Synthetic Lethality by Co-Inhibition of Androgen Receptor and Polyadenosine Diphosphate-Ribose in Metastatic Prostate Cancer
Abstract
1. Introduction
2. Homologous Recombination Repair Pathway in Prostate Cancer
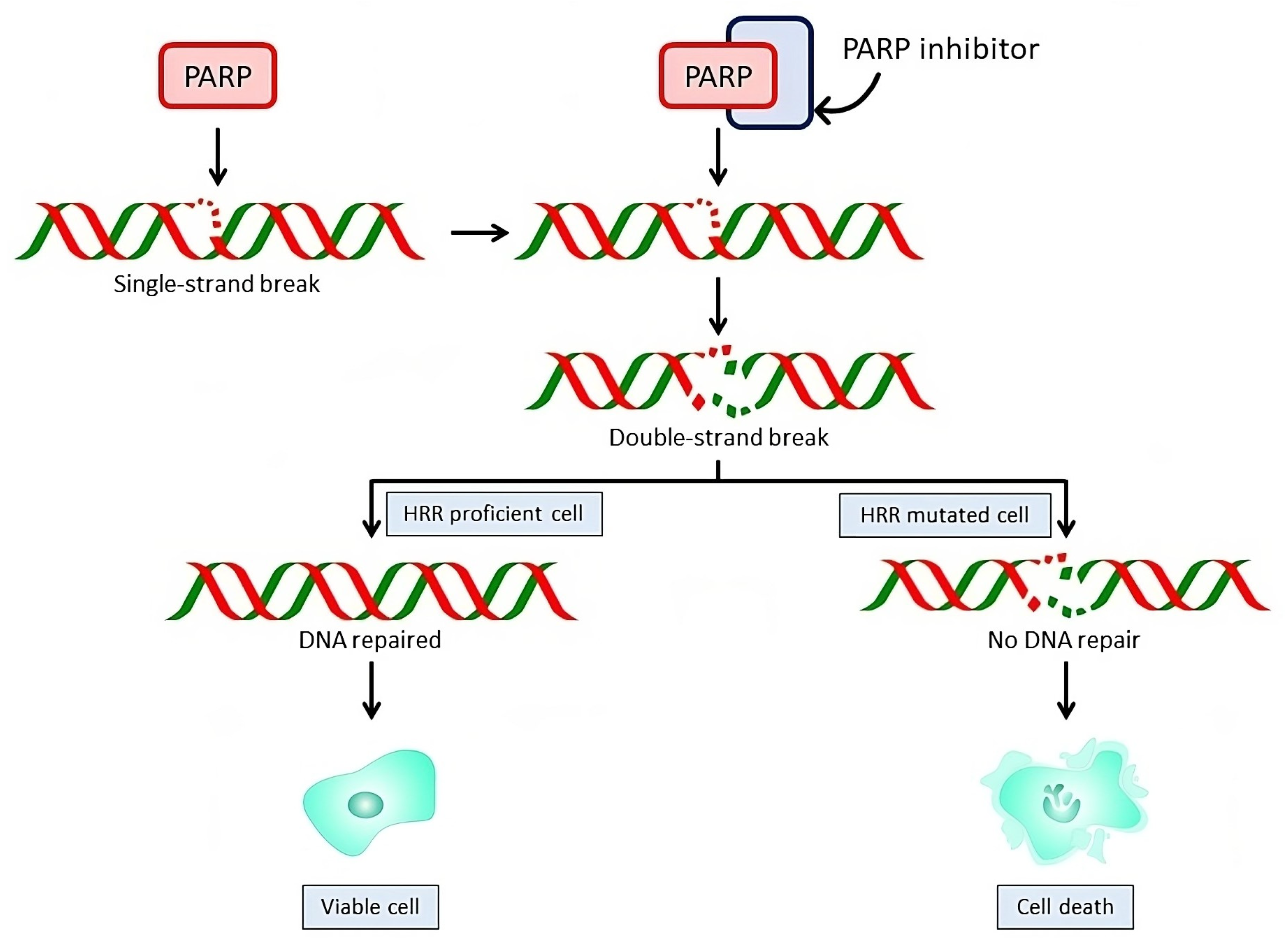
3. Mechanism of Action of PARP Inhibitors
4. PARP Inhibitor Monotherapy in Metastatic Castration-Resistant Prostate Cancer
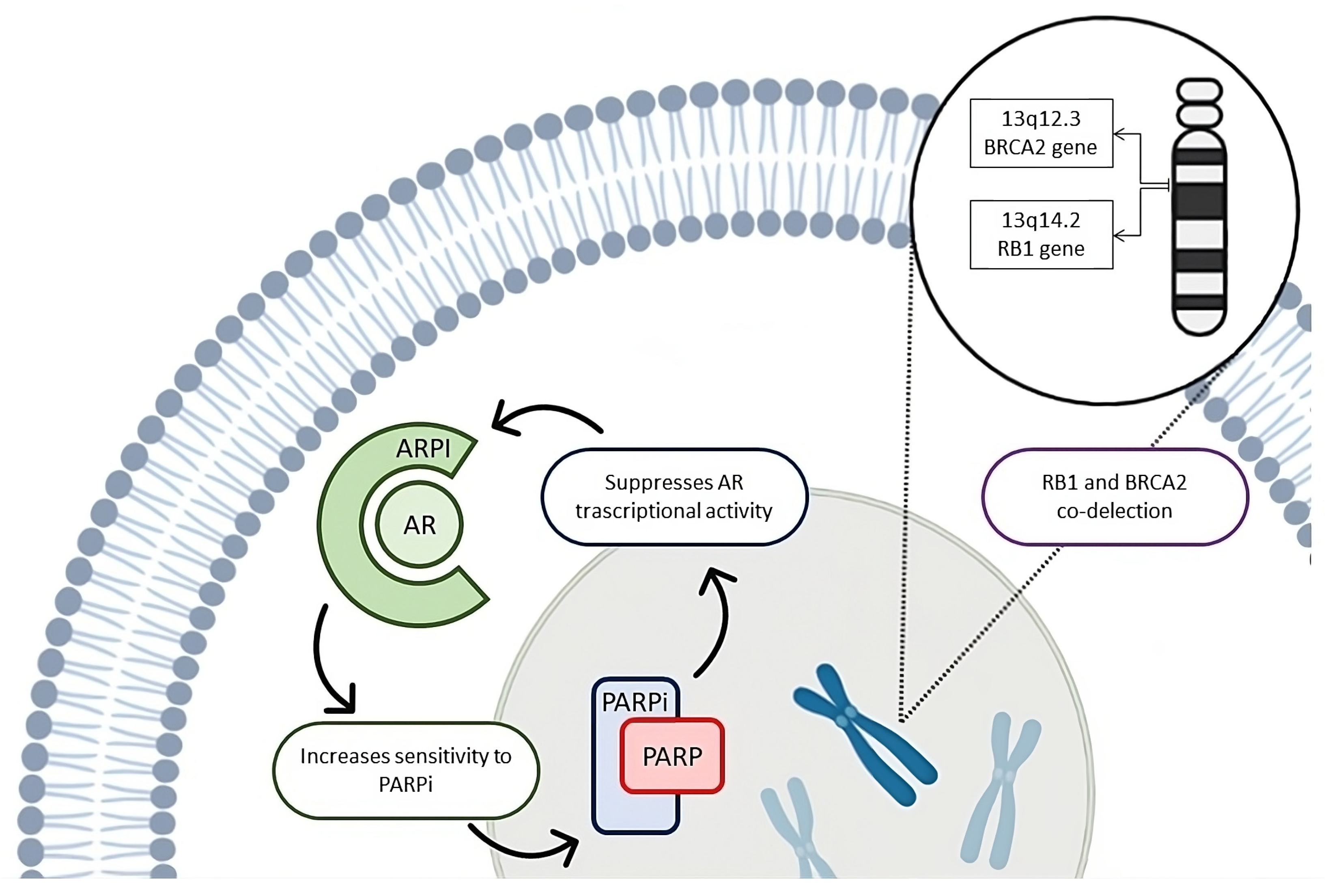
5. Biological Rationale for the ARPI–PARP Inhibitor Combination
6. Clinical Implications of the Co-Inhibition of ARPI and PARP-Inhibitors
7. Future Perspectives and Open Questions
8. Discussion
9. Conclusions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Prostate Cancer Early Detection, Diagnosis, and Staging; American Cancer Society: Hagerstown, MD, USA, 2023. [Google Scholar]
- Yamada, Y.; Beltran, H. The Treatment Landscape of Metastatic Prostate Cancer. Cancer Lett. 2021, 519, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Gravis, G.; Boher, J.-M.; Joly, F.; Soulié, M.; Albiges, L.; Priou, F.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel Versus ADT Alone in Metastatic Non Castrate Prostate Cancer: Impact of Metastatic Burden and Long-Term Survival Analysis of the Randomized Phase 3 GETUG-AFU15 Trial. Eur. Urol. 2016, 70, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of Docetaxel, Zoledronic Acid, or Both to First-Line Long-Term Hormone Therapy in Prostate Cancer (STAMPEDE): Survival Results from an Adaptive, Multiarm, Multistage, Platform Randomised Controlled Trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; De Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira De Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus Prednisone Added to Androgen Deprivation Therapy and Docetaxel in de Novo Metastatic Castration-Sensitive Prostate Cancer (PEACE-1): A Multicentre, Open-Label, Randomised, Phase 3 Study with a 2 × 2 Factorial Design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Verry, C.; Vincendeau, S.; Massetti, M.; Blachier, M.; Vimont, A.; Bazil, M.