Attenuation of PI3K-Akt-mTOR Pathway to Reduce Cancer Stemness on Chemoresistant Lung Cancer Cells by Shikonin and Synergy with BEZ235 Inhibitor
Abstract
1. Introduction
2. Results
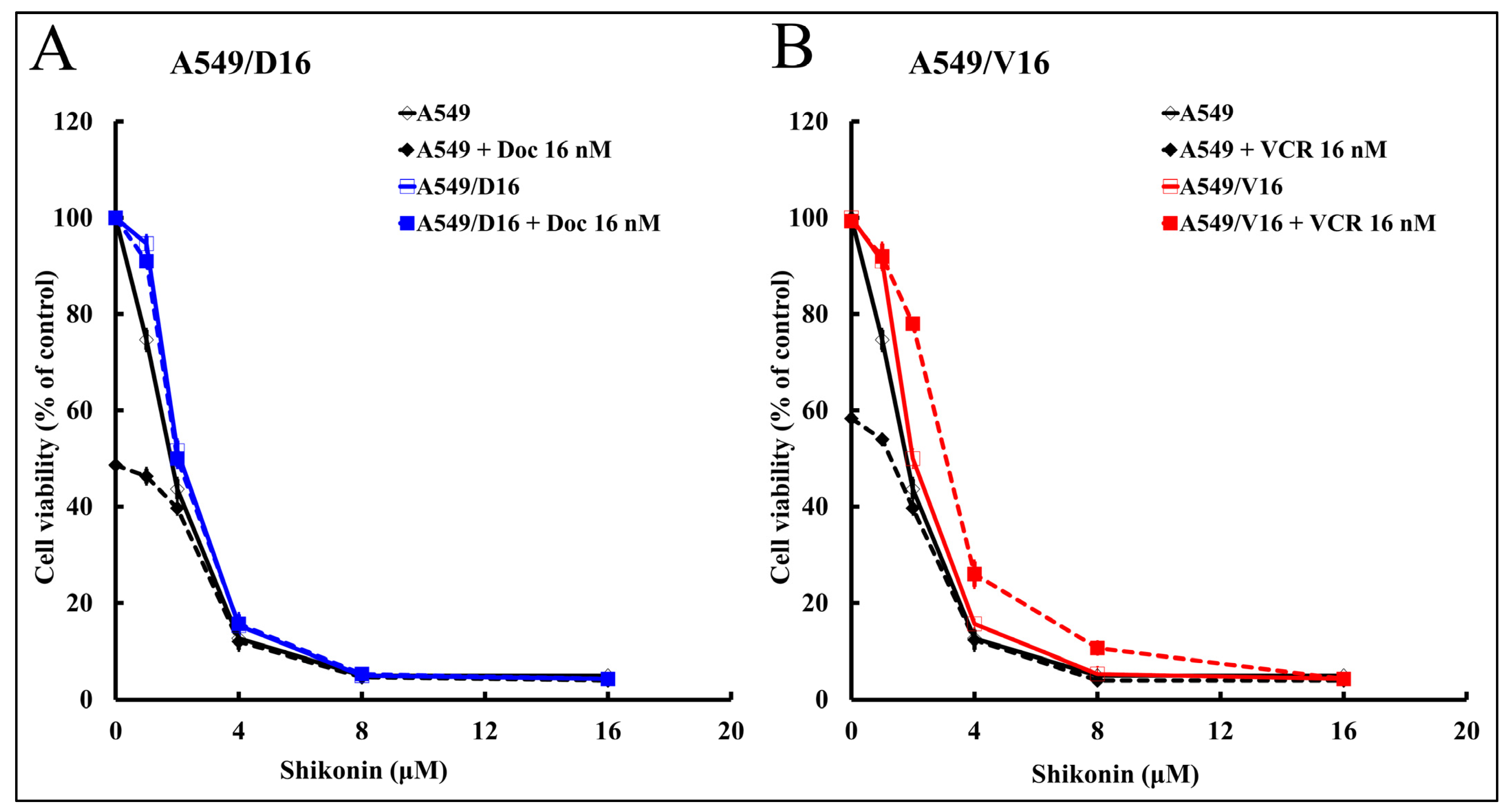
2.1. High Shikonin Sensitivity Was Observed from Parental A549 Lung Cancer and Its Chemoresistant Sublines
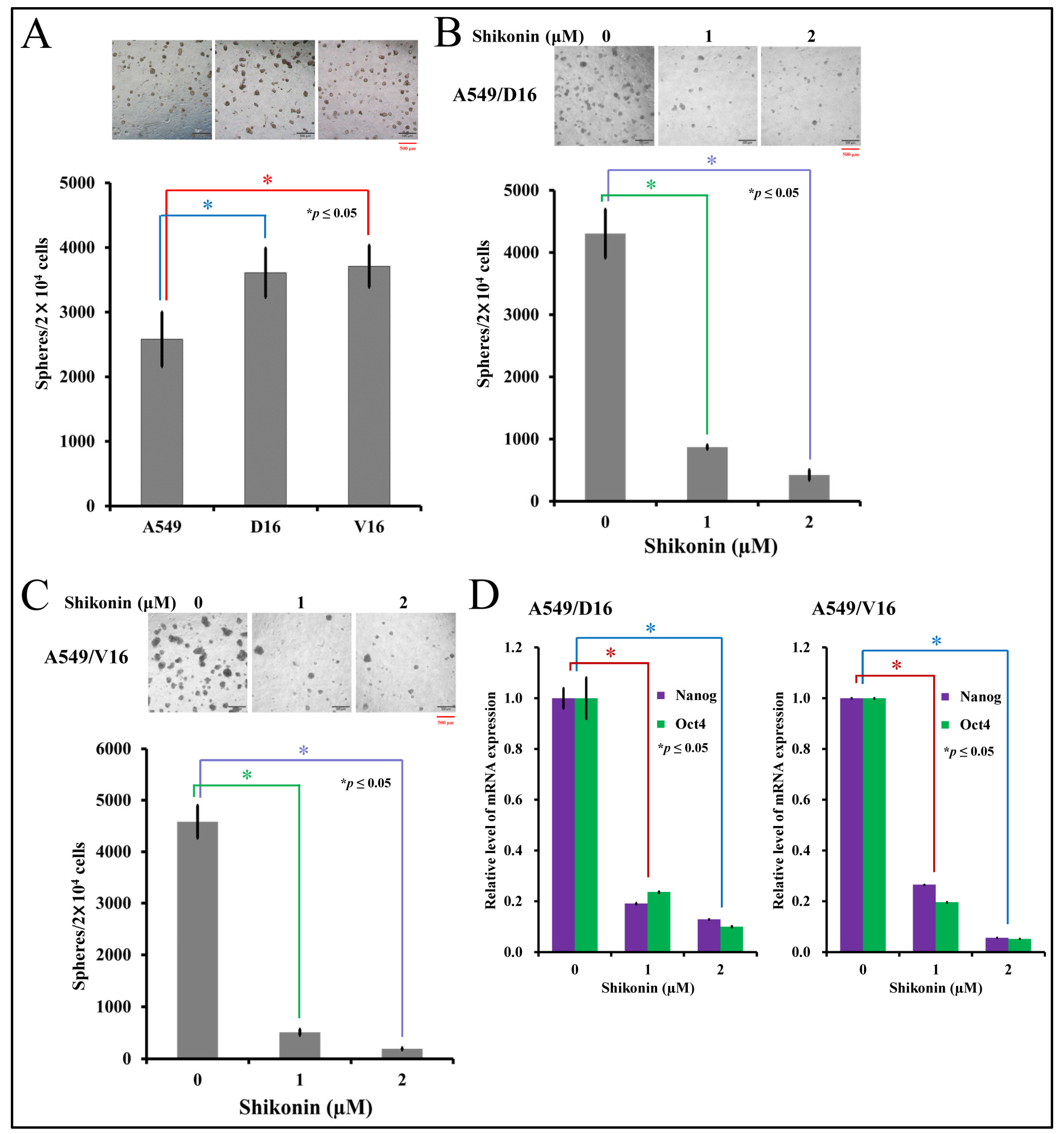
2.2. Shikonin Inhibits Spheroid Formation and Cancer Stemness Gene Expression
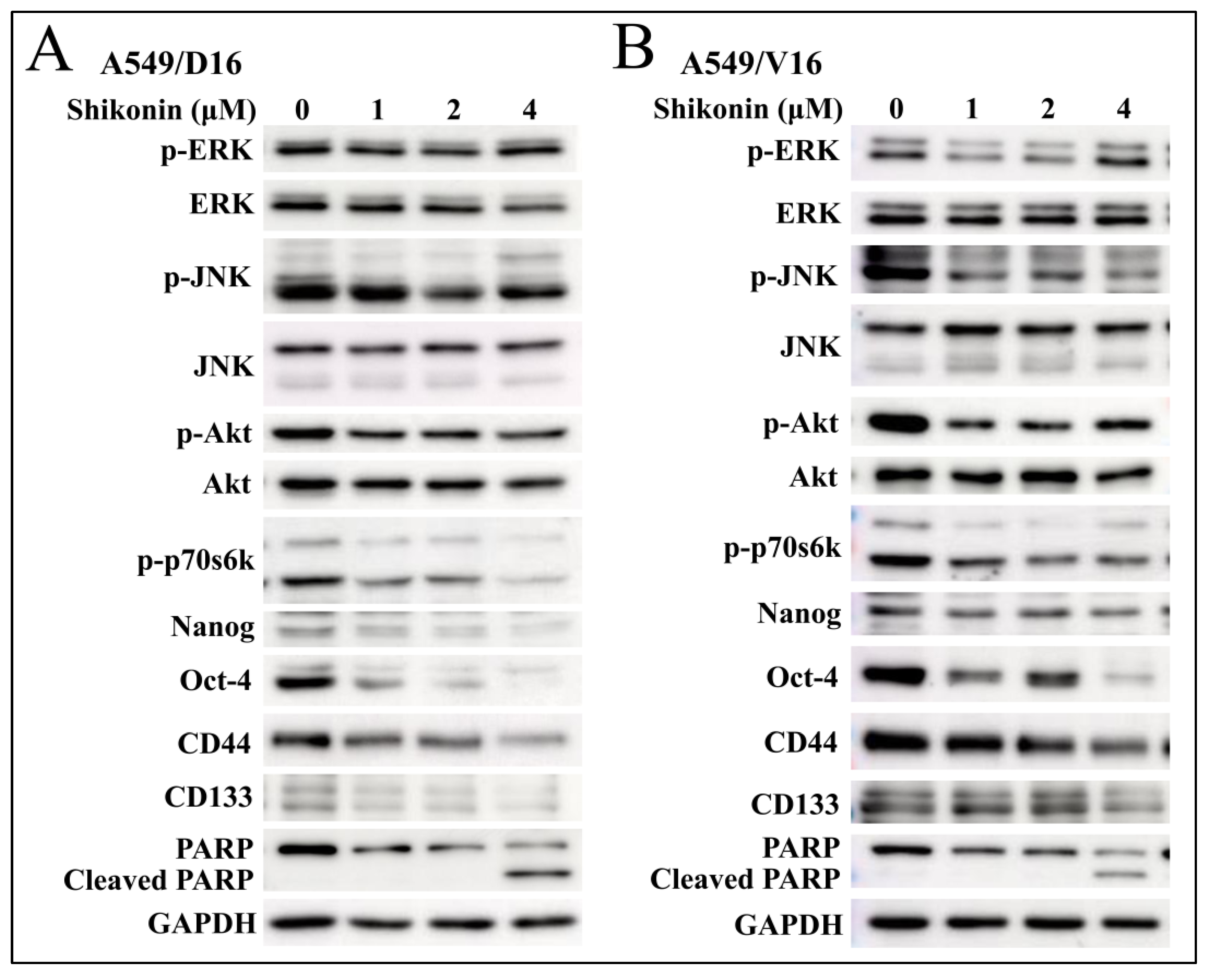
2.3. Shikonin Induces Apoptosis of Chemoresistant Sublines and Reduces Phosphorylated Akt and p70s6k Levels with Downregulation of Stemness Genes
2.4. Low Concentrations of Shikonin Have a Synergistic Effect When Combined with PI3K-Akt-mTOR Inhibitor (BEZ235) to Reduce the Survival of Chemoresistant Lung Cancer Cells
2.5. Combination of a Low Concentration of BEZ235 and Shikonin Significantly Reduced the Spheroid Formation on Chemoresistant Lung Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Cytotoxicity Assay (MTT Assay)
4.3. Quantitative Reverse Transcription Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. Primary and Secondary Sphere-Forming Assay
4.5. Protein Western Blot Assays
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Key, J.; Kim, Y.S.; Tatulli, F.; Palange, A.L.; O’Neill, B.; Aryal, S.; Ramirez, M.; Liu, X.; Ferrari, M.; Munden, R.; et al. Opportunities for NanoTheranosis in Lung Cancer and Pulmonary Metastasis. Clin. Transl. Imaging 2014, 2, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.; Costa, D.B.; Rangachari, D. Management of advanced non-small cell lung cancers with known mutations or rearrangements: Latest evidence and treatment approaches. Ther. Adv. Respir. Dis. 2016, 10, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Olaussen, K.A.; Postel-Vinay, S. Predictors of chemotherapy efficacy in non-small-cell lung cancer: A challenging landscape. Ann. Oncol. 2016, 27, 2004–2016. [Google Scholar] [CrossRef]
