Synthesis and Characterization of Poly(Butylene Sebacate-Co-Terephthalate) Copolyesters with Pentaerythritol as Branching Agent
Abstract
:1. Introduction
2. Results
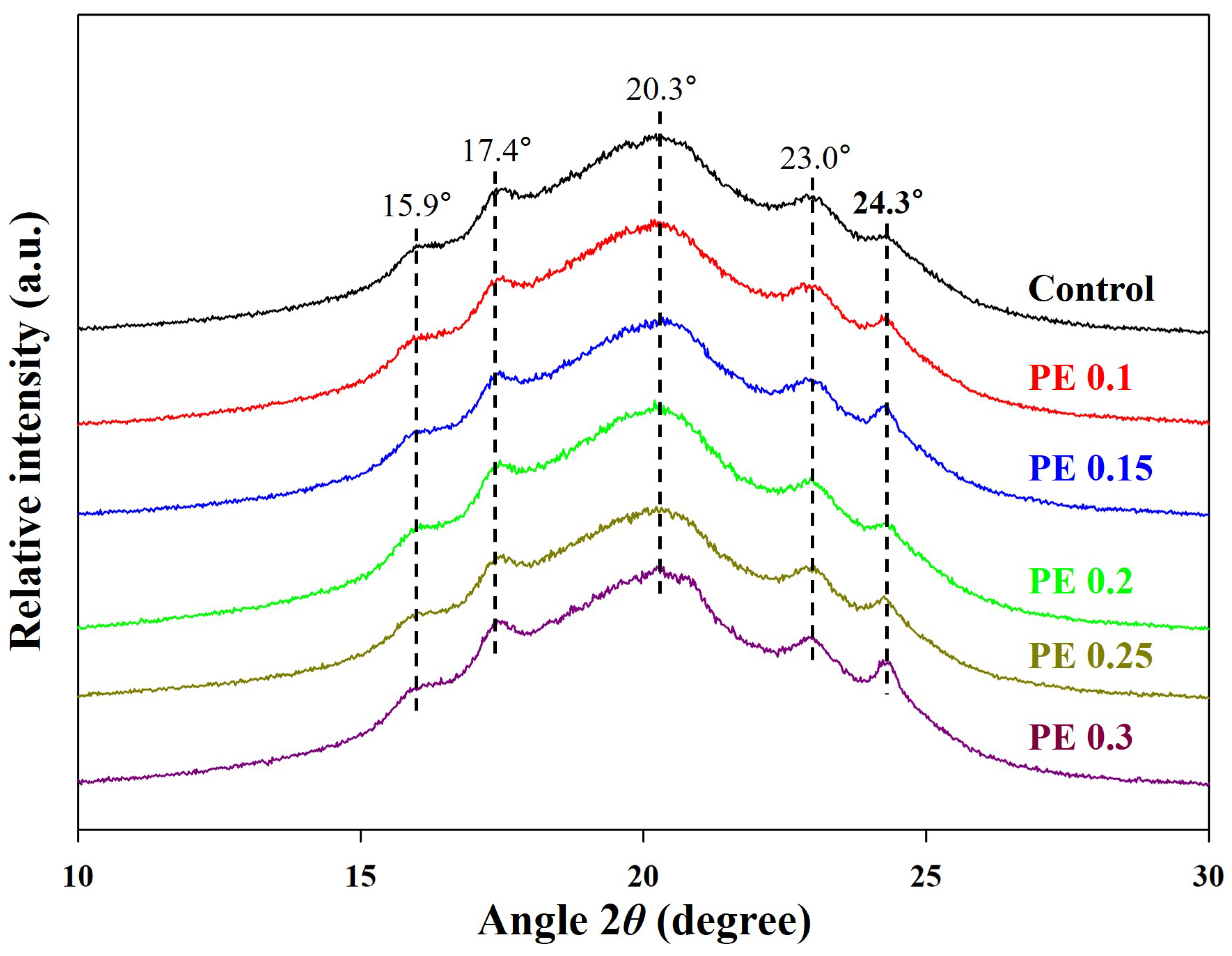
2.1. Synthesis and Structural Characterization of PBSeT
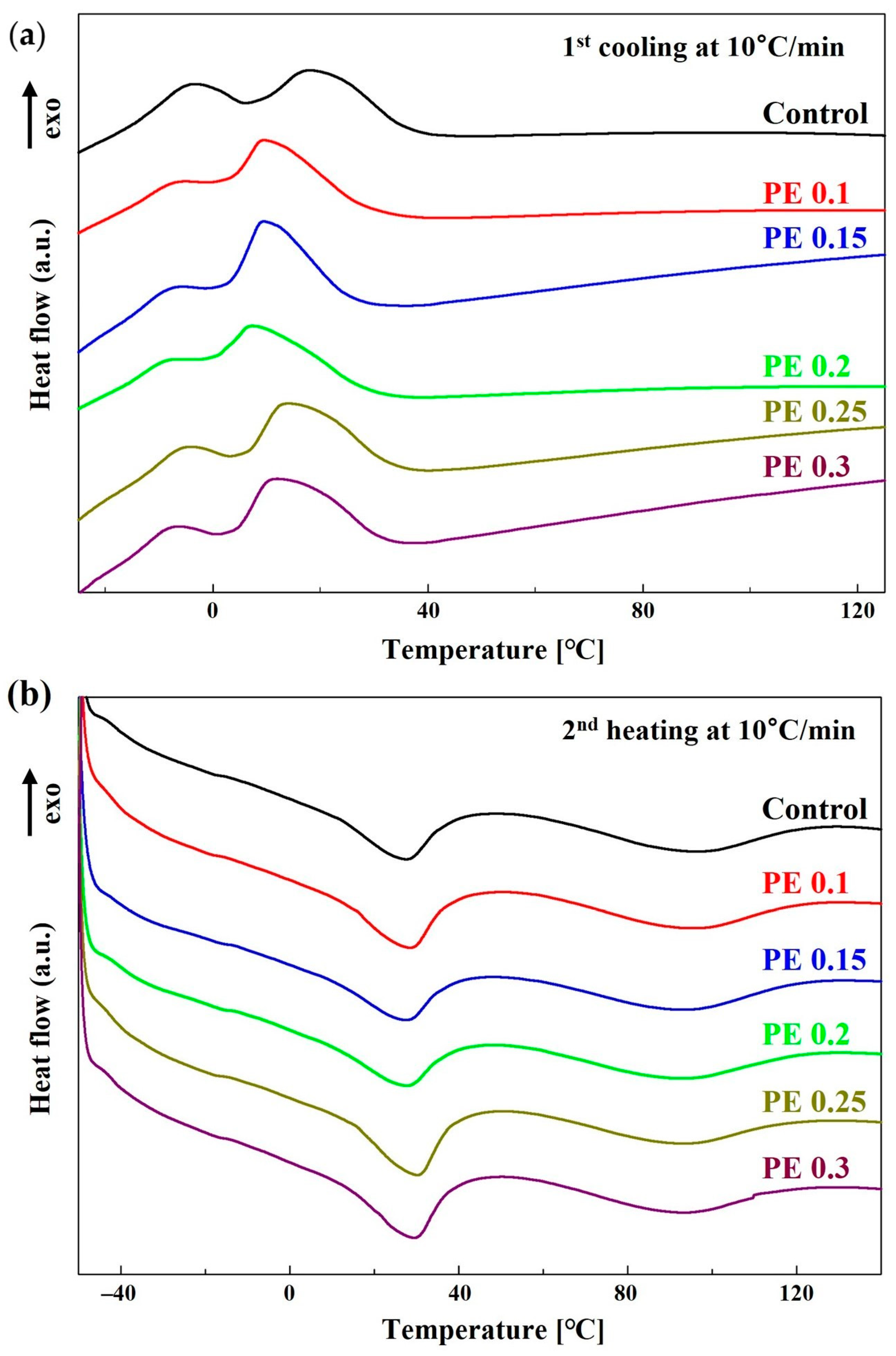
2.2. Thermal Transition of PBSeT Copolyesters
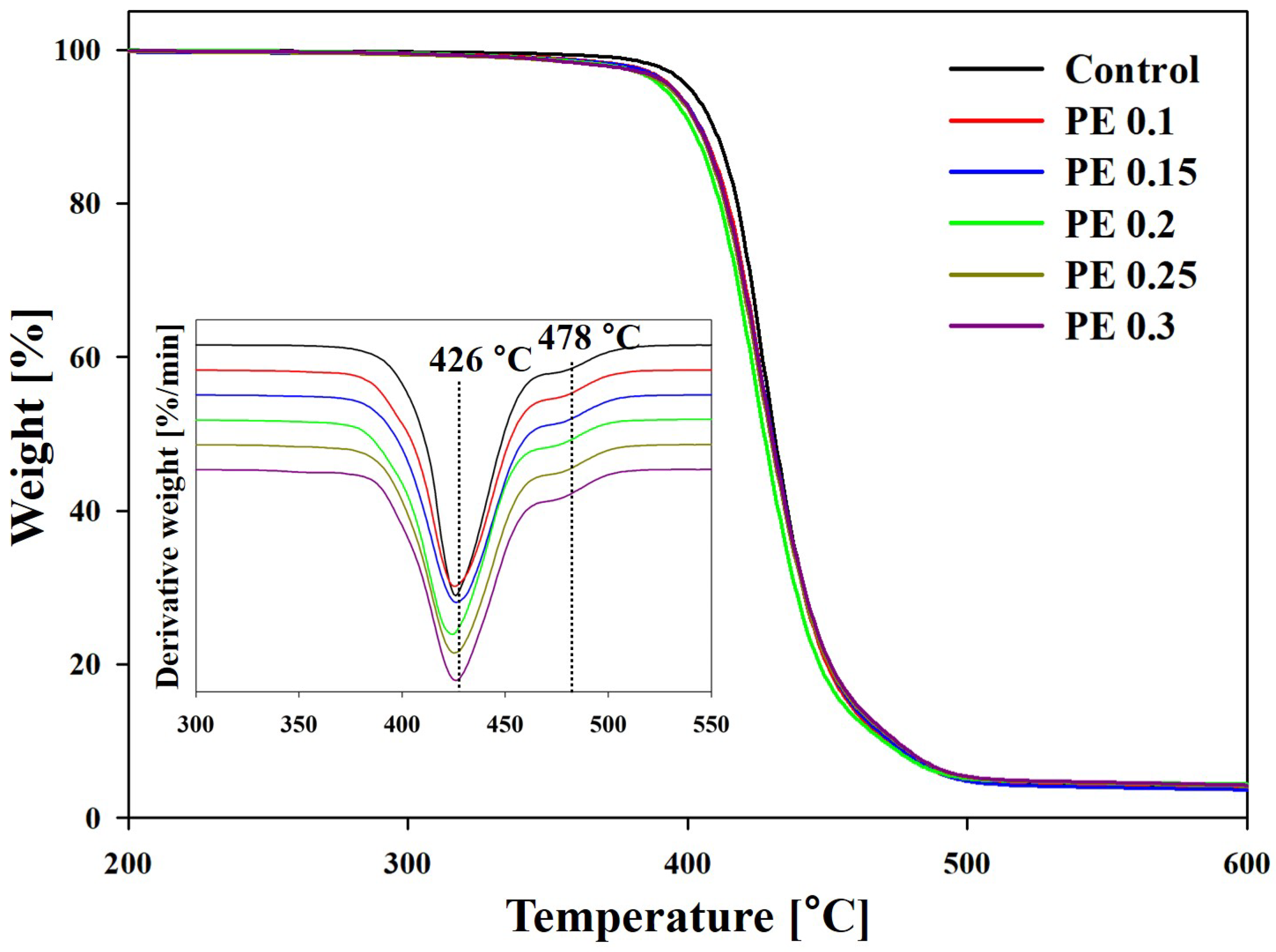
2.3. Thermal Stability of PBSeT Copolyesters
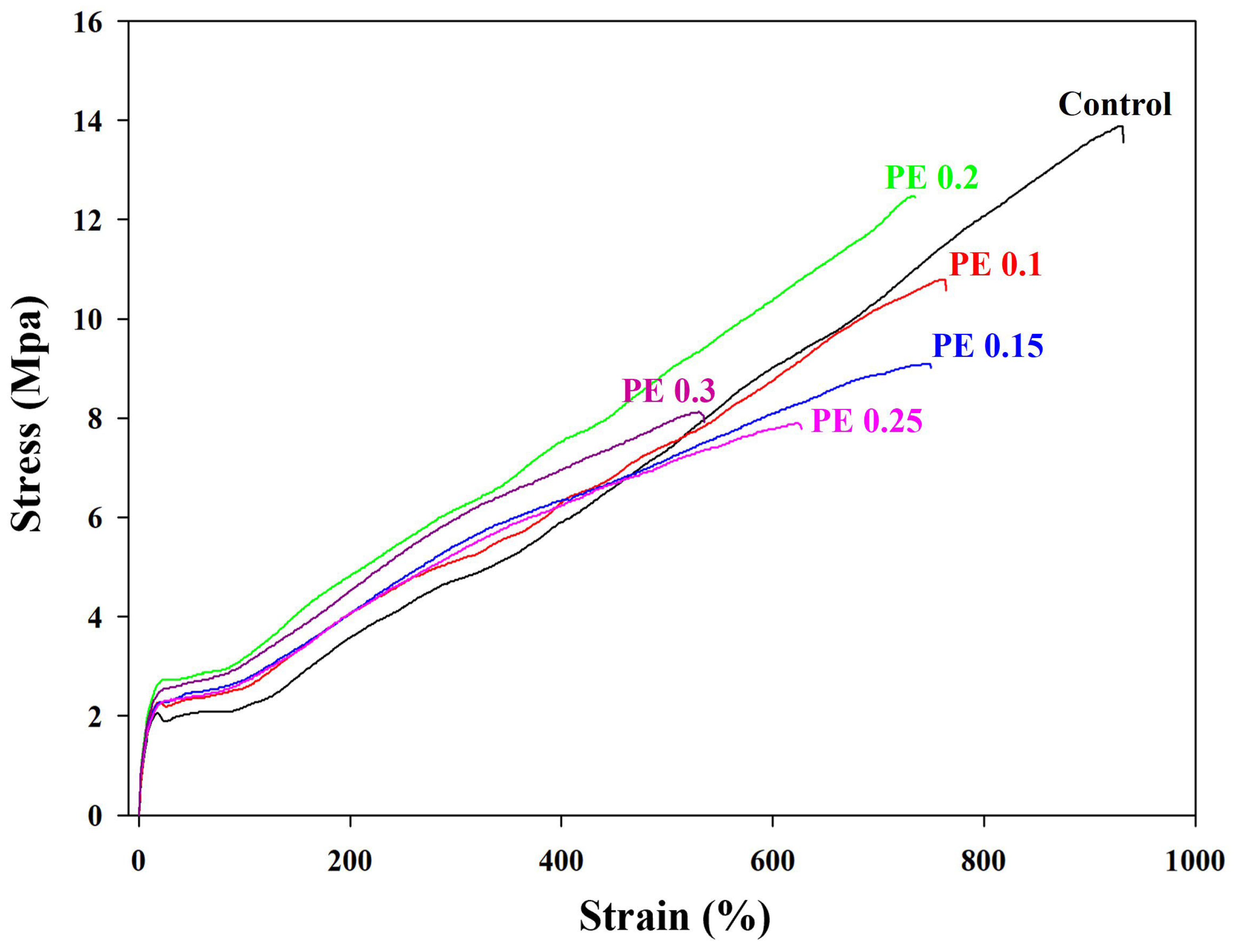
2.4. Mechanical Properties of Branched PBSeT Copolyesters
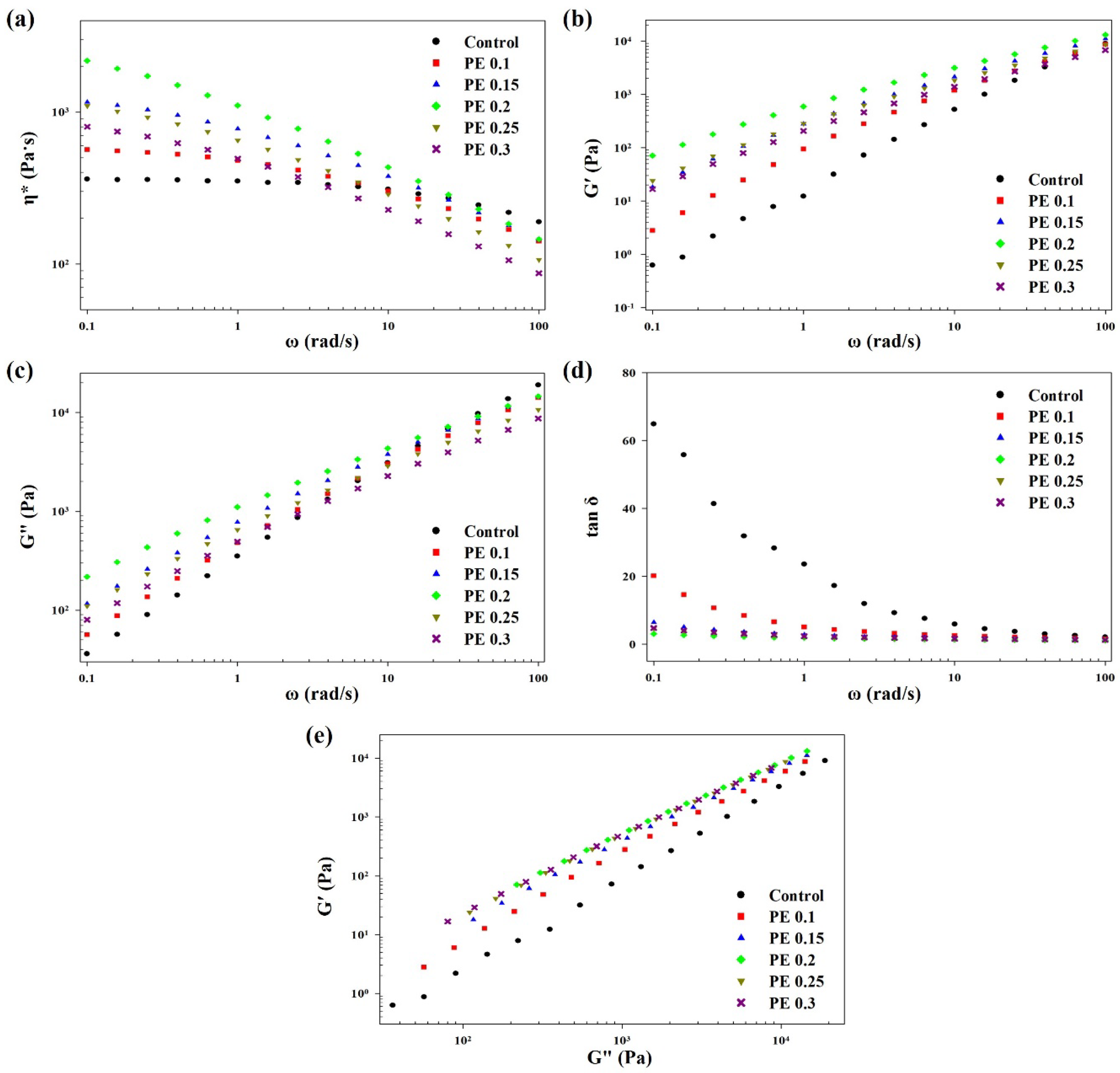
2.5. Rheological Properties of the Melts of PBSeT Copolyesters
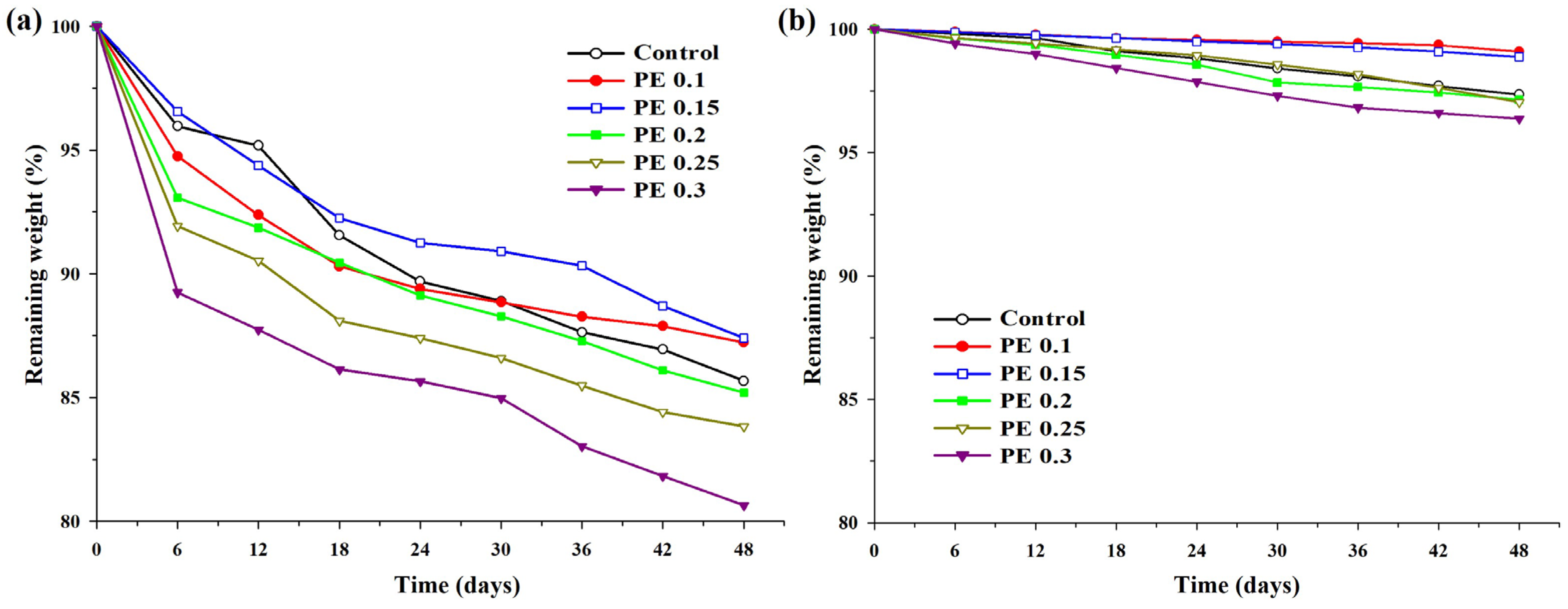
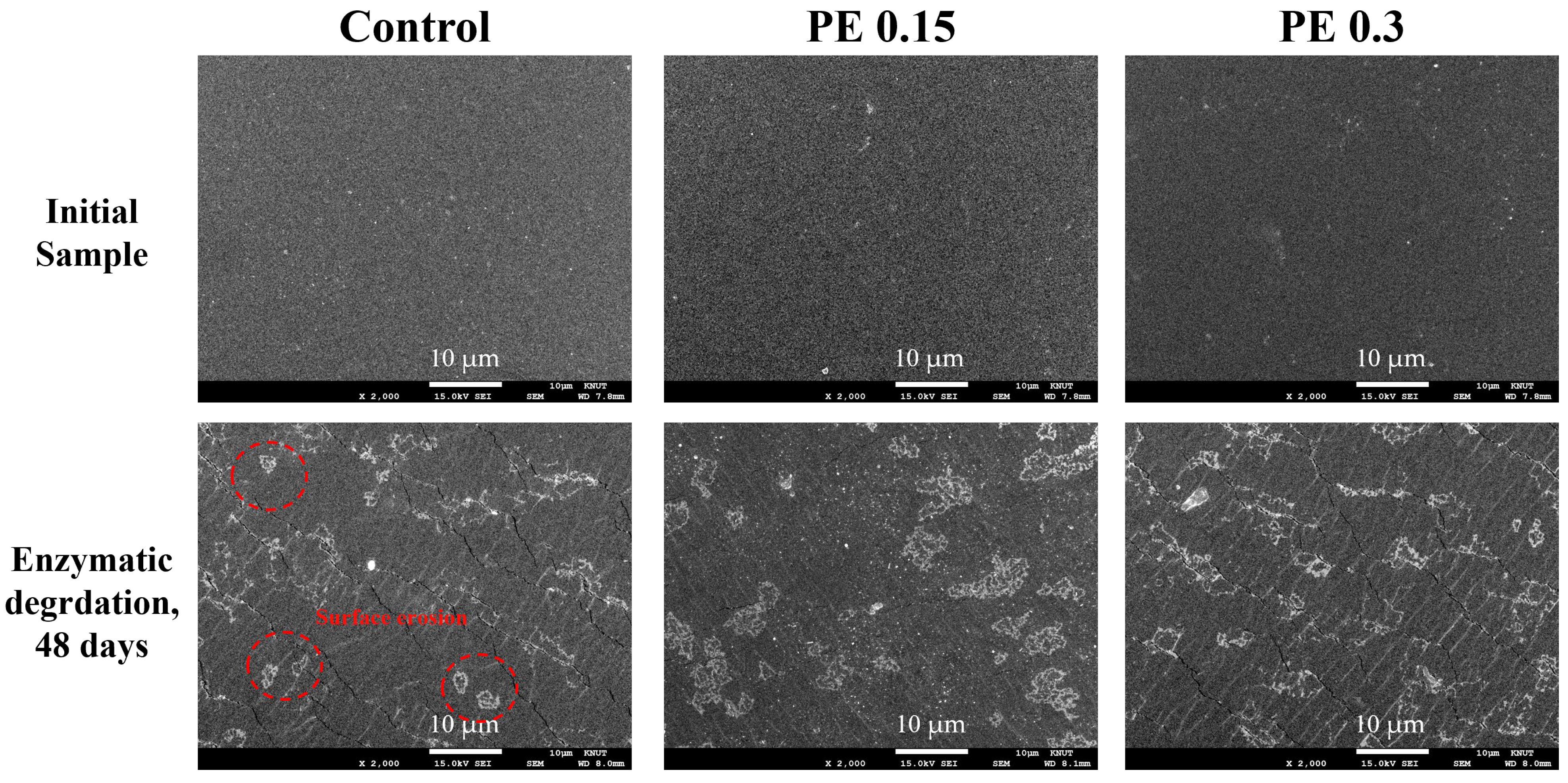
2.6. Enzymatic Degradation Test of PBSeT Copolyesters
3. Materials and Methods
3.1. Materials
3.2. Synthesis of PBSeT Copolyesters
3.3. Characterization of PBSeT Copolyesters
3.4. Enzymatic Degradation Test of PBSeT Copolyesters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Guo, B.-H. Poly(Butylene Succinate) and Its Copolymers: Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhuang, X.; Tang, Z.; Chen, X. Polylactic Acid (PLA): Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Thomas, N.L. Blending Poly(Butylene Succinate) with Poly(Lactic Acid): Ductility and Phase Inversion Effects. Eur. Polym. J. 2015, 71, 534–546. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Arrieta, M.P.; López-Martínez, J.; Samper, M.D. Improvement of PLA Film Ductility by Plasticization with Epoxidized Karanja Oil. Polym. Degrad. Stab. 2020, 179, 109259. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, L.; Chen, L.; Yan, Y.; Wang, X. Study on Biodegradable Aromatic/Aliphatic Copolyesters. Braz. J. Chem. Eng. 2008, 25, 321–335. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, S.W.; Lee, K.H. Properties of Potentially Biodegradable Copolyesters of (Succinic Acid–1,4-Butanediol)/(Dimethyl Terephthalate–1,4-Butanediol). Polym. Int. 1999, 48, 861–867. [Google Scholar] [CrossRef]