-L.; Bernardini, P.; Pettré, S.; Timsit, M.-O. Pattern of Clinical Progression Until Metastatic Castration-Resistant Prostate Cancer: An Epidemiological Study from the European Prostate Cancer Registry. Target. Oncol. 2022, 17, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; De Bono, J.S.; Molina, A.; Logothetis, C.J.; De Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Sartor, O.; Michels, R.M.; Massard, C.; De Bono, J.S. Novel Therapeutic Strategies for Metastatic Prostate Cancer in the Post-Docetaxel Setting. Oncologist 2011, 16, 1487–1497. [Google Scholar] [CrossRef][Green Version]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; De Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.A.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus Cabazitaxel or Mitoxantrone for Metastatic Castration-Resistant Prostate Cancer Progressing after Docetaxel Treatment: A Randomised Open-Label Trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; De Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer (TheraP): A Randomised, Open-Label, Phase 2 Trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Nijman, S.M.B. Synthetic Lethality: General Principles, Utility and Detection Using Genetic Screens in Human Cells. FEBS Lett. 2011, 585, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA Repair Defect in BRCA Mutant Cells as a Therapeutic Strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Branco, C.; Paredes, J. Inibidores Da PARP: Do Mecanismo de Ação à Prática Clínica. Acta Médica Port. 2022, 35, 135–143. [Google Scholar] [CrossRef]
- Lynparza (Olaparib), European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lynparza (accessed on 19 December 2023).