- Pilkington, G.; Boland, A.; Brown, T.; Oyee, J.; Bagust, A.; Dickson, R. A systematic review of the clinical effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer. Thorax 2015, 70, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.A.; Widodo, E.; Waltham, M.; Thompson, E.W. Breast cancer stem cells and epithelial mesenchymal plasticity—Implications for chemoresistance. Cancer Lett. 2013, 341, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Toledo, B.; Gonzalez-Titos, A.; Hernandez-Camarero, P.; Peran, M. A Brief Review on Chemoresistance; Targeting Cancer Stem Cells as an Alternative Approach. Int. J. Mol. Sci. 2023, 24, 4487. [Google Scholar] [CrossRef] [PubMed]
- Leon, G.; MacDonagh, L.; Finn, S.P.; Cuffe, S.; Barr, M.P. Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways. Pharmacol. Ther. 2016, 158, 71–90. [Google Scholar] [CrossRef]
- MacDonagh, L.; Gray, S.G.; Breen, E.; Cuffe, S.; Finn, S.P.; O’Byrne, K.J.; Barr, M.P. Lung cancer stem cells: The root of resistance. Cancer Lett. 2016, 372, 147–156. [Google Scholar] [CrossRef]
- Kim, J.; Chu, J.; Shen, X.; Wang, J.; Orkin, S.H. An extended transcriptional network for pluripotency of embryonic stem cells. Cell 2008, 132, 1049–1061. [Google Scholar] [CrossRef]
- Iida, H.; Suzuki, M.; Goitsuka, R.; Ueno, H. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. Int. J. Oncol. 2012, 40, 71–79. [Google Scholar]
- Chiou, S.H.; Wang, M.L.; Chou, Y.T.; Chen, C.J.; Hong, C.F.; Hsieh, W.J.; Chang, H.T.; Chen, Y.S.; Lin, T.W.; Hsu, H.S.; et al. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010, 70, 10433–10444. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, M.; Cui, S.; Wang, D.; Zhang, C.Y.; Zen, K.; Li, L. Shikonin Inhibits the Proliferation of Human Breast Cancer Cells by Reducing Tumor-Derived Exosomes. Molecules 2016, 21, 777. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kao, S.H.; Hunag, J.E.; Sheu, G.T.; Yeh, C.W.; Hseu, Y.C.; Wang, C.J.; Hsu, L.S. Shikonin time-dependently induced necrosis or apoptosis in gastric cancer cells via generation of reactive oxygen species. Chem. Biol. Interact. 2014, 211, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Li, Z.L.; Wu, J.Y.; Lu, F.J.; Chen, C.H. An oxidative stress mechanism of shikonin in human glioma cells. PLoS ONE 2014, 9, e94180. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Liu, T.J.; Lai, H.C. Shikonin Induces Apoptosis, Necrosis, and Premature Senescence of Human A549 Lung Cancer Cells through Upregulation of p53 Expression. Evid.-Based Complement. Altern. Med. 2015, 2015, 620383. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, C.; Wan, S.; Zhang, H.; Zhou, S.; Liu, G. Shikonin attenuates lung cancer cell adhesion to extracellular matrix and metastasis by inhibiting integrin beta1 expression and the ERK1/2 signaling pathway. Toxicology 2013, 308, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Liao, C.H.; Chen, W.S.; Pai, J.T.; Weng, M.S. Shikonin Inhibited Migration and Invasion of Human Lung Cancer Cells via Suppression of c-Met-Mediated Epithelial-to-Mesenchymal Transition. J. Cell Biochem. 2017, 118, 4639–4651. [Google Scholar] [CrossRef]
- Soga, T. Cancer metabolism: Key players in metabolic reprogramming. Cancer Sci. 2013, 104, 275–281. [Google Scholar] [CrossRef]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Liu, C.G.; Lu, Y.; Wang, B.B.; Zhang, Y.J.; Zhang, R.S.; Lu, Y.; Chen, B.; Xu, H.; Jin, F.; Lu, P. Clinical implications of stem cell gene Oct-4 expression in breast cancer. Ann. Surg. 2011, 253, 1165–1171. [Google Scholar] [CrossRef]
- Wu, H.; Xie, J.; Pan, Q.; Wang, B.; Hu, D.; Hu, X. Anticancer agent shikonin is an incompetent inducer of cancer drug resistance. PLoS ONE 2013, 8, e52706. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Liu, L.; Wang, Y.; Yan, G.; Zhang, Y. Long-term systemic toxicity of shikonin derivatives in Wistar rats. Pharm. Biol. 2014, 52, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Scrima, M.; De Marco, C.; Fabiani, F.; Franco, R.; Pirozzi, G.; Rocco, G.; Ravo, M.; Weisz, A.; Zoppoli, P.; Ceccarelli, M.; et al. Signaling networks associated with AKT activation in non-small cell lung cancer (NSCLC): New insights on the role of phosphatydil-inositol-3 kinase. PLoS ONE 2012, 7, e30427. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac. Cancer 2020, 11, 511–518. [Google Scholar] [CrossRef]