- Touchaleaume, F.; Martin-Closas, L.; Angellier-Coussy, H.; Chevillard, A.; Cesar, G.; Gontard, N.; Gastaldi, E. Performance and Environmental Impact of Biodegradable Polymers as Agricultural Mulching Films. Chemosphere 2016, 144, 433–439. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwak, H.W.; Kwon, S.; Jang, H.; Park, S. Synthesis, Characterization and Properties of Biodegradable Poly(Butylene Sebacate-Co-Terephthalate). Polymers 2020, 12, 2389. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Lei, H.; Feng, Y.; Wang, W.; Li, J.; Ding, T.; Yuan, B. The Effect of Synergistic/Inhibitory Mechanism of Terephthalic Acid and Glycerol on the Puncture, Tearing, and Degradation Properties of PBSeT Copolyesters. Adv. Compos. Hybrid. Mater. 2022, 5, 1335–1349. [Google Scholar] [CrossRef]
- Jang, H.; Kwon, S.; Kim, S.J.; Park, S. Maleic Anhydride-Grafted PLA Preparation and Characteristics of Compatibilized PLA/PBSeT Blend Films. Int. J. Mol. Sci. 2022, 23, 7166. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kwak, H.W.; Kwon, S.; Jang, H.; Park, S. Characterization of PLA/PBSeT Blends Prepared with Various Hexamethylene Diisocyanate Contents. Materials 2021, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fang, H.; Bai, J.; Zhang, Y.; Wang, Z. Preparation and Characterization of High-Melt-Strength Polylactide with Long-Chain Branched Structure through γ-Radiation-Induced Chemical Reactions. Ind. Eng. Chem. Res. 2014, 53, 1150–1159. [Google Scholar] [CrossRef]
- Lu, J.; Wu, L.; Li, B.-G. Long Chain Branched Poly(Butylene Succinate-Co-Terephthalate) Copolyesters Using Pentaerythritol as Branching Agent: Synthesis, Thermo-Mechanical, and Rheological Properties. J. Appl. Polym. Sci. 2017, 134, 44544. [Google Scholar] [CrossRef]
- Liu, M.-J.; Liu, M.-J.; Chen, S.-C.; Yang, K.-K.; Wang, Y.-Z. Biodegradable Polylactide Based Materials with Improved Crystallinity, Mechanical Properties and Rheological Behaviour by Introducing a Long-Chain Branched Copolymer. RSC Adv. 2015, 5, 42162–42173. [Google Scholar] [CrossRef]
- Ojijo, V.; Ray, S.S. Super Toughened Biodegradable Polylactide Blends with Non-Linear Copolymer Interfacial Architecture Obtained via Facile in-Situ Reactive Compatibilization. Polymer 2015, 80, 1–17. [Google Scholar] [CrossRef]
- Chen, C.-W.; Mao, H.-I.; Yang, Z.-Y.; Huang, K.-W.; Yan, H.-C.; Rwei, S.-P. Synthesis of Bio-Based Poly(Butylene Adipate-Co-Butylene Itaconate) Copolyesters with Pentaerythritol: A Thermal, Mechanical, Rheological, and Molecular Dynamics Simulation Study. Polymers 2020, 12, 2006. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Y.; Liao, X.; Yang, Q. The Molecular Structure Characteristics of Long Chain Branched Polypropylene and Its Effects on Non-Isothermal Crystallization and Mechanical Properties. Polymers 2013, 54, 1455–1462. [Google Scholar] [CrossRef]
- Wood-Adams, P.M.; Dealy, J.M.; de Groot, A.W.; Redwine, O.D. Effect of Molecular Structure on the Linear Viscoelastic Behavior of Polyethylene. Macromolecules 2000, 33, 7489–7499. [Google Scholar] [CrossRef]
- Hudson, N.; MacDonald, W.A.; Neilson, A.; Richards, R.W.; Sherrington, D.C. Synthesis and Characterization of Nonlinear PETs Produced via a Balance of Branching and End-Capping. Macromolecules 2000, 33, 9255–9261. [Google Scholar] [CrossRef]
- Chan, H.-W.; Cho, C.-J.; Hsu, K.-H.; He, C.-L.; Kuo, C.-C.; Chu, C.-C.; Chen, Y.-H.; Chen, C.-W.; Rwei, S.-P. Smart Wearable Textiles with Breathable Properties and Repeatable Shaping in In Vitro Orthopedic Support from a Novel Biomass Thermoplastic Copolyester. Macromol. Mater. Eng. 2019, 304, 1900103. [Google Scholar] [CrossRef]