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef]
- Polkinghorn, W.R.; Parker, J.S.; Lee, M.X.; Kass, E.M.; Spratt, D.E.; Iaquinta, P.J.; Arora, V.K.; Yen, W.-F.; Cai, L.; Zheng, D.; et al. Androgen Receptor Signaling Regulates DNA Repair in Prostate Cancers. Cancer Discov. 2013, 3, 1245–1253. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Alhmoud, J.F.; Woolley, J.F.; Al Moustafa, A.-E.; Malki, M.I. DNA Damage/Repair Management in Cancers. Cancers 2020, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.; Klein, H. Mechanism of Homologous Recombination: Mediators and Helicases Take on Regulatory Functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Moka, N.; Hamstra, D.A.; Ryan, C.J. Co-Inhibition of Androgen Receptor and PARP as a Novel Treatment Paradigm in Prostate Cancer—Where Are We Now? Cancers 2022, 14, 801. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J. DNA Repair Dysregulation from Cancer Driver to Therapeutic Target. Nat. Rev. Cancer 2012, 12, 801–817. [Google Scholar] [CrossRef]
- Cresta Morgado, P.; Mateo, J. Clinical Implications of Homologous Recombination Repair Mutations in Prostate Cancer. Prostate 2022, 82, S45–S59. [Google Scholar] [CrossRef]
- Jiang, M.; Jia, K.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; He, Y.; Zhou, C. Alterations of DNA Damage Response Pathway: Biomarker and Therapeutic Strategy for Cancer Immunotherapy. Acta Pharm. Sin. B 2021, 11, 2983–2994. [Google Scholar] [CrossRef]
- PCF/SU2C International Prostate Cancer Dream Team; Armenia, J.; Wankowicz, S.A.M.; Liu, D.; Gao, J.; Kundra, R.; Reznik, E.; Chatila, W.K.; Chakravarty, D.; Han, G.C.; et al. The Long Tail of Oncogenic Drivers in Prostate Cancer. Nat. Genet. 2018, 50, 645–651. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Mateo, J.; Seed, G.; Bertan, C.; Rescigno, P.; Dolling, D.; Figueiredo, I.; Miranda, S.; Nava Rodrigues, D.; Gurel, B.; Clarke, M.; et al. Genomics of Lethal Prostate Cancer at Diagnosis and Castration Resistance. J. Clin. Investig. 2020, 130, 1743–1751. [Google Scholar] [CrossRef]
- Warner, E.; Herberts, C.; Fu, S.; Yip, S.; Wong, A.; Wang, G.; Ritch, E.; Murtha, A.J.; Vandekerkhove, G.; Fonseca, N.M.; et al. BRCA2, ATM, and CDK12 Defects Differentially Shape Prostate Tumor Driver Genomics and Clinical Aggression. Clin. Cancer Res. 2021, 27, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Sivakumar, S.; Tukachinsky, H.; Coleman, I.; De Sarkar, N.; Yu, E.Y.; Konnick, E.Q.; Nelson, P.S.; Pritchard, C.C.; Montgomery, B. Concordance of DNA Repair Gene Mutations in Paired Primary Prostate Cancer Samples and Metastatic Tissue or Cell-Free DNA. JAMA Oncol. 2021, 7, 1378. [Google Scholar] [CrossRef] [PubMed]
- Kuzbari, Z.; Bandlamudi, C.; Loveday, C.; Garrett, A.; Mehine, M.; George, A.; Hanson, H.; Snape, K.; Kulkarni, A.; Allen, S.; et al. Germline-Focused Analysis of Tumour-Detected Variants in 49,264 Cancer Patients: ESMO Precision Medicine Working Group Recommendations. Ann. Oncol. 2023, 34, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.; Breen, K.; Nandakumar, S.; Sjoberg, D.D.; Kemel, Y.; Mehta, N.; Lenis, A.T.; Reisz, P.A.; Carruthers, J.; Benfante, N.; et al. Gene-Based Confirmatory Germline Testing Following Tumor-Only Sequencing of Prostate Cancer. Eur. Urol. 2023, 83, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Romero-Laorden, N.; Del Pozo, A.; Lozano, R.; Medina, A.; Puente, J.; Piulats, J.M.; Lorente, D.; Saez, M.I.; Morales-Barrera, R.; et al. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019, 37, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Goh, C.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Dadaev, T.; Govindasami, K.; Guy, M.; Ellis, S.; Frost, D.; et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-Specific Survival After Radical Treatment for Localised Prostate Cancer. Eur. Urol. 2015, 68, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Annala, M.; Struss, W.J.; Warner, E.W.; Beja, K.; Vandekerkhove, G.; Wong, A.; Khalaf, D.; Seppälä, I.-L.; So, A.; Lo, G.; et al. Treatment Outcomes and Tumor Loss of Heterozygosity in Germline DNA Repair–Deficient Prostate Cancer. Eur. Urol. 2017, 72, 34–42. [Google Scholar] [CrossRef]
- Carter, H.B.; Helfand, B.; Mamawala, M.; Wu, Y.; Landis, P.; Yu, H.; Wiley, K.; Na, R.; Shi, Z.; Petkewicz, J.; et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. Eur. Urol. 2019, 75, 743–749. [Google Scholar] [CrossRef]
- Olmos, D.; Lorente, D.; Alameda, D.; Cattrini, C.; Romero-Laorden, N.; Lozano, R.; Lopez-Casas, P.P.; Capone, C.; Vanden Broecke, A.M.; Trevisan, M.; et al. Presence of Somatic/Germline Homologous Recombination Repair (HRR) Mutations and Outcomes in Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients (Pts) Receiving First-Line (1L) Treatment Stratified by BRCA Status. J. Clin. Oncol. 2023, 41 (Suppl. S16), 5003. [Google Scholar] [CrossRef]
- Cheng, H.H.; Pritchard, C.C.; Boyd, T.; Nelson, P.S.; Montgomery, B. Biallelic Inactivation of BRCA2 in Platinum-Sensitive Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2016, 69, 992–995. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Mayer, E.L.; He, L.; Traina, T.A.; Carey, L.A.; Krag, K.J.; Rugo, H.S.; Liu, M.C.; Stearns, V.; Come, S.E.; et al. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy with Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2015, 33, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Jubin, T.; Kadam, A.; Jariwala, M.; Bhatt, S.; Sutariya, S.; Gani, A.R.; Gautam, S.; Begum, R. The PARP Family: Insights into Functional Aspects of Poly (ADP-Ribose) Polymerase-1 in Cell Growth and Survival. Cell Prolif. 2016, 49, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.A.; Kraus, W.L. New Insights into the Molecular and Cellular Functions of Poly(ADP-Ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 2012, 13, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Geenen, J.J.J.; Linn, S.C.; Beijnen, J.H.; Schellens, J.H.M. PARP Inhibitors in the Treatment of Triple-Negative Breast Cancer. Clin. Pharmacokinet. 2018, 57, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Huang, S.N.; Das, B.B.; Renaud, A.; Zhang, Y.; Doroshow, J.H.; Ji, J.; Takeda, S.; Pommier, Y. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012, 72, 5588–5599. [Google Scholar] [CrossRef]
- Mateo, J.; Lord, C.J.; Serra, V.; Tutt, A.; Balmaña, J.; Castroviejo-Bermejo, M.; Cruz, C.; Oaknin, A.; Kaye, S.B.; De Bono, J.S. A Decade of Clinical Development of PARP Inhibitors in Perspective. Ann. Oncol. 2019, 30, 1437–1447. [Google Scholar] [CrossRef]
- Rudolph, J.; Jung, K.; Luger, K. Inhibitors of PARP: Number Crunching and Structure Gazing. Proc. Natl. Acad. Sci. USA 2022, 119, e2121979119. [Google Scholar] [CrossRef]
- Marković, J.; Grdović, N.; Dinić, S.; Karan-Djurašević, T.; Uskoković, A.; Arambašić, J.; Mihailović, M.; Pavlović, S.; Poznanović, G.; Vidaković, M. PARP-1 and YY1 Are Important Novel Regulators of CXCL12 Gene Transcription in Rat Pancreatic Beta Cells. PLoS ONE 2013, 8, e59679. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Q.; Ling, X.; Tan, Q.; Liang, H.; Chen, J.; Lin, L.; Xiao, Y.; Chen, W.; Liu, L.; et al. PARP-1 May Be Involved in Hydroquinone-Induced Apoptosis by Poly ADP-Ribosylation of ZO-2. Mol. Med. Rep. 2017, 16, 8076–8084. [Google Scholar] [CrossRef]
- Unlu, S.; Kim, J.W. Emerging Role of PARP Inhibitors in Metastatic Prostate Cancer. Curr. Oncol. Rep. 2022, 24, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Campbell, D.; Patnaik, A.; Shapiro, J.D.; Sautois, B.; Vogelzang, N.J.; Voog, E.G.; Bryce, A.H.; McDermott, R.; Ricci, F.; et al. Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin. Cancer Res. 2020, 26, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U.; et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in Patients with Metastatic Castration-Resistant Prostate Cancer with DNA Repair Gene Aberrations (TOPARP-B): A Multicentre, Open-Label, Randomised, Phase 2 Trial. Lancet Oncol. 2020, 21, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 383, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Mehra, N.; Scagliotti, G.V.; Castro, E.; Dorff, T.; Stirling, A.; Stenzl, A.; Fleming, M.T.; Higano, C.S.; Saad, F.; et al. Talazoparib Monotherapy in Metastatic Castration-Resistant Prostate Cancer with DNA Repair Alterations (TALAPRO-1): An Open-Label, Phase 2 Trial. Lancet Oncol. 2021, 22, 1250–1264. [Google Scholar] [CrossRef]
- Smith, M.R.; Scher, H.I.; Sandhu, S.; Efstathiou, E.; Lara, P.N.; Yu, E.Y.; George, D.J.; Chi, K.N.; Saad, F.; Ståhl, O.; et al. Niraparib in Patients with Metastatic Castration-Resistant Prostate Cancer and DNA Repair Gene Defects (GALAHAD): A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2022, 23, 362–373. [Google Scholar] [CrossRef]
- Li, L.; Karanika, S.; Yang, G.; Wang, J.; Park, S.; Broom, B.M.; Manyam, G.C.; Wu, W.; Luo, Y.; Basourakos, S.; et al. Androgen Receptor Inhibitor–Induced “BRCAness” and PARP Inhibition Are Synthetically Lethal for Castration-Resistant Prostate Cancer. Sci. Signal. 2017, 10, eaam7479. [Google Scholar] [CrossRef]
- Asim, M.; Tarish, F.; Zecchini, H.I.; Sanjiv, K.; Gelali, E.; Massie, C.E.; Baridi, A.; Warren, A.Y.; Zhao, W.; Ogris, C.; et al. Synthetic Lethality between Androgen Receptor Signalling and the PARP Pathway in Prostate Cancer. Nat. Commun. 2017, 8, 374. [Google Scholar] [CrossRef]