- Carlo, M.I.; Molina, A.M.; Lakhman, Y.; Patil, S.; Woo, K.; DeLuca, J.; Lee, C.H.; Hsieh, J.J.; Feldman, D.R.; Motzer, R.J.; et al. A Phase Ib Study of BEZ235, a Dual Inhibitor of Phosphatidylinositol 3-Kinase (PI3K) and Mammalian Target of Rapamycin (mTOR), in Patients With Advanced Renal Cell Carcinoma. Oncologist 2016, 21, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Watanabe, K.; Amagasaki, T.; Natsume, K.; Takeuchi, H.; Quadt, C.; Shirao, K.; Minami, H. A phase I study of single-agent BEZ235 special delivery system sachet in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2019, 83, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Wise-Draper, T.M.; Moorthy, G.; Salkeni, M.A.; Karim, N.A.; Thomas, H.E.; Mercer, C.A.; Beg, M.S.; O’Gara, S.; Olowokure, O.; Fathallah, H.; et al. A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib (BEZ235) Combined with Everolimus in Patients with Advanced Solid Malignancies. Target. Oncol. 2017, 12, 323–332. [Google Scholar] [CrossRef]
- Zhu, H.; Shi, Y.; Jiao, X.; Yang, G.; Wang, R.; Yuan, Y. Synergistic antitumor effect of dual PI3K and mTOR inhibitor NVP-BEZ235 in combination with cisplatin on drug-resistant non-small cell lung cancer cell. Oncol. Lett. 2020, 20, 326. [Google Scholar] [CrossRef]
- Xu, C.X.; Li, Y.; Yue, P.; Owonikoko, T.K.; Ramalingam, S.S.; Khuri, F.R.; Sun, S.Y. The combination of RAD001 and NVP-BEZ235 exerts synergistic anticancer activity against non-small cell lung cancer in vitro and in vivo. PLoS ONE 2011, 6, e20899. [Google Scholar] [CrossRef]
- Chou, T.C. The mass-action law based algorithm for cost-effective approach for cancer drug discovery and development. Am. J. Cancer Res. 2011, 1, 925–954. [Google Scholar]
- Chiu, L.Y.; Ko, J.L.; Lee, Y.J.; Yang, T.Y.; Tee, Y.T.; Sheu, G.T. L-type calcium channel blockers reverse docetaxel and vincristine-induced multidrug resistance independent of ABCB1 expression in human lung cancer cell lines. Toxicol. Lett. 2010, 192, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Jeung, Y.J.; Kim, H.G.; Ahn, J.; Lee, H.J.; Lee, S.B.; Won, M.; Jung, C.R.; Im, J.Y.; Kim, B.K.; Park, S.K.; et al. Shikonin induces apoptosis of lung cancer cells via activation of FOXO3a/EGR1/SIRT1 signaling antagonized by p300. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2584–2593. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yuan, Z.; Jiang, J.; Rao, Y. Anti-tumor activity of Shikonin against afatinib resistant non-small cell lung cancer via negative regulation of PI3K/Akt signaling pathway. Biosci. Rep. 2018, 38, BSR20181693. [Google Scholar] [CrossRef]
- Zang, F.; Rao, Y.; Zhu, X.; Wu, Z.; Jiang, H. Shikonin suppresses NEAT1 and Akt signaling in treating paclitaxel-resistant non-small cell of lung cancer. Mol. Med. 2020, 26, 28. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Xu, X.Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: From basic research to clinical application. Am. J. Cancer Res. 2015, 5, 1602–1609. [Google Scholar] [PubMed]
- Wu, M.F.; Huang, Y.H.; Chiu, L.Y.; Cherng, S.H.; Sheu, G.T.; Yang, T.Y. Curcumin Induces Apoptosis of Chemoresistant Lung Cancer Cells via ROS-Regulated p38 MAPK Phosphorylation. Int. J. Mol. Sci. 2022, 23, 8248. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Guo, C.Y.; Yan, C.; Luo, L.; Goto, S.; Urata, Y.; Xu, J.J.; Wen, X.M.; Kuang, Y.K.; Tou, F.F.; Li, T.S. Enhanced expression of PKM2 associates with the biological properties of cancer stem cells from A549 human lung cancer cells. Oncol. Rep. 2017, 37, 2161–2166. [Google Scholar] [CrossRef]
- Tang, J.C.; Ren, Y.G.; Zhao, J.; Long, F.; Chen, J.Y.; Jiang, Z. Shikonin enhances sensitization of gefitinib against wild-type EGFR non-small cell lung cancer via inhibition PKM2/stat3/cyclinD1 signal pathway. Life Sci. 2018, 204, 71–77. [Google Scholar] [CrossRef]
- Herrera, V.A.; Zeindl-Eberhart, E.; Jung, A.; Huber, R.M.; Bergner, A. The dual PI3K/mTOR inhibitor BEZ235 is effective in lung cancer cell lines. Anticancer Res. 2011, 31, 849–854. [Google Scholar]