- Liu, J.; Lou, L.; Yu, W.; Liao, R.; Li, R.; Zhou, C. Long Chain Branching Polylactide: Structures and Properties. Polymer 2010, 51, 5186–5197. [Google Scholar] [CrossRef]
- Wu, D.; Xu, R.; Zhang, Y.; Shi, S. Influence of Branching on the Mechanical, Rheological and Crystallization Properties of Poly(Butylene Adipate-Co-Terephthalate). J. Therm. Anal. Calorim. 2022, 147, 9539–9557. [Google Scholar] [CrossRef]
- Kim, E.K.; Bae, J.S.; Im, S.S.; Kim, B.C.; Han, Y.K. Preparation and Properties of Branched Polybutylenesuccinate. J. Appl. Polym. Sci. 2001, 80, 1388–1394. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Dong, X.; Wang, W.; Zhu, Y.-C.; Murugadoss, V.; Song, G.; Naik, N.; Pan, D.; Guo, Z. Synthesis, Characterization and Properties of Poly(Butanediol Sebacate–Butanediol Terephthalate) (PBSeT) Copolyesters Using Glycerol as Cross-Linking Agent. Mater. Today Commun. 2021, 28, 102557. [Google Scholar] [CrossRef]
- Chen, C.-W.; Hsu, T.-S.; Huang, K.-W.; Rwei, S.-P. Effect of 1,2,4,5-Benzenetetracarboxylic Acid on Unsaturated Poly(Butylene Adipate-Co-Butylene Itaconate) Copolyesters: Synthesis, Non-Isothermal Crystallization Kinetics, Thermal and Mechanical Properties. Polymers 2020, 12, 1160. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R.; Behnken, G. Biodegradable Hyperbranched Aliphatic Polyesters Derived from Pentaerythritol. Macromolecules 2008, 41, 5651–5657. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, L.; Bu, Z.; Li, B.-G.; Li, N.; Dai, J. Synthesis and Thermomechanical and Rheological Properties of Biodegradable Long-Chain Branched Poly(Butylene Succinate-Co-Butylene Terephthalate) Copolyesters. Ind. Eng. Chem. Res. 2014, 53, 10380–10386. [Google Scholar] [CrossRef]
- Xu, M.; Lu, J.; Zhao, J.; Wei, L.; Liu, T.; Zhao, L.; Park, C.B. Rheological and Foaming Behaviors of Long-Chain Branched Polyamide 6 with Controlled Branch Length. Polymer 2021, 224, 123730. [Google Scholar] [CrossRef]
- McKee, M.G.; Unal, S.; Wilkes, G.L.; Long, T.E. Branched Polyesters: Recent Advances in Synthesis and Performance. Prog. Polym. Sci. 2005, 30, 507–539. [Google Scholar] [CrossRef]
- Wang, L.; Jing, X.; Cheng, H.; Hu, X.; Yang, L.; Huang, Y. Rheology and Crystallization of Long-Chain Branched Poly(l-Lactide)s with Controlled Branch Length. Ind. Eng. Chem. Res. 2012, 51, 10731–10741. [Google Scholar] [CrossRef]
- Acierno, S.; van Puyvelde, P. Effect of Short Chain Branching upon the Crystallization of Model Polyamides-11. Polymer 2005, 46, 10331–10338. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Ye, X.; Li, Z.; Wang, W.; Liu, T.; Azab, I.H.l; Mersal, G.A.M.; Ibrahim, M.M.; El-Bahy, Z.M.; et al. Synthesis and Characterization of 2,5-Furandicarboxylic Acid Poly(Butanediol Sebacate-Butanediol) Terephthalate (PBSeT) Segment Copolyesters with Excellent Water Vapor Barrier and Good Mechanical Properties. J. Mater. Sci. 2022, 57, 10997–11012. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; He, M.; Wang, W.; Li, J. Effects of the Species of Crosslinking Reagents on the Structures and Properties of Biodegradable Poly (Butanediol Sebacate—Butanediol Terephthalate) Copolyester. J. Appl. Polym. Sci. 2022, 139, 52145. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Jiang, T.; Xi, S.; Li, Y.; Guo, J.; Huang, M.; Algadi, H.; Ye, X.; Jiang, Q. Improvement of Thermodynamic Properties of Poly(Butanediol Sebacate-Butanediol Terephthalate) (PBSeT) Composites Based on the Dispersion of PCaCO3@tannic Acid Formed by Complexation of Tannic Acid and Ti. Adv. Compos. Hybrid. Mater. 2022, 5, 2787–2800. [Google Scholar] [CrossRef]
- Liu, G.C.; Zhang, W.Q.; Zhou, S.L.; Wang, X.L.; Wang, Y.Z. Improving Crystallization and Processability of PBS: Via Slight Cross-Linking. RSC Adv. 2016, 6, 68942–68951. [Google Scholar] [CrossRef]
- Jordens, K.; Wilkes, G.L.; Janzen, J.; Rohlfing, D.C.; Welch, M.B. The Influence of Molecular Weight and Thermal History on the Thermal, Rheological, and Mechanical Properties of Metallocene-Catalyzed Linear Polyethylenes. Polymer 2000, 41, 7175–7192. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Liu, T.; Ye, X.; He, M.; Zhao, L.; Li, H.; Ren, J.; Algadi, H.; Li, Y.; et al. Interfacial Interaction Enhancement between Biodegradable Poly (Butylene Adipate-Co-Terephthalate) and Microcrystalline Cellulose Based on Covalent Bond for Improving Puncture, Tearing, and Enzymatic Degradation Properties. Adv. Compos. Hybrid. Mater. 2023, 6, 69. [Google Scholar] [CrossRef]
- Cai, W.; Liu, L. Shape-Memory Effect of Poly (Glycerol–Sebacate) Elastomer. Mater. Lett. 2008, 62, 2171–2173. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, M.; Ding, T.; Shi, R.; Zhang, L. Preparation and Characterization of a Biodegradable Polyester Elastomer with Thermal Processing Abilities. J. Appl. Polym. Sci. 2005, 98, 2033–2041. [Google Scholar] [CrossRef]
- Pfefferkorn, D.; Pulst, M.; Naolou, T.; Busse, K.; Balko, J.; Kressler, J. Crystallization and Melting of Poly(Glycerol Adipate)-Based Graft Copolymers with Single and Double Crystallizable Side Chains. J. Polym. Sci. B Polym. Phys. 2013, 51, 1581–1591. [Google Scholar] [CrossRef]
- Münstedt, H. Rheological Properties and Molecular Structure of Polymer Melts. Soft Matter 2011, 7, 2273–2283. [Google Scholar] [CrossRef]
- Tian, J.; Yu, W.; Zhou, C. The Preparation and Rheology Characterization of Long Chain Branching Polypropylene. Polymer 2006, 47, 7962–7969. [Google Scholar] [CrossRef]
- Chuang, H.-K.; Han, C.D. Rheological Behavior of Polymer Blends. J. Appl. Polym. Sci. 1984, 29, 2205–2229. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Qi, J.; Wu, J.; Chen, J.; Wang, H. An Investigation of the Thermal and (Bio)Degradability of PBS Copolyesters Based on Isosorbide. Polym. Degrad. Stab. 2019, 160, 229–241. [Google Scholar] [CrossRef]
- Billmeyer, F.W., Jr. Methods for Estimating Intrinsic Viscosity. J. Polym. Sci. 1949, 4, 83–86. [Google Scholar] [CrossRef]
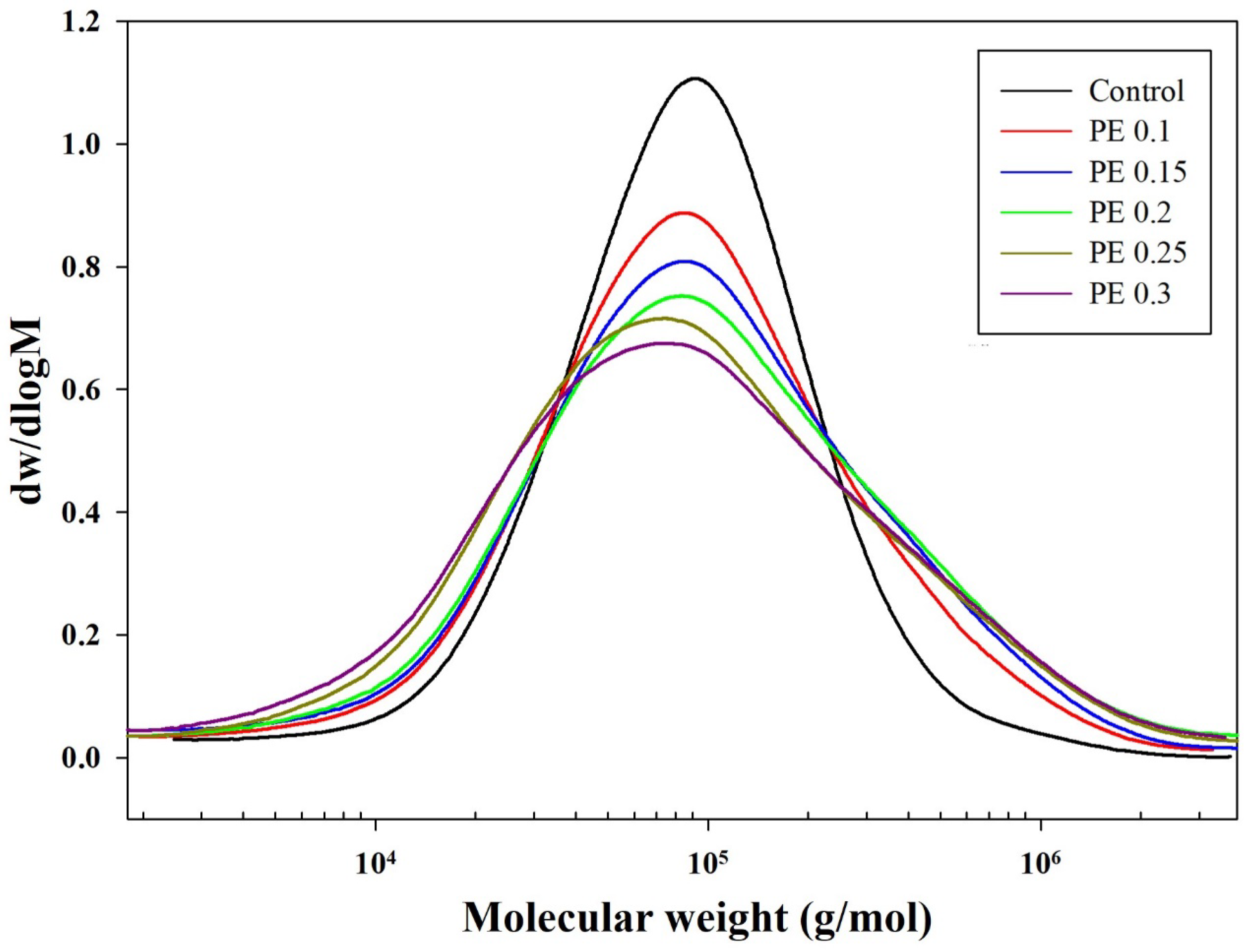
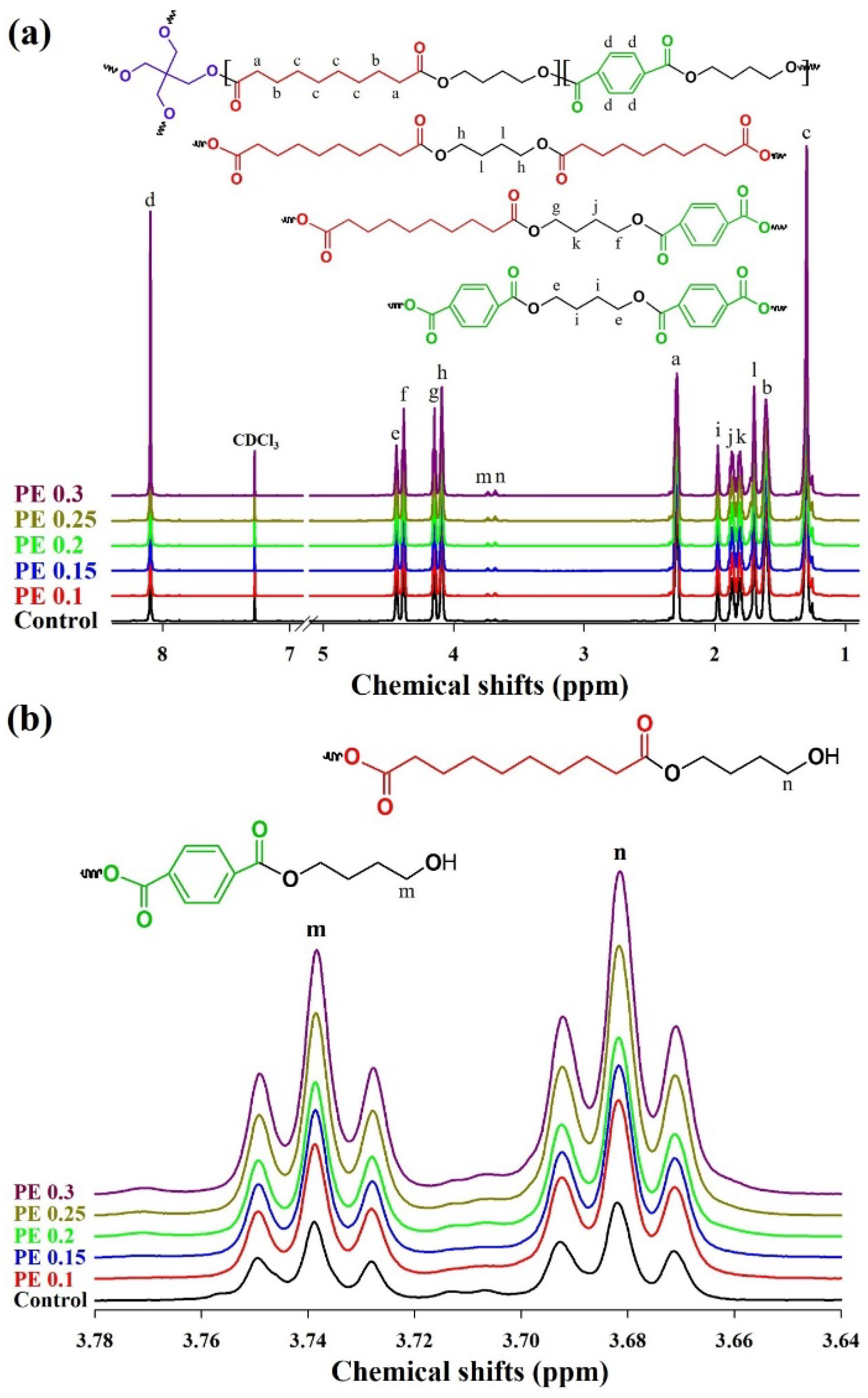


| Sample | ϕPE (%) b | tes (min) c | tpc (min) d | 1H NMR | [η] (dL/g) e | GPC | MFI h (g/10 min) | ||
|---|---|---|---|---|---|---|---|---|---|
| ϕBT (%) | Mn (g/mol) f | Mw (g/mol) f | Đ g | ||||||
| Control | 0 | 110 | 360 | 42.7 | 1.19 | 50,500 | 143,000 | 2.84 | 33.3 |
| PE 0.1 | 0.1 | 110 | 350 | 42.4 | 1.23 | 39,100 | 158,000 | 4.04 | 26.7 |
| PE 0.15 | 0.15 | 110 | 325 | 42.5 | 1.30 | 37,500 | 178,000 | 4.74 | 18.7 |
| PE 0.2 | 0.2 | 110 | 285 | 42.5 | 1.58 | 37,800 | 211,000 | 5.57 | 7.3 |
| PE 0.25 | 0.25 | 110 | 255 | 42.3 | 1.42 | 32,000 | 194,000 | 6.06 | 20.1 |
| PE 0.3 | 0.3 | 110 | 250 | 42.5 | 1.32 | 29,100 | 189,000 | 6.49 | 21.7 |
| Sample | Thermal Transition (°C) | ΔHm (J/g) | ΔHc (J/g) | Xc (%) a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg | Tm1 | Tm2 | Tc1 | Tc2 | ||||||||
| Control | −41 | 28 | 95 | 18 | −3 | 6.2 | / | 9.1 | 10.3 | / | 5.5 | 13.6 |
| PE 0.1 | −42 | 29 | 94 | 10 | −5 | 7.5 | / | 9.0 | 12.0 | / | 4.0 | 14.0 |
| PE 0.15 | −41 | 28 | 93 | 9 | −6 | 5.8 | / | 9.3 | 13.0 | / | 4.0 | 15.6 |
| PE 0.2 | −40 | 28 | 93 | 8 | −8 | 5.5 | / | 9.0 | 10.8 | / | 2.6 | 13.2 |
| PE 0.25 | −42 | 29 | 93 | 14 | −4 | 8.1 | / | 7.9 | 12.1 | / | 4.3 | 15.2 |
| PE 0.3 | −42 | 29 | 93 | 12 | −7 | 8.1 | / | 8.6 | 13.4 | / | 5.6 | 17.0 |
| Sample | Tonset (°C) | T50 a (°C) | T75 (°C) | Tmax (°C) | Residue at 600 °C (%) | |
|---|---|---|---|---|---|---|
| Step 1 | Step 2 | |||||
| Control | 411 | 431 | 445 | 426 | 478 | 4.0 |
| PE 0.1 | 407 | 430 | 444 | 429 | 476 | 3.6 |
