Abstract
Natural compounds continue to serve as the most fruitful source of new antimicrobials. Analysis of bacterial genomes have revealed that the biosynthetic potential of antibiotic producers by far exceeds the number of already discovered structures. However, due to the repeated discovery of known substances, it has become necessary to change both approaches to the search for antibiotics and the sources of producer strains. The pressure of natural selection and the diversity of interactions in symbiotic communities make animal microbiomes promising sources of novel substances. Here, microorganisms associated with various animals were examined in terms of their antimicrobial agents. The application of alternative cultivation techniques, ultrahigh-throughput screening, and genomic analysis facilitated the investigation of compounds produced by unique representatives of the animal microbiota. We believe that new strategies of antipathogen defense will be discovered by precisely studying cell–cell and host–microbe interactions in microbiomes in the wild.
1. Introduction
Known compound rediscovery is the major obstacle in the search for novel antibiotics from the pool of natural products. However, secondary metabolites of various microorganisms are far from depleted as a source of active compounds [1,2]. Potential improvements of systematic approaches to antibiotic discovery include (i) the development of new cultivation methods [3,4], (ii) the mining of antibiotic biosynthetic gene clusters (BGCs) of secondary metabolites followed by the activation of silent biosynthetic pathways [5,6], (iii) the creation of platforms to increase screening throughput [7,8,9,10], and (iv) the usage of less studied sources of producer strains [11,12,13].
The versatility of metabolic pathways makes microbes a fundamental component of any ecosystem [14,15]. However, their ecology remains poorly studied because of the complexity and methodological limitations [14]. Environmental conditions, including osmotic pressure, pH, temperature, limited resources, and biotic interactions, shape ecological niches [16]. Depending on the environment, microorganisms apply several basic survival strategies: outgrowing competitor strains through adaptation, mutualistic cooperation, and suppression of competitor strains [15]. Thus, the variety of ecological niches provides a variety of phenotypes, including metabolic profiles [16]. Antimicrobial production is one of the most common mechanisms of this suppression. