Fertility Protection, A Novel Concept: Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Protect against Chemotherapy-Induced Testicular Cytotoxicity
Abstract
1. Introduction
2. Results
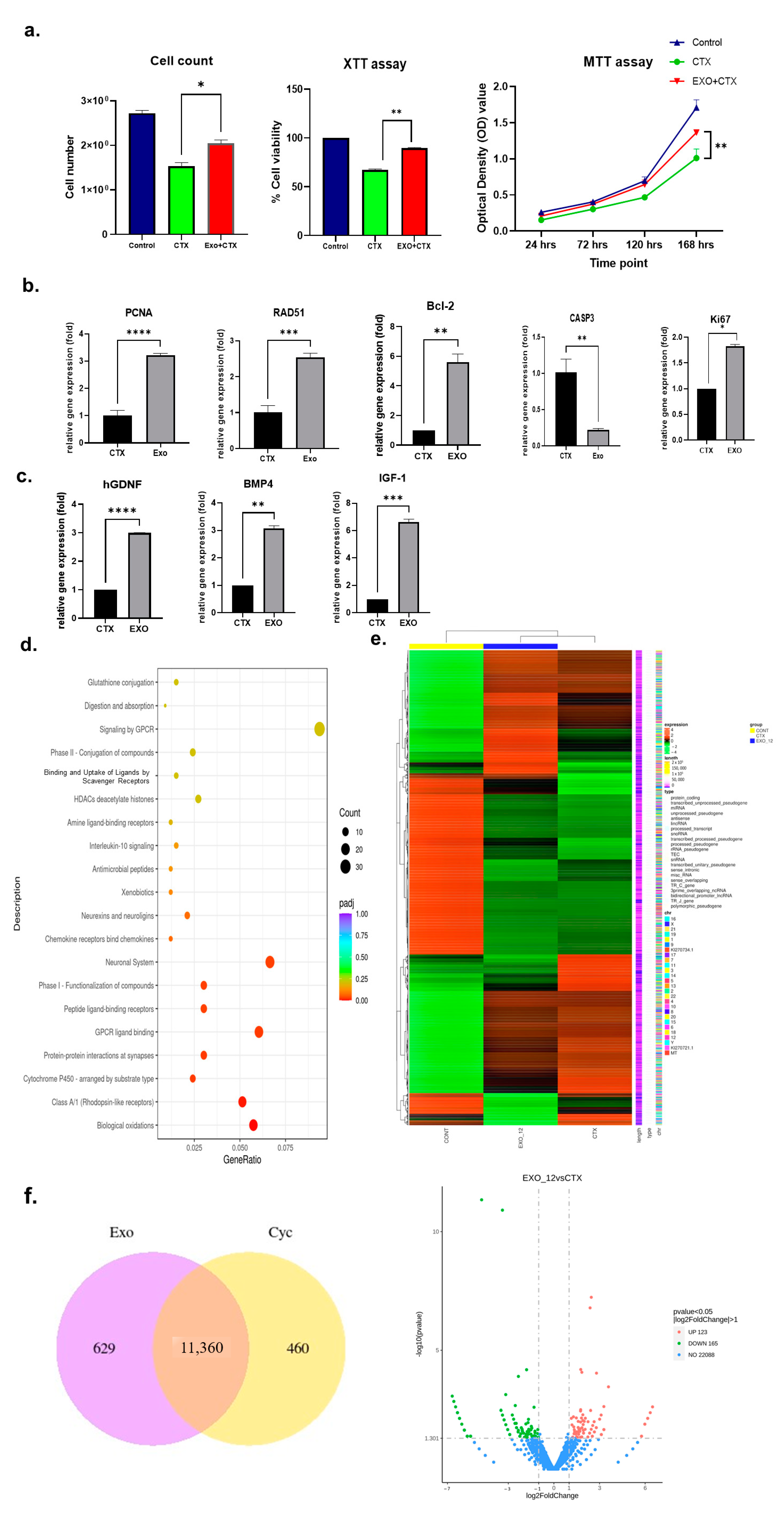
2.1. Effects of h-UCMSC-Exos on Human Sertoli Cells
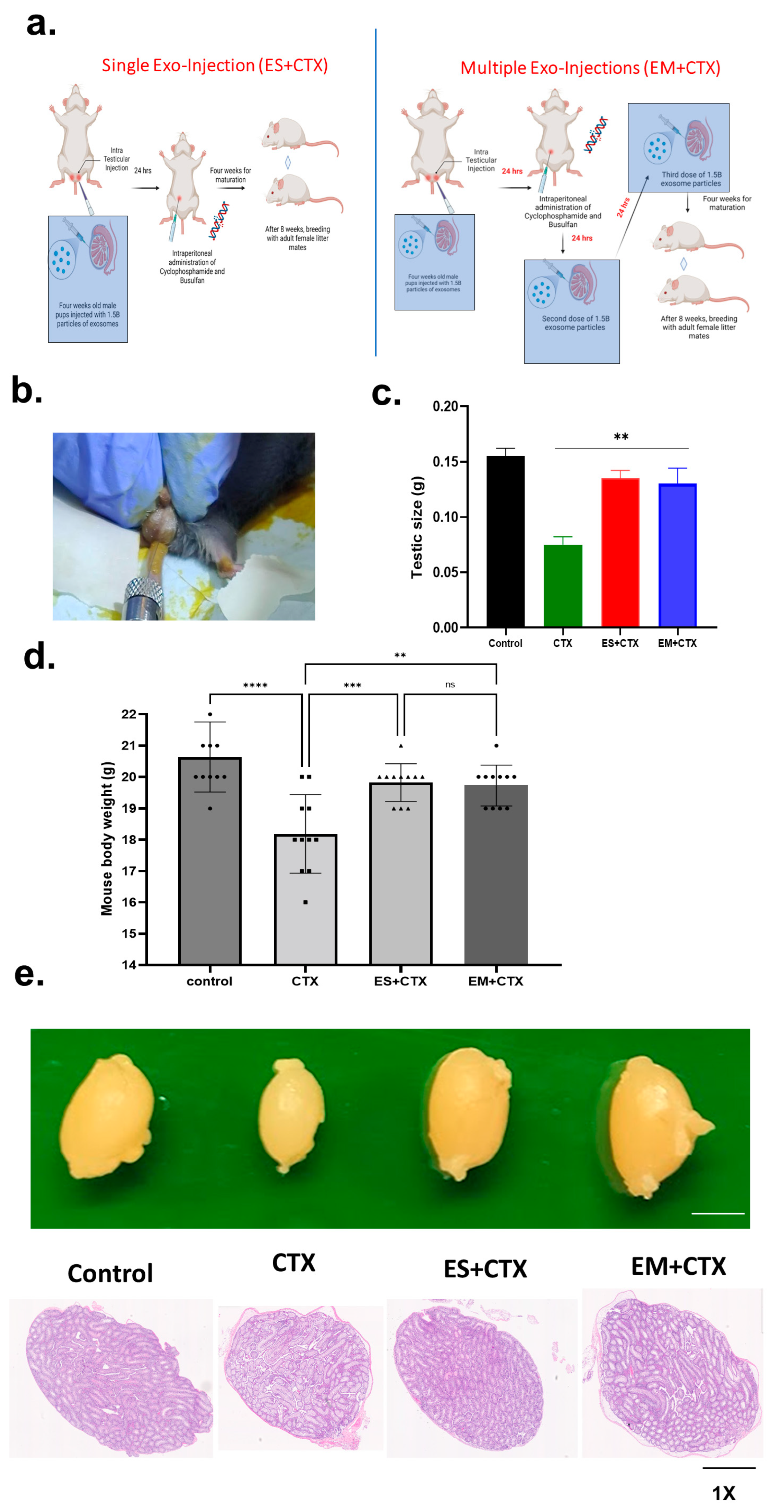
2.2. Pretreatment of h-UCMSC-Exo Improved Total Body Weight, Testicular Size, and Testicular Weight after Polychemotherapy-Induced TD
2.3. h-UCMSC-Exo Protects Testes during Polychemotherapy and Preserves Fertility in a TD Mouse Model
2.4. h-UCMSC-Exo Preserve Ferility, Enhance Spermatogenesis-Associated Gene Markers, and Regulate Associated Pathways
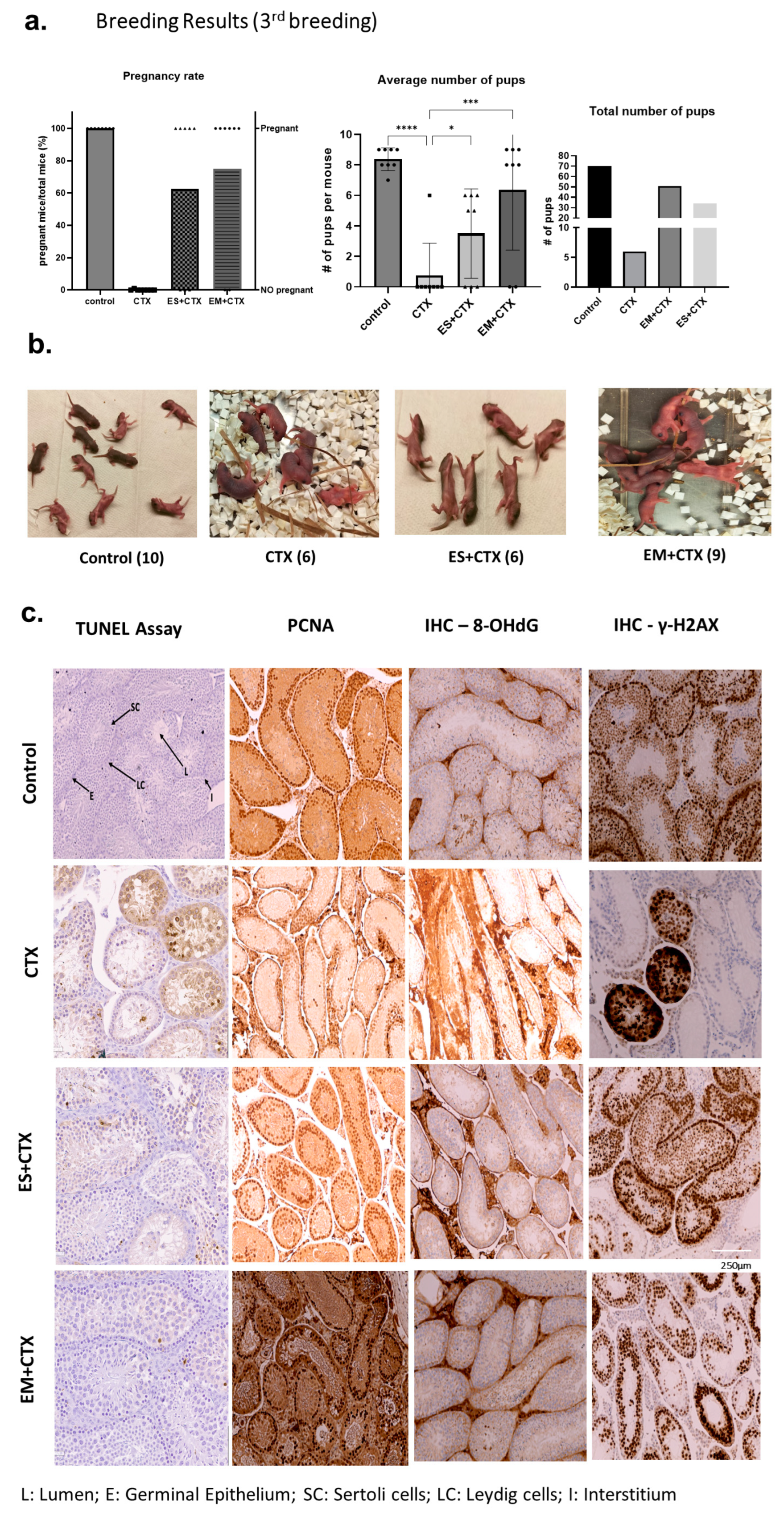
2.5. h-UCMSC-Exo Permanently Restore Ferility through Continued Breeding and Alleviate Testicular Damage via Various Biological Pathways
3. Discussion
4. Materials and Methods
4.1. In Vitro Testicular Toxicity (TT) Cell Model Using Human Sertoli Cells (hSerC)
4.2. Preparation of the h-UCMSC−Derived Exosomes
4.3. MTT and XTT Assays
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Western Blot
4.6. RNA Sequencing
4.7. Development of In-House Prepubescent Mice Colony and Mouse TD Model
4.8. Serum Hormone Measurements
4.9. Breeding Experiments
4.10. Histological Assays
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Ahmedin Jemal Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Fotouh, G.I.; Abdel-Dayem, M.M.; Ismail, D.I.; Mohamed, H.H. Histological Study on the Protective Effect of Endogenous Stem Cell Mobilization in Busulfan-Induced Testicular Injury in Albino Rats. J. Microsc. Ultrastruct. 2018, 6, 197–204. [Google Scholar] [PubMed]
- Delessard, M.; Saulnier, J.; Rives, A.; Dumont, L.; Rondanino, C.; Rives, N. Exposure to Chemotherapy during Childhood or Adulthood and Consequences on Spermatogenesis and Male Fertility. Int. J. Mol. Sci. 2020, 21, 1454. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish, R.; Emadi, L.; Akhtardanesh, B.; Azizi, S.; Imani, M.; Mahmoodabadi, F.; Irani, F.; Shokrizadeh, H. Effects of ciprofloxacin on testicular tissue and sperm quality in rabbits. Asian Pac. J. Reprod. 2020, 9, 83–88. [Google Scholar]
- Brougham, M.F.H.; Wallace, W.H.B. Subfertility in children and young people treated for solid and haematological malignancies. Br. J. Haematol. 2005, 131, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Suttorp, M.; Millot, F. Treatment of pediatric chronic myeloid leukemia in the year 2010: Use of tyrosine kinase inhibitors and stem-cell transplantation. Hematol. Am. Soc. Hematol. Educ. Program 2010, 368–376. [Google Scholar] [CrossRef][Green Version]
- Galaup, A.; Paci, A. Pharmacology of dimethanesulfonate alkylating agents: Busulfan and treosulfan. Expert. Opin. Drug Metab. Toxicol. 2013, 9, 333–347. [Google Scholar] [CrossRef]
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of male survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2010, 28, 332–339. [Google Scholar] [CrossRef]
- Mulder, R.L.; Font-Gonzalez, A.; Hudson, M.M.; Van Santen, H.M.; Loeffen, E.A.; Burns, K.C.; Quinn, G.P.; van Dulmen-den Broeder, E.; Byrne, J.; Haupt, R.; et al. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e57–e67. [Google Scholar] [CrossRef]
- Anderson, R.A.; Mitchell, R.T.; Kelsey, T.W.; Spears, N.; Telfer, E.E.; Wallace, W.H. Cancer treatment and gonadal function: Experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015, 3, 556–567. [Google Scholar] [CrossRef]
- Nagano, M.C. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol. Reprod. 2003, 69, 701–707. [Google Scholar] [CrossRef]
- Goossens, E.; Bilgec, T.; Van Saen, D.; Tournaye, H. Mouse germ cells go through typical epigenetic modifications after intratesticular tissue grafting. Hum. Reprod. 2011, 26, 3388–3400. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, Y.; Ohta, H.; Sato, T.; Murase, Y.; Yabuta, Y.; Kojima, Y.; Yamashiro, C.; Nakamura, T.; Yamamoto, T.; Ogawa, T.; et al. In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell 2021, 28, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Roy, P.; Corbitt, C.; Kakar, S.S. Application of Stem Cell Therapy for Infertility. Cells 2021, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transpl. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.J.C.; Noël, D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 2013, 95, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Chen, S.R.; Liu, Y.X. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 2015, 149, R159–R167. [Google Scholar] [CrossRef]
- Porubska, B.; Vasek, D.; Somova, V.; Hajkova, M.; Hlaviznova, M.; Tlapakova, T.; Holan, V.; Krulova, M. Sertoli Cells Possess Immunomodulatory Properties and the Ability of Mitochondrial Transfer Similar to Mesenchymal Stromal Cells. Stem Cell Rev. Rep. 2021, 17, 1905–1916. [Google Scholar] [CrossRef]
- Gong, D.; Zhang, C.; Li, T.; Zhang, J.; Zhang, N.; Tao, Z.; Zhu, W.; Sun, X. Are Sertoli cells a kind of mesenchymal stem cells? Am. J. Transl. Res. 2017, 9, 1067–1074. [Google Scholar]
- Köse, S.; Yersal, N.; Önen, S.; Korkusuz, P. Comparison of hematopoietic and spermatogonial stem cell niches from the regenerative medicine aspect. Adv. Exp. Med. Biol. 2018, 1107, 15–40. [Google Scholar] [PubMed]
- Yang, R.-F.; Liu, T.-H.; Zhao, K.; Xiong, C. Enhancement of mouse germ cell-associated genes expression by injection of human umbilical cord mesenchymal stem cells into the testis of chemical-induced azoospermic mice. Asian J. Androl. 2014, 16, 698–704. [Google Scholar] [PubMed]
- Cakici, C.; Buyrukcu, B.; Duruksu, G.; Haliloglu, A.H.; Aksoy, A.; Isık, A.; Uludag, O.; Ustun, H.; Subası, C.; Karaoz, E. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: The sperm generation. Biomed. Res. Int. 2013, 2013, 529589. [Google Scholar] [CrossRef] [PubMed]
- Monsefi, M.; Fereydouni, B.; Rohani, L.; Talaei, T. Mesenchymal stem cells repair germinal cells of seminiferous tubules of sterile rats. Iran. J. Reprod. Med. 2013, 11, 537. [Google Scholar] [PubMed]
- Tamadon, A.; Mehrabani, D.; Rahmanifar, F.; Jahromi, A.R.; Panahi, M.; Zare, S.; Khodabandeh, Z.; Jahromi, I.R.; Tanideh, N.; Dianatpour, M.; et al. Induction of Spermatogenesis by Bone Marrow-derived Mesenchymal Stem Cells in Busulfan-induced Azoospermia in Hamster. Int. J. Stem Cells 2015, 8, 134–145. [Google Scholar] [CrossRef]
- Ghasemzadeh-Hasankolaei, M.; Batavani, R.; Eslaminejad, M.B.; Sayahpour, F. Transplantation of autologous bone marrow mesenchymal stem cells into the testes of infertile male rats and new germ cell formation. Int. J. Stem Cells 2016, 9, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Peng, J.; He, D.; Lin, T.; Zhu, J.; Li, X.; Zhang, Y.; Wei, G. Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model. Int. J. Mol. Sci. 2014, 15, 13151–13165. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Q.L.; Wu, X.Y.; Xie, L.C.; Lin, L.M.; Ho, G.Y.; Ma, L. Differentiation of human umbilical cord mesenchymal stem cells into germ-like cells in mouse seminiferous tubules. Mol. Med. Rep. 2015, 12, 819–828. [Google Scholar] [CrossRef]
- Vahdati, A.; Fathi, A.; Hajihoseini, M.; Aliborzi, G.; Hosseini, E. The Regenerative effect of bone marrow-derived stem cells in spermatogenesis of infertile hamster. World J. Plastic Surg. 2017, 6, 18–25. [Google Scholar]
- Prihatno, S.A.; Padeta, I.; Larasati, A.D.; Sundari, B.; Hidayati, A.; Fibrianto, Y.H.; Budipitojo, T. Effects of secretome on cisplatin-induced testicular dysfunction in rats. Vet. World 2018, 11, 1349–1356. [Google Scholar] [CrossRef]
- Mehrabani, D.; Hassanshahi, M.A.; Tamadon, A.; Zare, S.; Keshavarz, S.; Rahmanifar, F.; Dianatpour, M.; Khodabandeh, Z.; Jahromi, I.; Tanideh, N.; et al. Adipose tissue-derived mesenchymal stem cells repair germinal cells of seminiferous tubules of busulfan-induced azoospermic rats. J. Hum. Reprod. Sci. 2015, 8, 103–110. [Google Scholar] [PubMed]
- Aghamir, S.M.; Salavati, A.; Yousefie, R.; Tootian, Z.; Ghazaleh, N.; Jamali, M.; Azimi, P. Does bone marrow-derived mesenchymal stem cell transfusion prevent antisperm antibody production after traumatic testis rupture? Urology 2014, 84, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Yan, J.; Zou, X.-L.; Guo, K.-J.; Zhao, Y.; Meng, C.-Y.; Yin, F.; Guo, L. Bone marrow mesenchymal stem cells repair cadmium-induced rat testis injury by inhibiting mitochondrial apoptosis. Chem. Biol. Interact. 2017, 271, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Önen, S.; Köse, S.; Yersal, N.; Korkusuz, P. Mesenchymal stem cells promote spermatogonial stem/progenitor cell pool and spermatogenesis in neonatal mice in vitro. Sci. Rep. 2022, 12, 11494. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, Q.W.; Sun, Y.; Chen, X.F. The emerging role of extracellular vesicles in the testis. Hum. Reprod. 2023, 38, 334–351. [Google Scholar] [CrossRef]
- Elliott, R.O. HM Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef]
- Cai, Y.T.; Xiong, C.L.; Shen, S.L.; Rao, J.P.; Liu, T.S.; Qiu, F. Mesenchymal stem cell-secreted factors delayed spermatogenesis injuries induced by busulfan involving intercellular adhesion molecule regulation. Andrologia 2019, 51, e13285. [Google Scholar] [CrossRef]
- Deng, C.; Xie, Y.; Zhang, C.; Ouyang, B.; Chen, H.; Lv, L.; Yao, J.; Liang, X.; Zhang, Y.; Sun, X.; et al. Urine-Derived Stem Cells Facilitate Endogenous Spermatogenesis Restoration of Busulfan-Induced Nonobstructive Azoospermic Mice by Paracrine Exosomes. Stem Cells Dev. 2019, 28, 1322–1333. [Google Scholar] [CrossRef]
- Guo, X.B.; Zhai, J.W.; Xia, H.; Yang, J.K.; Zhou, J.H.; Guo, W.B.; Yang, C.; Xia, M.; Xue, K.Y.; Liu, C.D.; et al. Protective effect of bone marrow mesenchymal stem cell-derived exosomes against the reproductive toxicity of cyclophosphamide is associated with the p38MAPK/ERK and AKT signaling pathways. Asian J. Androl. 2021, 23, 386–391. [Google Scholar] [PubMed]
- Liang, H.-Y.; Peng, F.; Pan, M.-J.; Liao, S.-L.; Wei, C.; Wei, G.-Y.; Xie, X.; Xue, K.-Y.; Chen, M.-K.; Yang, J.-K.; et al. Exosomes derived from BMSCs ameliorate cyclophosphamide-induced testosterone deficiency by enhancing the autophagy of Leydig cells via the AMPK-mTOR signaling pathway. Asian J. Androl. 2023, 25, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, H.; Heidarpour, M.; Rahbarghazi, R.; Nouri, M.; Mahdipour, M. Amniotic fluid-derived exosomes improved spermatogenesis in a rat model of azoospermia. Life Sci. 2021, 274, 119336. [Google Scholar] [CrossRef] [PubMed]
- Aslam, I.; Fishel, S.; Moore, H.; Dowell, K.; Thornton, S. Fertility preservation of boys undergoing anti-cancer therapy: A review of the existing situation and prospects for the future. Hum. Reprod. 2000, 15, 2154–2159. [Google Scholar] [CrossRef]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Green, D.M.; Liu, W.; Kutteh, W.H.; Ke, R.W.; Shelton, K.C.; Sklar, C.A.; Chemaitilly, W.; Pui, C.-H.; Klosky, J.L.; Spunt, S.L.; et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Lancet Oncol 2014, 15, 1215–1223. [Google Scholar] [CrossRef]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E. Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Tharmalingam, M.D.; Matilionyte, G.; Wallace, W.H.B.; Stukenborg, J.-B.; Jahnukainen, K.; Oliver, E.; Goriely, A.; Lane, S.; Guo, J.; Cairns, B.; et al. Cisplatin and carboplatin result in similar gonadotoxicity in immature human testis with implications for fertility preservation in childhood cancer. BMC Med. 2020, 18, 374. [Google Scholar] [CrossRef]
- Qian, C.; Meng, Q.; Lu, J.; Zhang, L.; Li, H.; Huang, B. Human amnion mesenchymal stem cells restore spermatogenesis in mice with busulfan-induced testis toxicity by inhibiting apoptosis and oxidative stress. Stem Cell Res. Ther. 2020, 11, 290. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, L.; Mohsin, A.; Ahmed, W.; Xu, C.; Peng, Y.; Hang, H.; Zhuang, Y.; Chu, J.; Guo, M. Efficient generation of male germ-like cells derived during co-culturing of adipose-derived mesenchymal stem cells with Sertoli cells under retinoic acid and testosterone induction. Stem Cell Res. Ther. 2019, 10, 91. [Google Scholar] [CrossRef]
- Kadam, P.; Van Saen, D.; Goossens, E. Can mesenchymal stem cells improve spermatogonial stem cell transplantation efficiency? Andrology 2017, 5, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhai, C.; Li, Y.; Ma, B.; Li, Z.; Wang, J. Sertoli cells-derived exosomal miR-30a-5p regulates ubiquitin E3 ligase Zeb2 to affect the spermatogonial stem cells proliferation and differentiation. Reprod. Toxicol. 2023, 117, 108340. [Google Scholar] [CrossRef] [PubMed]
- Stukenborg, J.-B.; Jahnukainen, K.; Hutka, M.; Mitchell, R.T. Cancer treatment in childhood and testicular function: The importance of the somatic environment. Endocr. Connect. 2018, 7, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.; Mandili, G.; Witzmann, F.A.; Novelli, F.; Zimmers, T.A.; Bonetto, A. Cancer and Chemotherapy Contribute to Muscle Loss by Activating Common Signaling Pathways. Front. Physiol. 2016, 19, 472. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.; Waning, D.L.; Gao, H.; Liu, Y.; Zimmers, T.A.; Bonetto, A. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 2016, 7, 43442–43460. [Google Scholar] [CrossRef] [PubMed]
- Kadam, P.; Ntemou, E.; Baert, Y.; Van Laere, S.; Van Saen, D.; Goossens, E. Co-transplantation of mesenchymal stem cells improves spermatogonial stem cell transplantation efficiency in mice. Stem Cell Res. Ther. 2018, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Bhartiya, D.; Sriraman, K.; Mallick, A. Underlying Mechanisms that Restore Spermatogenesis on Transplanting Healthy Niche Cells in Busulphan Treated Mouse Testis. Stem Cell Rev. Rep. 2016, 12, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.H.; El-Din, E.Y.S.; El-Dakdoky, M.H.; Ahmed, T.A. The impact of mesenchymal stem cells on doxorubicin-induced testicular toxicity and progeny outcome of male prepubertal rats. Birth Defects Res. 2019, 111, 906–919. [Google Scholar] [CrossRef]
- Erma Safitri, H.P. Effectiveness of mesenchymal stem cells cultured under hypoxia to increase the fertility rate in rats (Rattus norvegicus). Vet. World 2021, 14, 3056–3064. [Google Scholar] [CrossRef]
- Ibrahim, D.; Abozied, N.; Maboud, S.A.; Alzamami, A.; Alturki, N.A.; Jaremko, M.; Alanazi, M.K.; Alhuthali, H.M.; Seddek, A. Therapeutic potential of bone marrow mesenchymal stem cells in cyclophosphamide-induced infertility. Front. Pharmacol. 2023, 14, 1122175. [Google Scholar] [CrossRef]