- Wu, Y.Y.; Wu, H.C.; Wu, J.E.; Huang, K.Y.; Yang, S.C.; Chen, S.X.; Tsao, C.J.; Hsu, K.F.; Chen, Y.L.; Hong, T.M. The dual PI3K/mTOR inhibitor BEZ235 restricts the growth of lung cancer tumors regardless of EGFR status, as a potent accompanist in combined therapeutic regimens. J. Exp. Clin. Cancer Res. 2019, 38, 282. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Li, Q.; Sun, Q.; Yang, L.; Liu, X.; Zhao, Y.; Shi, M.; Li, X.; Luo, K. Promising Nanomedicines of Shikonin for Cancer Therapy. Int. J. Nanomed. 2023, 18, 1195–1218. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Lambert, K.A.; Norris, D.A.; Shellman, Y.G. Enrichment of Melanoma Stem-Like Cells via Sphere Assays. Methods Mol. Biol. 2021, 2265, 185–199. [Google Scholar] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-H.; Chiu, L.-Y.; Tseng, J.-S.; Hsu, K.-H.; Chen, C.-H.; Sheu, G.-T.; Yang, T.-Y. Attenuation of PI3K-Akt-mTOR Pathway to Reduce Cancer Stemness on Chemoresistant Lung Cancer Cells by Shikonin and Synergy with BEZ235 Inhibitor. Int. J. Mol. Sci. 2024, 25, 616. https://doi.org/10.3390/ijms25010616
Huang Y-H, Chiu L-Y, Tseng J-S, Hsu K-H, Chen C-H, Sheu G-T, Yang T-Y. Attenuation of PI3K-Akt-mTOR Pathway to Reduce Cancer Stemness on Chemoresistant Lung Cancer Cells by Shikonin and Synergy with BEZ235 Inhibitor. International Journal of Molecular Sciences. 2024; 25(1):616. https://doi.org/10.3390/ijms25010616
Chicago/Turabian StyleHuang, Yen-Hsiang, Ling-Yen Chiu, Jeng-Sen Tseng, Kuo-Hsuan Hsu, Chang-Han Chen, Gwo-Tarng Sheu, and Tsung-Ying Yang. 2024. "Attenuation of PI3K-Akt-mTOR Pathway to Reduce Cancer Stemness on Chemoresistant Lung Cancer Cells by Shikonin and Synergy with BEZ235 Inhibitor" International Journal of Molecular Sciences 25, no. 1: 616. https://doi.org/10.3390/ijms25010616
APA StyleHuang, Y.-H., Chiu, L.-Y., Tseng, J.-S., Hsu, K.-H., Chen, C.-H., Sheu, G.-T., & Yang, T.-Y. (2024). Attenuation of PI3K-Akt-mTOR Pathway to Reduce Cancer Stemness on Chemoresistant Lung Cancer Cells by Shikonin and Synergy with BEZ235 Inhibitor. International Journal of Molecular Sciences, 25(1), 616. https://doi.org/10.3390/ijms25010616