- Bolla, M.; Gonzalez, D.; Warde, P.; Dubois, J.B.; Mirimanoff, R.-O.; Storme, G.; Bernier, J.; Kuten, A.; Sternberg, C.; Gil, T.; et al. Improved Survival in Patients with Locally Advanced Prostate Cancer Treated with Radiotherapy and Goserelin. N. Engl. J. Med. 1997, 337, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; Collette, L.; Blank, L.; Warde, P.; Dubois, J.B.; Mirimanoff, R.-O.; Storme, G.; Bernier, J.; Kuten, A.; Sternberg, C.; et al. Long-Term Results with Immediate Androgen Suppression and External Irradiation in Patients with Locally Advanced Prostate Cancer (an EORTC Study): A Phase III Randomised Trial. Lancet 2002, 360, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; De Reijke, T.M.; Van Tienhoven, G.; Van Den Bergh, A.C.M.; Oddens, J.; Poortmans, P.M.P.; Gez, E.; Kil, P.; Akdas, A.; Soete, G.; et al. Duration of Androgen Suppression in the Treatment of Prostate Cancer. N. Engl. J. Med. 2009, 360, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Zietman, A.L. Why Does Androgen Deprivation Enhance the Results of Radiation Therapy? Urol. Oncol. Semin. Orig. Investig. 2008, 26, 522–529. [Google Scholar] [CrossRef]
- Goodwin, J.F.; Schiewer, M.J.; Dean, J.L.; Schrecengost, R.S.; De Leeuw, R.; Han, S.; Ma, T.; Den, R.B.; Dicker, A.P.; Feng, F.Y.; et al. A Hormone–DNA Repair Circuit Governs the Response to Genotoxic Insult. Cancer Discov. 2013, 3, 1254–1271. [Google Scholar] [CrossRef]
- Tarish, F.L.; Schultz, N.; Tanoglidi, A.; Hamberg, H.; Letocha, H.; Karaszi, K.; Hamdy, F.C.; Granfors, T.; Helleday, T. Castration Radiosensitizes Prostate Cancer Tissue by Impairing DNA Double-Strand Break Repair. Sci. Transl. Med. 2015, 7, 312re11. [Google Scholar] [CrossRef]
- Kounatidou, E.; Nakjang, S.; McCracken, S.R.C.; Dehm, S.M.; Robson, C.N.; Jones, D.; Gaughan, L. A Novel CRISPR-Engineered Prostate Cancer Cell Line Defines the AR-V Transcriptome and Identifies PARP Inhibitor Sensitivities. Nucleic Acids Res. 2019, 47, 5634–5647. [Google Scholar] [CrossRef]
- Schiewer, M.J.; Goodwin, J.F.; Han, S.; Brenner, J.C.; Augello, M.A.; Dean, J.L.; Liu, F.; Planck, J.L.; Ravindranathan, P.; Chinnaiyan, A.M.; et al. Dual Roles of PARP-1 Promote Cancer Growth and Progression. Cancer Discov. 2012, 2, 1134–1149. [Google Scholar] [CrossRef]
- Yin, Y.; Li, R.; Xu, K.; Ding, S.; Li, J.; Baek, G.; Ramanand, S.G.; Ding, S.; Liu, Z.; Gao, Y.; et al. Androgen Receptor Variants Mediate DNA Repair after Prostate Cancer Irradiation. Cancer Res. 2017, 77, 4745–4754. [Google Scholar] [CrossRef]
- Chakraborty, G.; Armenia, J.; Mazzu, Y.Z.; Nandakumar, S.; Stopsack, K.H.; Atiq, M.O.; Komura, K.; Jehane, L.; Hirani, R.; Chadalavada, K.; et al. Significance of BRCA2 and RB1 Co-Loss in Aggressive Prostate Cancer Progression. Clin. Cancer Res. 2020, 26, 2047–2064. [Google Scholar] [CrossRef]
- Abida, W.; Cyrta, J.; Heller, G.; Prandi, D.; Armenia, J.; Coleman, I.; Cieslik, M.; Benelli, M.; Robinson, D.; Van Allen, E.M.; et al. Genomic Correlates of Clinical Outcome in Advanced Prostate Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11428–11436. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Castro, E.; Lopez-Campos, F.; Thorne, H.; Ramirez-Backhaus, M.; Aragon, I.M.; Cendón-Florez, Y.; Gutierrez-Pecharroman, A.; Salles, D.C.; Romero-Laorden, N.; et al. Impact of Concurrent Tumour Events on the Prostate Cancer Outcomes of Germline BRCA2 Mutation Carriers. Eur. J. Cancer 2023, 185, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Daignault-Newton, S.; Twardowski, P.W.; Albany, C.; Stein, M.N.; Kunju, L.P.; Siddiqui, J.; Wu, Y.-M.; Robinson, D.; Lonigro, R.J.; et al. Targeting Androgen Receptor and DNA Repair in Metastatic Castration-Resistant Prostate Cancer: Results from NCI 9012. J. Clin. Oncol. 2018, 36, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Fléchon, A.; Redfern, C.; et al. Olaparib Combined with Abiraterone in Patients with Metastatic Castration-Resistant Prostate Cancer: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Oncol. 2018, 19, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.H.; Adelman, C.; Barnicle, A.; Kozarewa, I.; Luke, S.; Lai, Z.; Hollis, S.; Dougherty, B.; Harrington, E.A.; Kang, J.; et al. Homologous Recombination Repair Gene Mutation Characterization by Liquid Biopsy: A Phase II Trial of Olaparib and Abiraterone in Metastatic Castrate-Resistant Prostate Cancer. Cancers 2021, 13, 5830. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.; Loredo, E.; Procopio, G.; De Menezes, J.; Girotto, G.; Arslan, C.; et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid. 2022, 1, EVIDoa2200043. [Google Scholar] [CrossRef]
- Saad, F.; Clarke, N.W.; Oya, M.; Shore, N.; Procopio, G.; Guedes, J.D.; Arslan, C.; Mehra, N.; Parnis, F.; Brown, E.; et al. Olaparib plus Abiraterone versus Placebo plus Abiraterone in Metastatic Castration-Resistant Prostate Cancer (PROpel): Final Prespecified Overall Survival Results of a Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2023, 24, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.D.; Procopio, G.; Guedes, J.D.C.; Arslan, C.; Mehra, N.; Parnis, F.; et al. Final Overall Survival (OS) in PROpel: Abiraterone (Abi) and Olaparib (Ola) versus Abiraterone and Placebo (Pbo) as First-Line (1L) Therapy for Metastatic Castration-Resistant Prostate Cancer (mCRPC). J. Clin. Oncol. 2023, 41 (Suppl. 6), LBA16. [Google Scholar] [CrossRef]
- Chi, K.N.; Sandhu, S.; Smith, M.R.; Attard, G.; Saad, M.; Olmos, D.; Castro, E.; Roubaud, G.; Pereira De Santana Gomes, A.J.; Small, E.J.; et al. Niraparib plus Abiraterone Acetate with Prednisone in Patients with Metastatic Castration-Resistant Prostate Cancer and Homologous Recombination Repair Gene Alterations: Second Interim Analysis of the Randomized Phase III MAGNITUDE Trial. Ann. Oncol. 2023, 34, 772–782. [Google Scholar] [CrossRef]
- Chi, K.N.; Rathkopf, D.; Smith, M.R.; Efstathiou, E.; Attard, G.; Olmos, D.; Lee, J.Y.; Small, E.J.; Pereira De Santana Gomes, A.J.; Roubaud, G.; et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2023, 18, 3339–3351. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; De Giorgi, U.; Young Joung, J.; Fong, P.C.C.; et al. Talazoparib plus Enzalutamide in Men with First-Line Metastatic Castration-Resistant Prostate Cancer (TALAPRO-2): A Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2023, 402, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Azad, A.; Shore, N.D.; Carles, J.; Fay, A.P.; Dunshee, C.; Karsh, L.I.; Paccagnella, M.L.; Santo, N.D.; Elmeliegy, M.; et al. Talazoparib plus Enzalutamide in Metastatic Castration-Resistant Prostate Cancer: TALAPRO-2 Phase III Study Design. Future Oncol. 2022, 18, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.H.A.; Kocherginsky, M.; Agarwal, N.; Zhang, J.; Adra, N.; Paller, C.J.; Picus, J.; Reichert, Z.R.; Szmulewitz, R.Z.; Tagawa, S.T.; et al. BRCAAWAY: A Randomized Phase 2 Trial of Abiraterone, Olaparib, or Abiraterone + Olaparib in Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) with DNA Repair Defects. J. Clin. Oncol. 2022, 40 (Suppl. S16), 5018. [Google Scholar] [CrossRef]
- Messina, C.; Giunta, E.F.; Signori, A.; Rebuzzi, S.E.; Banna, G.L.; Maniam, A.; Buti, S.; Cattrini, C.; Fornarini, G.; Bauckneht, M.; et al. Combining PARP Inhibitors and Androgen Receptor Signalling Inhibitors in Metastatic Prostate Cancer: A Quantitative Synthesis and Meta-Analysis. Eur. Urol. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Peyraud, F.; Italiano, A. Combined PARP Inhibition and Immune Checkpoint Therapy in Solid Tumors. Cancers 2020, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Wu, Z.; Ding, W.; Gao, S.; Gao, Y.; Xu, C. Mechanisms of Enzalutamide Resistance in Castration-resistant Prostate Cancer and Therapeutic Strategies to Overcome It. Br. J. Pharmacol. 2021, 178, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Y.; Xiang, S.; Chan, F.L. Towards Understanding Androgen Receptor-Independent Prostate Cancer: An Evolving Paradigm. Transl. Cancer Res. 2020, 9, 415–417. [Google Scholar] [CrossRef]
- Scott, R.J.; Mehta, A.; Macedo, G.S.; Borisov, P.S.; Kanesvaran, R.; El Metnawy, W. Genetic Testing for Homologous Recombination Repair (HRR) in Metastatic Castration-Resistant Prostate Cancer (mCRPC): Challenges and Solutions. Oncotarget 2021, 12, 1600–1614. [Google Scholar] [CrossRef]
- Fu, X.; Li, P.; Zhou, Q.; He, R.; Wang, G.; Zhu, S.; Bagheri, A.; Kupfer, G.; Pei, H.; Li, J. Mechanism of PARP Inhibitor Resistance and Potential Overcoming Strategies. Genes Dis. 2024, 11, 306–320. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Nindra, U.; Hong, J.H.; Balakrishnar, B.; Pal, A.; Chua, W. Review of Toxicities of PARP Inhibitors in Metastatic Castrate Resistant Prostate Cancer. Clin. Genitourin. Cancer 2023, 21, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.-J.; Wienke, A.; Surov, A. Incidental Pulmonary Embolism in Oncologic Patients—A Systematic Review and Meta-Analysis. Support. Care Cancer 2021, 29, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- LaFargue, C.J.; Dal Molin, G.Z.; Sood, A.K.; Coleman, R.L. Exploring and Comparing Adverse Events between PARP Inhibitors. Lancet Oncol. 2019, 20, e15–e28. [Google Scholar] [CrossRef] [PubMed]
- Beije, N.; Abida, W.; Antonarakis, E.S.; Castro, E.; De Wit, R.; Fizazi, K.; Gillessen, S.; Hussain, M.; Mateo, J.; Morris, M.J.; et al. PARP Inhibitors for Prostate Cancer: Tangled up in PROfound and PROpel (and TALAPRO-2) Blues. Eur. Urol. 2023, 84, 253–256. [Google Scholar] [CrossRef]
| Study | Phase | Patients | Treatment | Response Rate | PFS | OS |
|---|---|---|---|---|---|---|
| TOPARP A (NCT01682772) | 2 | mCRPC with prior chemotherapy unselected for DDR gene aberrations (n = 50), including biomarker negative (n = 33) and biomarker positive (n = 16) | Single arm: olaparib 400 mg BID | 33% in all evaluable patients 88% in biomarker positive vs. 6% in biomarker negative patients. | 9.8 months in biomarker positive vs. 2.7 months in biomarker negative (HR 0.24; 95% CI 0.11–0.50; p < 0.001) | 13.8 months in biomarker positive vs. 7.5 months in biomarker negative (HR 0.47; 95% CI 0.22–1.021; p = 0.05) |
| TOPARP B (NCT01682772) | 2 | mCRPC with prior chemotherapy selected for DDR gene aberrations (n = 98) | Olaparib 400 mg BID (n = 49) vs. Olaparib 300 mg BID (n = 49) | 400 mg BID cohort: 54.3% 300 mg BID cohort: 39.1% | 400 mg BID cohort: 5.5 months (95% CI 4.4–8.3) 300 mg BID cohort: 5.6 months (95% CI 3.7–7.7) | 400 mg BID cohort: 14.3 months (95% CI 9.7–18.9) 300 mg BID cohort: 10.1 months (95% CI 9.0–17.7) |