- Cetinkaya-Un, B.; Un, B.; Akpolat, M.; Andic, F.; Yazir, Y. Human Amnion Membrane-Derived Mesenchymal Stem Cells and Conditioned Medium Can Ameliorate X-Irradiation-Induced Testicular Injury by Reducing Endoplasmic Reticulum Stress and Apoptosis. Reprod. Sci. 2022, 29, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Abd El Dayem, S.M.; Arafa, M.M.; Foda, F.M.; Helal, M.A.; Zahra, F.A.; Haggag, N.Z. Role of bone marrow mesenchymal stem cells (MSCs) on restoration of fertility in male rats after exposure to endocrine disrupter. Int. J. Pharm. Rev. Res. 2015, 5, 158–169. [Google Scholar]
- AbdRabou, M.A.; Mehany, A.; Farrag, I.M.; Belal, A.; Abdelzaher, O.F.; El-Sharkawy, A.; El-Azez, A.; Asmaa, M.; EL-Sharkawy, S.M.; Al Badawi, M.H. Therapeutic Effect of Murine Bone Marrow-Derived Mesenchymal Stromal/Stem Cells and Human Placental Extract on Testicular Toxicity Resulting from Doxorubicin in Rats. Biomed. Res. Int. 2021, 2021, 9979670. [Google Scholar] [CrossRef] [PubMed]
- El-Fiky, B.A. Fertility in induced azoospermic mice. J. Biosci. Appl. Res. 2016, 2, 626–633. [Google Scholar] [CrossRef]
- Keivan, M.; Torghabeh, F.M.; Davoodi, S.; Maryamneghari, S.M.; Dadfar, R. Single intratesticular injection of blood-serum-derived exosomes can potentially alleviate testopathy following testicular torsion. Animal Model. Exp. Med. 2022, 5, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Fan, X.; Hao, R.; Dong, L.; Xue, M.; Tan, L.; Yang, C.; Li, X.; Ren, X. Human embryonic stem cell-derived extracellular vesicles alleviate retinal degeneration by upregulating Oct4 to promote retinal Müller cell retrodifferentiation via HSP90. Stem Cell Res. Ther. 2021, 12, 21. [Google Scholar] [CrossRef]
- Hers, I.; Vincent, E.E.; Tavare, J.M. Akt signalling in health and disease. Cell Signal 2011, 23, 115–127. [Google Scholar] [CrossRef]
- Iqubal, A.; Iqubal, M.K.; Sharma, S.; Ansari, M.A.; Najmi, A.K.; Ali, S.M.; Ali, J.; Haque, S.E. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci. 2019, 218, 112–131. [Google Scholar] [CrossRef]
- Zhang, B.F.; Hu, Y.; Liu, X.; Cheng, Z.; Lei, Y.; Liu, Y.; Zhao, X.; Mu, M.; Yu, L.; Cheng, M.L. The role of AKT and FOXO3 in preventing ovarian toxicity induced by cyclophosphamide. PLoS ONE 2018, 13, e0201136. [Google Scholar] [CrossRef]
- Ng, D.C.; Bogoyevitch, M.A. The mechanism of heat shock activation of ERK mitogen-activated protein kinases in the interleukin 3-dependent ProB cell line BaF3. J. Biol. Chem. 2000, 275, 40856–40866. [Google Scholar] [CrossRef]
- Li, M.W.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int. J. Biochem. Cell Biol. 2009, 11, 2302–2314. [Google Scholar] [CrossRef] [PubMed]
- Salas-Ramirez, K.Y.; Bagnall, C.; Frias, L.; AAbdali, S.; Ahles, T.A.; Hubbard, K. Doxorubicin and cyclophosphamide induce cognitive dysfunction and activate the ERK and AKT signaling pathways. Behav. Brain Res. 2015, 292, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.P.; Yang, X.M.; Duan, Z.H.; Luo, P.; Shang, J.H.; Xiao, W.; Tao, Y.X.; Zhang, D.Y.; Zhang, Y.B.; Liu, H.Z. Inhibition of chemotherapy-induced apoptosis of testicular cells by squid ink polysaccharide. Exp. Ther. Med. 2017, 14, 5889–5895. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, H.-S.; Chugh, R.M.; Elsharoud, A.; Ulin, M.; Esfandyari, S.; Aboalsoud, A.; Bakir, L.; Al-Hendy, A. Safety of Intraovarian Injection of Human Mesenchymal Stem Cells in a Premature Ovarian Insufficiency Mouse Model. Cell Transpl. 2021, 30, 963689720988502. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liakath Ali, F.; Park, H.-S.; Beckman, A.; Eddy, A.C.; Alkhrait, S.; Ghasroldasht, M.M.; Al-Hendy, A.; Raheem, O. Fertility Protection, A Novel Concept: Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Protect against Chemotherapy-Induced Testicular Cytotoxicity. Int. J. Mol. Sci. 2024, 25, 60. https://doi.org/10.3390/ijms25010060
Liakath Ali F, Park H-S, Beckman A, Eddy AC, Alkhrait S, Ghasroldasht MM, Al-Hendy A, Raheem O. Fertility Protection, A Novel Concept: Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Protect against Chemotherapy-Induced Testicular Cytotoxicity. International Journal of Molecular Sciences. 2024; 25(1):60. https://doi.org/10.3390/ijms25010060
Chicago/Turabian StyleLiakath Ali, Farzana, Hang-Soo Park, Analea Beckman, Adrian C. Eddy, Samar Alkhrait, Mohammad Mousaei Ghasroldasht, Ayman Al-Hendy, and Omer Raheem. 2024. "Fertility Protection, A Novel Concept: Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Protect against Chemotherapy-Induced Testicular Cytotoxicity" International Journal of Molecular Sciences 25, no. 1: 60. https://doi.org/10.3390/ijms25010060
APA StyleLiakath Ali, F., Park, H.-S., Beckman, A., Eddy, A. C., Alkhrait, S., Ghasroldasht, M. M., Al-Hendy, A., & Raheem, O. (2024). Fertility Protection, A Novel Concept: Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Protect against Chemotherapy-Induced Testicular Cytotoxicity. International Journal of Molecular Sciences, 25(1), 60. https://doi.org/10.3390/ijms25010060