| PE 0.15 | 406 | 430 | 445 | 426 | 478 | 3.6 |
| PE 0.2 | 405 | 428 | 444 | 424 | 476 | 3.9 |
| PE 0.25 | 405 | 429 | 445 | 425 | 476 | 4.0 |
| PE 0.3 | 406 | 430 | 446 | 427 | 477 | 4.2 |
| Sample | Young’s Modulus (MPa) | Tensile Strength (MPa) | Tear Strength (N/mm) | Elongation at Break (%) |
|---|---|---|---|---|
| Control | 20.8 ± 0.6 | 14.7 ± 0.2 | 50.9 ± 1.0 | 917.6 ± 26.4 |
| PE 0.1 | 22.0 ± 0.9 | 11.5 ± 0.1 | 57.1 ± 1.7 | 756.5 ± 10.2 |
| PE 0.15 | 22.1 ± 0.9 | 8.9 ± 0.2 | 59.5 ± 1.5 | 739.2 ± 11.6 |
| PE 0.2 | 25.6 ± 0.8 | 12.2 ± 0.2 | 61.9 ± 1.1 | 725.4 ± 19.5 |
| PE 0.25 | 21.8 ± 0.9 | 8.0 ± 0.1 | 56.3 ± 1.6 | 638.9 ±11.6 |
| PE 0.3 | 24.4 ± 1.0 | 8.4 ± 0.2 | 53.3 ± 1.4 | 566.9 ± 34.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Kwon, S.; Kim, S.J.; Kim, Y.-T.; Park, S.-i. Synthesis and Characterization of Poly(Butylene Sebacate-Co-Terephthalate) Copolyesters with Pentaerythritol as Branching Agent. Int. J. Mol. Sci. 2024, 25, 55. https://doi.org/10.3390/ijms25010055
Jang H, Kwon S, Kim SJ, Kim Y-T, Park S-i. Synthesis and Characterization of Poly(Butylene Sebacate-Co-Terephthalate) Copolyesters with Pentaerythritol as Branching Agent. International Journal of Molecular Sciences. 2024; 25(1):55. https://doi.org/10.3390/ijms25010055
Chicago/Turabian StyleJang, Hyunho, Sangwoo Kwon, Sun Jong Kim, Young-Teck Kim, and Su-il Park. 2024. "Synthesis and Characterization of Poly(Butylene Sebacate-Co-Terephthalate) Copolyesters with Pentaerythritol as Branching Agent" International Journal of Molecular Sciences 25, no. 1: 55. https://doi.org/10.3390/ijms25010055
APA StyleJang, H., Kwon, S., Kim, S. J., Kim, Y.-T., & Park, S.-i. (2024). Synthesis and Characterization of Poly(Butylene Sebacate-Co-Terephthalate) Copolyesters with Pentaerythritol as Branching Agent. International Journal of Molecular Sciences, 25(1), 55. https://doi.org/10.3390/ijms25010055