| PROfound (NCT02987543) | 3 | mCRPC with prior NHA, chemotherapy-naïve, selected for DDR gene aberrations (n = 387) Cohort A (n = 245): at least one alteration in BRCA1, BRCA2, or ATM Cohort B (n = 142): alterations in any of the other DDR genes | Experimental arm: olaparib 300 mg BID Control arm: physician choice ARPI (enza 160 mg/day or AA 1000 mg/day) Cohort A: Olaparib (n = 162) vs. physician choice ARPI (n = 83) Cohort A+B: Olaparib (n = 256) vs. physician choice ARPI (131) | Cohort A: 33% in experimental arm vs. 2% in control arm Cohort A+B: 22% in experimental arm vs. 4% in control arm | Cohort A: 7.4 vs. 3.6 months (HR 0.34; 95% CI 0.25–0.47; p < 0.001) Cohort A+B: 5.8 vs. 3.5 months (HR 0.49; 95% CI 0.38–0.63; p < 0.001) | Cohort A: 19.1 vs. 14.7 months (HR 0.69; 95% CI 0.50–0.97; p = 0.02) Cohort A+B: 17.3 vs. 14.0, (HR 0.55; 95% CI 0.29–1.06, crossover adjusted analysis) |
| TRITON2 (NCT02952534) | 2 | mCRPC with prior NHA and chemotherapy, selected for DDR gene alterations (n = 203) BRCA1/2 subgroup: at least one germinal or somatic aberration in BRCA1 or BRCA2 (n = 115) | Single arm: rucaparib 600 mg BID | 43.5% in BRCA1/2 subgroup 13.5% in other DDR gene alterations | 9.0 months (95% CI 8.3–13.5 months) in BRCA1/2 subgroup | 12-month OS 73% (95% CI 62.9%–80.7%, maturity 41%) in BRCA1/2 subgroup |
| TRITON3 * (NCT02975934) | 3 | mCRPC with prior NHA, chemotherapy-naïve, selected for mutations in any of BRCA1/2 or ATM genes (n = 405) BRCA1/2 subgroup: at least one germinal or somatic aberration in BRCA1 or BRCA2 (n = 302) | Experimental arm: rucaparib 600 mg BID (n = 270) Control arm: physician choice of ARPI (n = 60) or Docetaxel (75) | - | ITT population: 10.2 vs. 6.4 months (HR 0.61, 95% CI 0.47–0.80, p = 0.0003) BRCA1/2 subgroup: 11.2 vs. 6.4 months (HR 0.50, 95% CI 0.36–0.69; p < 0.0001) | ITT population: 23.6 months vs. 20.9 months (HR 0.94, 95% CI 0.72–1.23, p = 0.67, maturity 59%) BRCA1/2 subgroup: 24.3 months vs. 20.8 months (HR 0.81, 95% CI 0.58–1.12; p = 0.21, maturity 54%) |
| TALAPRO 1 (NCT03148795) | 2 | mCRPC with prior NHA and chemotherapy, selected for DDR gene aberrations (n = 128) BRCA1/2 subgroup: at least one germinal or somatic aberration in BRCA1 or BRCA2 (n = 61) | Single arm: talazoparib 1 mg/day | 29.8% in all evaluable patients 45.9% in BRCA1/2 subgroup | 5.6 months (95% CI 3.7–8.8) BRCA1/2 subgroup: 11.2 months (95% CI 7.5–19.2) | 16.4 months (95% CI 12.2–19.9) |
| GALAHAD (NCT02854436) | 2 | mCRPC with prior NHA and chemotherapy, selected for DDR gene aberrations (n = 223) BRCA cohort: germline pathogenic or biallelic pathogenic alterations in BRCA1 or BRCA2 (n = 142), including measurable (n = 76) and non-measurable disease (n = 66) Non-BRCA cohort: alterations in any of the other DDR genes (n = 81), including measurable (n = 47) and non-measurable disease (n = 34) | Single arm: niraparib 300 mg/day | 34.2% in measurable BRCA cohort 10.6% in measurable non-BRCA cohort | BRCA cohort: 8.08 months (95% CI 5.55–8.38) Measurable BRCA cohort: 5.52 months (95% CI 5.29–7.59) Non-BRCA cohort: 3.71 months (95% CI 1.97–5.49) | BRCA cohort: 13.01 months (95% CI 11.04–14.29) Measurable BRCA cohort: 10.87 months (95% CI 9.49–13.77) Non-BRCA cohort: 9.63 months (95% CI 8.05–13.44) |
| Study | Phase | Patients | Treatment | Response Rate | PFS | OS |
|---|---|---|---|---|---|---|
| PROpel (NCT03732820) | 3 | mCRPC. Chemotherapy and ARPI allowed in mHSPC setting. No AA. Other NHAs allowed if stopped >12 months prior to enrollment. Patients unselected for DDR mutation (n = 796) AA + olaparib (n = 399) vs. AA + pbo (n = 397) | 1:1 randomisation. Experimental arm (AA + olaparib): AA 1000 mg/day + prednisone 5 mg BID + olaparib 300 mg BID. Control arm (AA + pbo): AA 1000 mg/day + prednisone 5 mg BID + pbo. | 40.3% AA + olaparib: ORR 58.4% (94/161) AA + pbo: ORR 48.1% (77/160) (OR 1.60; 95% CI, 1.02–2.53) | rPFS AA + olaparib vs. AA + pbo. ITT population: 24.8 vs. 16.6 months HR 0.66 (95% CI 0.54–0.81) p < 0.0001 mHRR: NR vs. 13.9 months, HR 0.5 (95% CI 0.34–0.73) nmHRR: 24.1 vs. 19 months, HR 0.76 (95% CI 0.6–0.97) mBRCA: NR vs. 8.4 months, HR 0.23 (95% CI 0.12–0.43) | 47.9% maturity OS 42.1 vs. 34.7 months, HR 0.81 (95% CI 0.67–1); p = 0.0544 mHRR: NR vs. 28.5 months, HR 0.66 (95% CI 0.45–0.95) nmHRR: 42.1 vs. 38.9 months, HR 0.89 (95% CI 0.7–1.14) mBRCA: NR vs. 23 months, HR 0.29 (95% CI 0.14–0.56) |
| MAGNITUDE (NCT03748641) | 3 | mCRPC. Docetaxel and AA allowed in mHSPC. AA in m0CRPC allowed if given <4 months. Patients unselected for DDR mutation (n = 946) Cohort 1 (mHRR cohort, n = 423): niraparib + AA (n= 212) vs. AA + pbo (n = 211) Cohort 2 (nmHRR cohort, n = 247): niraparib + AA (n= 117) vs. AA + pbo (n = 116), closed for futility | 1:1 randomisation. Experimental arm (niraparib + AA): niraparib 200 mg/daily + AA 1000 mg/day + prednisone 5 mg BID Control arm (pbo + AA): AA 1000 mg/day plus prednisone 5 mg BID + pbo | mHRR: niraparib + AA ORR 59.7% (55/92) vs. pbo + AA ORR 28% (28/82). RR 2.13, p < 0.001 BRCA1/2: niraparib + AA ORR 51.8% (29/56) vs. pbo + AA ORR 31.3% (15/48). RR 1.66, p = 0.04 | mHRRm: 16.7 vs. 13.7 months HR 0.76 (95% CI 0.60–0.97); p= 0.0280 mBRCA: 19.5 vs. 10.9 months HR 0.55 (95% CI 0.39–0.78); p = 0.0007 nmHRRm HR 1.09; (95% CI 0.75 to 1.57); p = 5.66 | mHRR: HR 1.01 (95% CI 0.75–1.36); p = 0.948 mBRCA: 0.88 (0.58, 1.34) p = 0.5505 |
