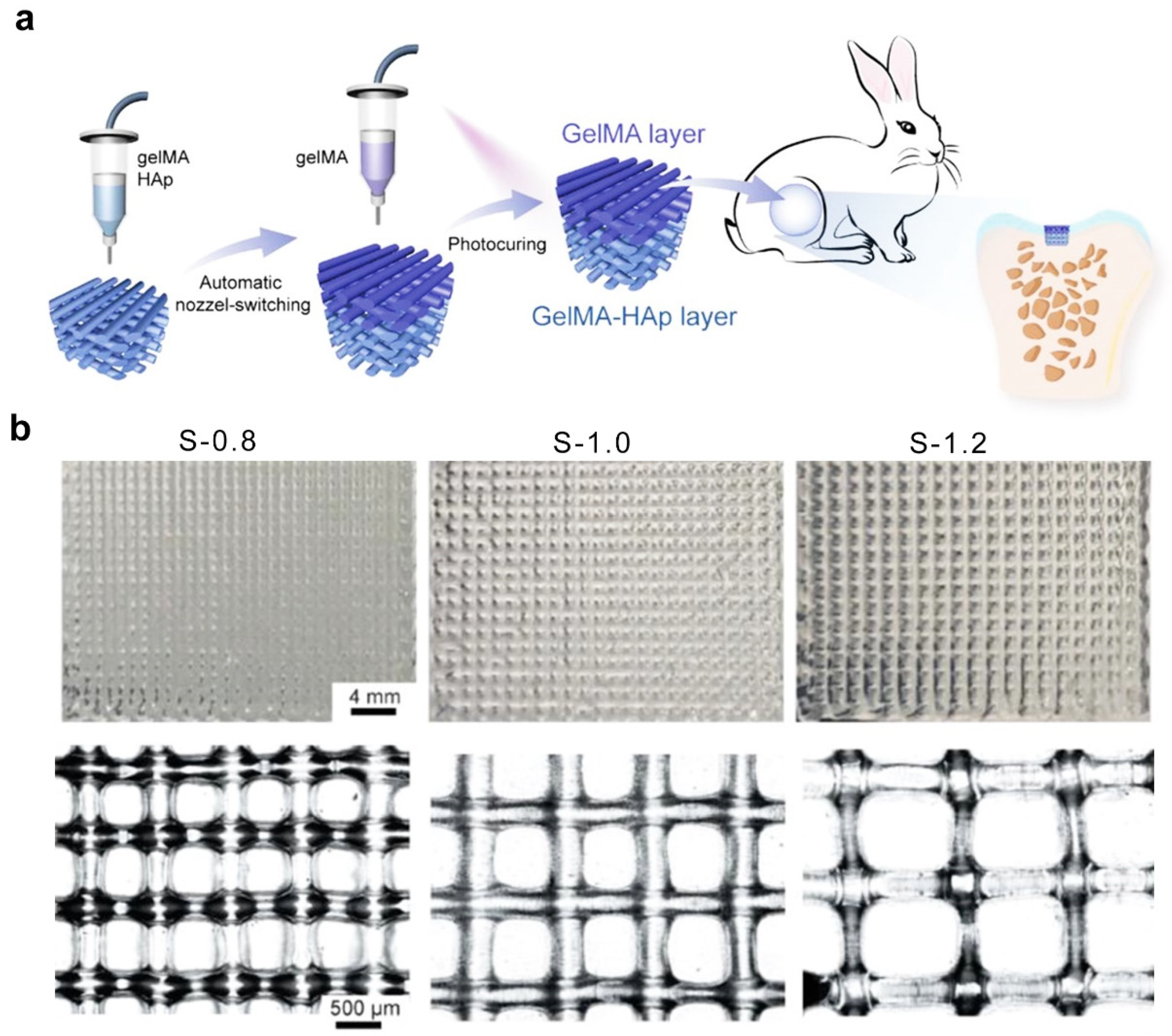
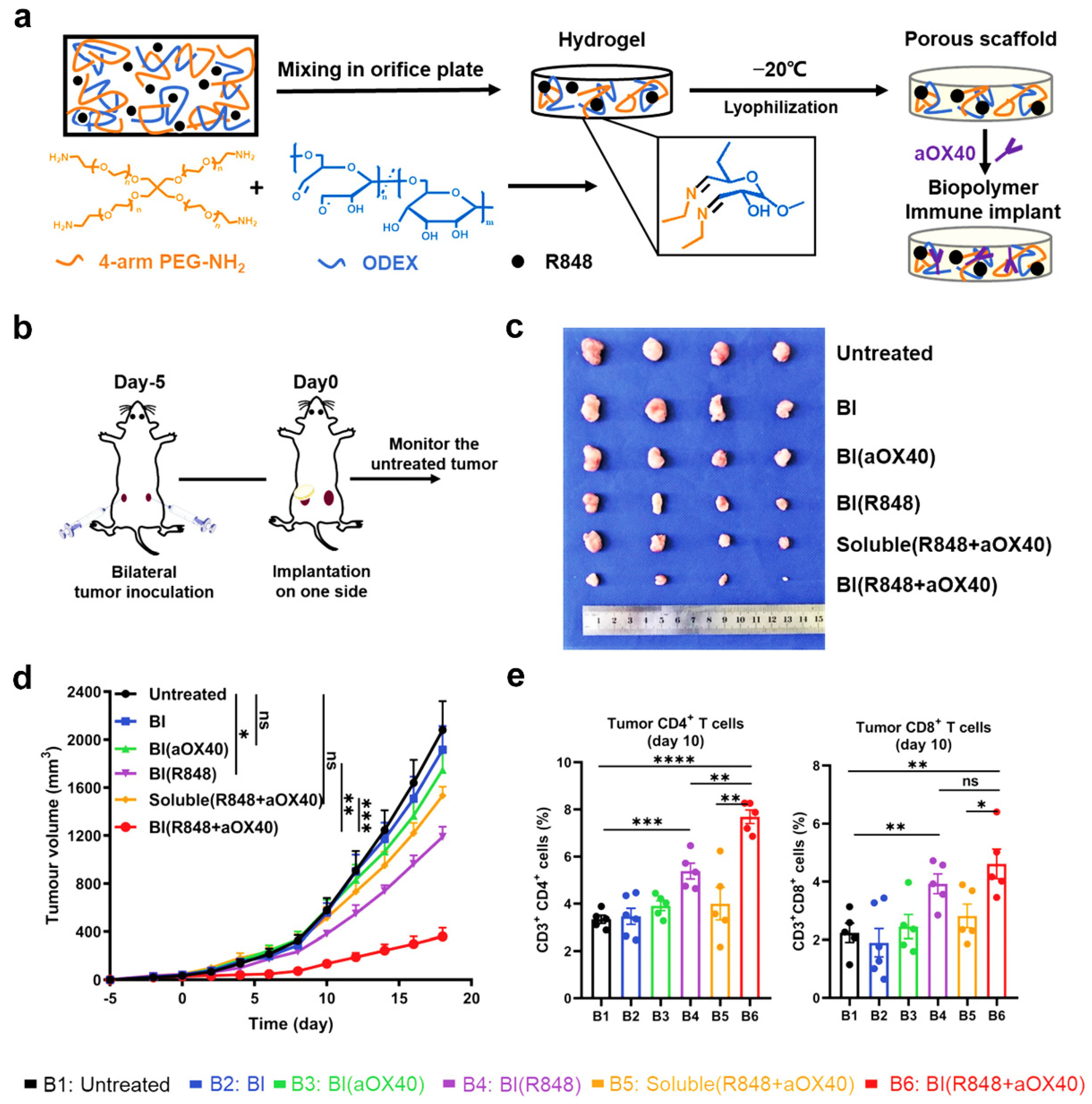

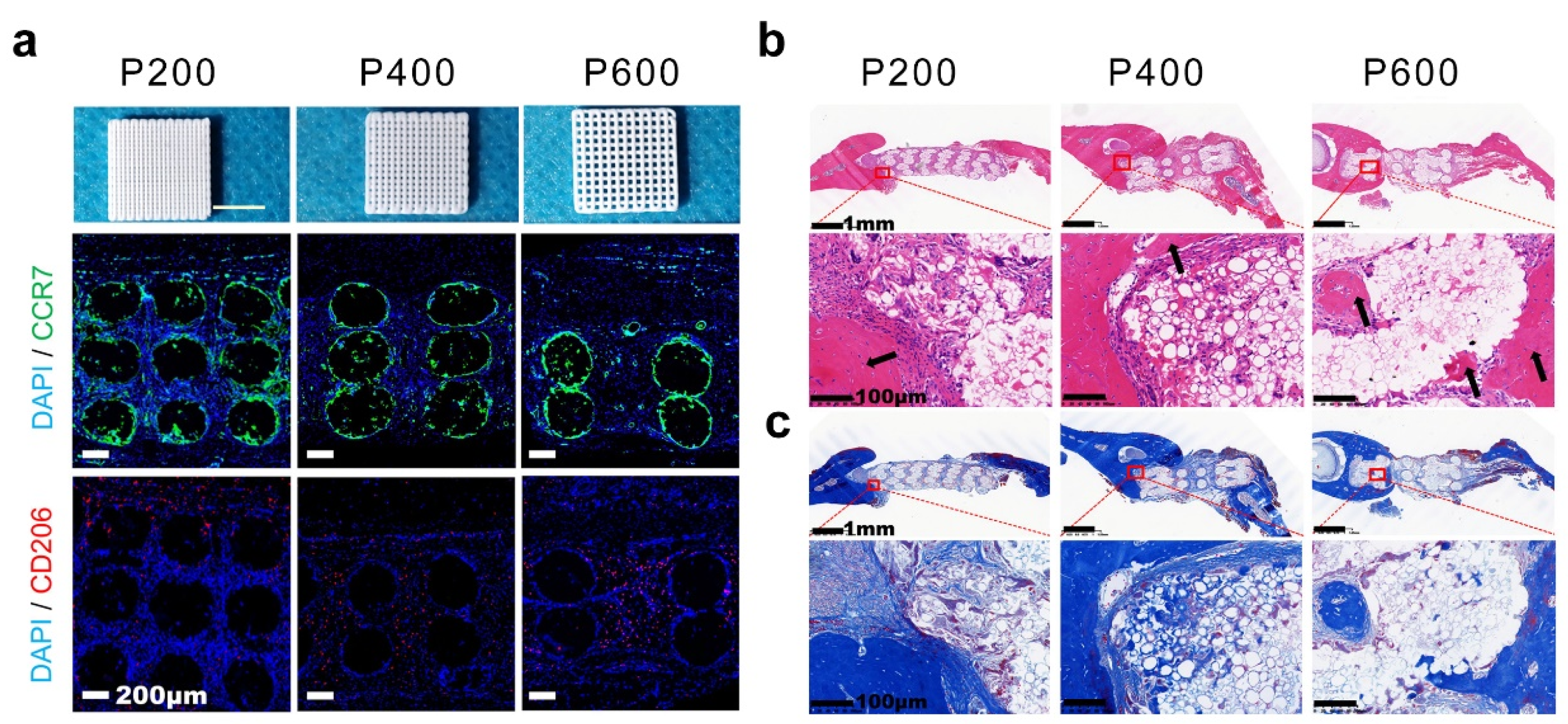
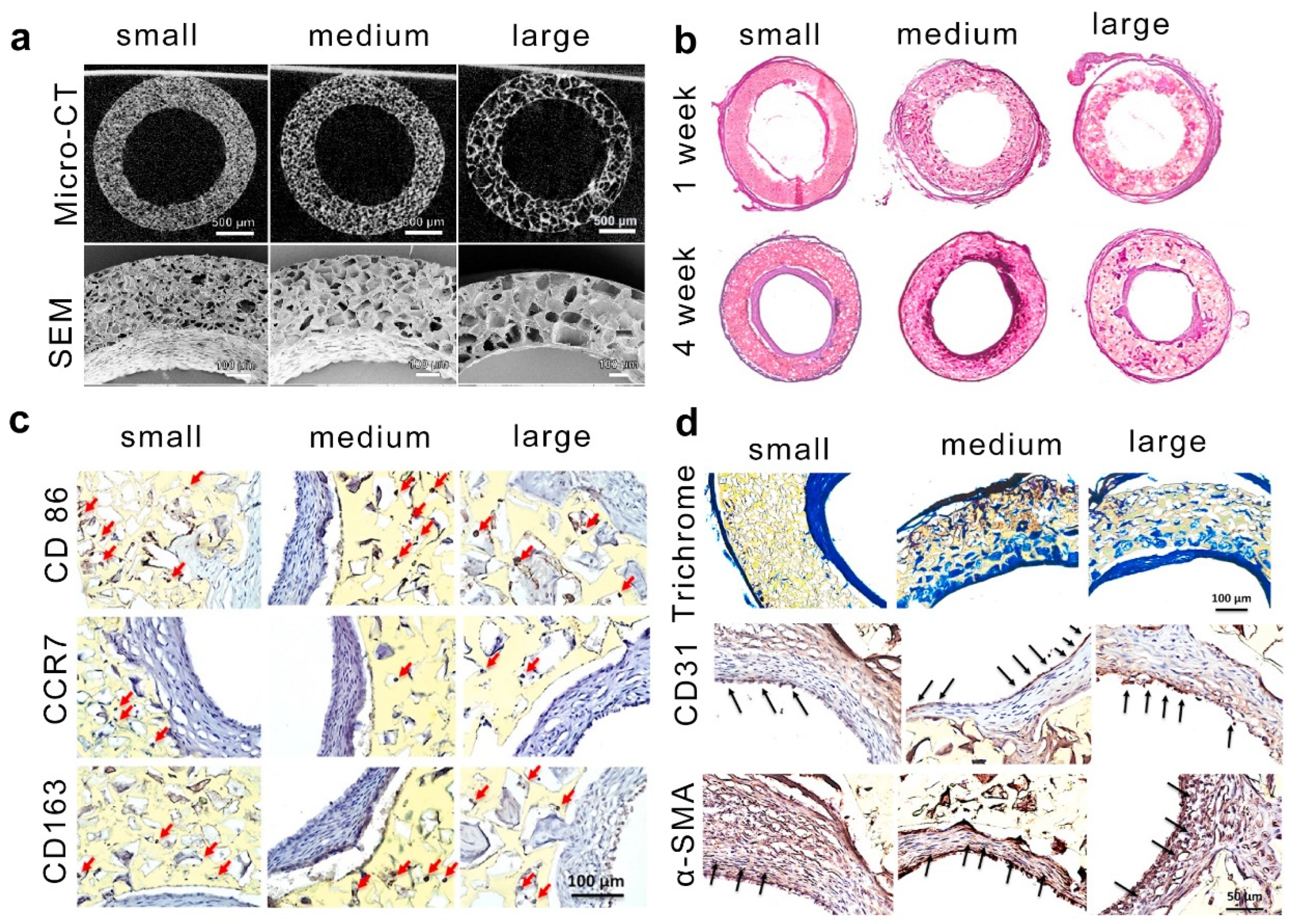
| Strategies | Hydrogel | Pore Size (μm) | Gelation Mechanisms | Cell Type | Cellular Response | Refs. |
|---|---|---|---|---|---|---|
| Ice templating | Gelatin/PA | 26–155 | covalent interactions | Macrophages | Macrophages within smaller and softer pores exhibit proinflammatory phenotype, whereas anti-inflammatory phenotype is induced by larger and stiffer pores. | [33,38,40] |
| Pickering emulsions templating | GelMA | 50–150 | covalent interactions | BMSCs | Macroporous hydrogel speeds up