| TALAPRO-2 (NCT03395197) | 3 | mCRPC. Docetaxel and AA allowed in mHSPC. Patients selected for DDR mutation (n = 805) Talazoparib + enza (n = 402) vs. pbo + enza (n = 403) | 1:1 randomisation. Experimental arm (talalazoparib + enza): talazoparib 0.5 mg/daily + enza 160 mg/daily Control arm (pbo + enza): enza 160 mg/daily + pbo | Talazoparib + enza: ORR 62% (74/120), (95% CI 52.4–70.4) vs. pbo + enza: ORR 44% (58/132), (95% CI 35.3–52.8); p = 0.005 | ITT population: NR vs. 21.9 months HR 0.66 (95% CI 0.49–0.91); p = 0.009 mHRR: 27.9 vs. 16.4 months, HR 0.46 (95% CI 0.3–0.7); p < 0.01 nmHRR: NR vs. 22.5 months, HR 0.70 (95% CI 0.54–0.89): p = 0.04 mBRCA: HR 0.23 (95% CI 0.10–0.53); p < 0.001 | 36.4 vs. NR, HR 0.89 (95% CI 0.69–1.14) p = 0.35 (31% maturity) |
| Study | Phase | Setting | Treatment Arms | HHR Mutational Status | Primary Endpoint |
|---|---|---|---|---|---|
| CASPAR trial (NCT04455750) | 3 | mCRPC with no prior chemotherapy or NHA in castration resistant setting (Docetaxel or NHA treatment allowed in mHSPC) | Experimental arm: rucaparib 600 mg BID + enza 160 mg/die Control arm: pbo + enza 160 mg/die | Unselected | rPFS and OS |
| AMPLITUDE trial (NCT04497844) | 3 | First line mHSPC | Experimental arm: niraparib 200 mg/die + AA 1000 mg/die + prednisone 5 mg BID Control arm: abiraterone 1000 mg/die + prednisone 5 mg BID + pbo | Selected | rPFS |
| TALAPRO-3 trial (NCT04821622) | 3 | First line mHSPC | Experimental arm: talazoparib 0.5 mg/die + enza 160 mg/die Control arm: pbo + enza 160 mg/die | Selected | rPFS |
| NCT04734730 | 2 | First line mHSPC | Single arm: talazoparib 1 mg/die + AA 1000 mg/die + prednisone 5 mg BID | Unselected | PSA nadir < 0.2 |
| ZZ First Trial (NCT04332744) | 2 | First line mHSPC | Experimental arm: talazoparib 0.5 mg/die + enza 160 mg/die Control arm: pbo+ enza 160 mg/die | Unselected | PSA-CR |
| NCT05167175 | 2 | First line mHSPC | Single arm: olaparib 300 mg BID + AA 1000 mg/die + prednisone 5 mg BID | Selected | rPFS |
| NCT04691804 | 3 | mCRPC with any systemic anti-tumor treatment during the mCRPC stage or non-metastatic CRPC stage | Experimental arm: fuzuloparib 150 mg BID + AA 1000 mg/die+ prednisone 5 mg BID Control arm: pbo + AA 1000 mg/die + prednisone 5 mg BID | Cohort 1: unselected Cohort 2: selected | rPFS |
| NCT05405439 | 1b/2 | mCRPC with no prior NHA in mHSPC and nmCRPC. | Experimental arm: TQB3823 tablets (PARPi) + AA 1000 mg/die + prednisone 5 mg BID | Unselected | DLT, RP2D, rPFS |
| PETRANHA (NCT05367440) | 1/2 | mPC with no prior platinum or NHA | Arm 1: AZD5305 tablets (PARPi) + enza 160 mg/die Arm 2: AZD5305 tablets (PARPi) + AA 1000 mg/die + prednisone 5 mg BID Arm 3: AZD5305 tablets (PARPi) + daro 600 mg BID | Unselected | DLT, ORR, DoR, rPFS |
| NCT04108247 | 1 | mCRPC with 4 weeks of wash out of any anti-tumor therapy | Experimental arm: SHR3162 (PARPi) + AA 1000 mg/die + prednisone 5 mg BID | Unselected | Incidence of AE and PK characteristics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, M.; Saporita, I.; Turco, F.; Gillessen, S.; Castro, E.; Vogl, U.M.; Di Stefano, R.F.; Carfì, F.M.; Poletto, S.; Farinea, G.; et al. Synthetic Lethality by Co-Inhibition of Androgen Receptor and Polyadenosine Diphosphate-Ribose in Metastatic Prostate Cancer. Int. J. Mol. Sci. 2024, 25, 78. https://doi.org/10.3390/ijms25010078
Calabrese M, Saporita I, Turco F, Gillessen S, Castro E, Vogl UM, Di Stefano RF, Carfì FM, Poletto S, Farinea G, et al. Synthetic Lethality by Co-Inhibition of Androgen Receptor and Polyadenosine Diphosphate-Ribose in Metastatic Prostate Cancer. International Journal of Molecular Sciences. 2024; 25(1):78. https://doi.org/10.3390/ijms25010078
Chicago/Turabian StyleCalabrese, Mariangela, Isabella Saporita, Fabio Turco, Silke Gillessen, Elena Castro, Ursula Maria Vogl, Rosario Francesco Di Stefano, Federica Maria Carfì, Stefano Poletto, Giovanni Farinea, and et al. 2024. "Synthetic Lethality by Co-Inhibition of Androgen Receptor and Polyadenosine Diphosphate-Ribose in Metastatic Prostate Cancer" International Journal of Molecular Sciences 25, no. 1: 78. https://doi.org/10.3390/ijms25010078
APA StyleCalabrese, M., Saporita, I., Turco, F., Gillessen, S., Castro, E., Vogl, U. M., Di Stefano, R. F., Carfì, F. M., Poletto, S., Farinea, G., Tucci, M., & Buttigliero, C. (2024). Synthetic Lethality by Co-Inhibition of Androgen Receptor and Polyadenosine Diphosphate-Ribose in Metastatic Prostate Cancer. International Journal of Molecular Sciences, 25(1), 78. https://doi.org/10.3390/ijms25010078