stem cell migration to bone defects, promoting osteogenic differentiation and bone regeneration. | [48,51] |
| Microgel templating | Gelatin/GHS | 10–100 | covalent interactions | Osteoblast-like Saos-2 cells | A higher ratio of microgel-matrix would result in higher metabolic activity and a faster proliferation rate. | [54,57] |
| Phase separation | PEG and high viscous polysaccharides | 0.5–50 | noncovalent interactions | DRGs | The macroporous gels supported axonal growth in a rat sciatic nerve injury model. | [63] |
| Salt templating | HEMA copolymerized with EOEMA | 185–485 | covalent interactions | Osteoblast-like MG63 cells | The growth and survival of MG63 cells are mainly influenced by the higher elasticity of HEMA/EOEMA hydrogels and lack of positive charge, with pore size having a minimal impact. | [66] |
| Gas foaming | Gelatin | 5–30 | covalent interactions | L929 | Macro-porous hydrogel can promote cell vitality and proliferation. | [69] |
| 3D printing technique | GelMA | 800–1200 | covalent interactions | - | - | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zhang, H.; Guo, Y.; Sun, X.; Hu, Z.; Teng, L.; Zeng, Z. Porous Hydrogels for Immunomodulatory Applications. Int. J. Mol. Sci. 2024, 25, 5152. https://doi.org/10.3390/ijms25105152
Wu C, Zhang H, Guo Y, Sun X, Hu Z, Teng L, Zeng Z. Porous Hydrogels for Immunomodulatory Applications. International Journal of Molecular Sciences. 2024; 25(10):5152. https://doi.org/10.3390/ijms25105152
Chicago/Turabian StyleWu, Cuifang, Honghong Zhang, Yangyang Guo, Xiaomin Sun, Zuquan Hu, Lijing Teng, and Zhu Zeng. 2024. "Porous Hydrogels for Immunomodulatory Applications" International Journal of Molecular Sciences 25, no. 10: 5152. https://doi.org/10.3390/ijms25105152
APA StyleWu, C., Zhang, H., Guo, Y., Sun, X., Hu, Z., Teng, L., & Zeng, Z. (2024). Porous Hydrogels for Immunomodulatory Applications. International Journal of Molecular Sciences, 25(10), 5152. https://doi.org/10.3390/ijms25105152








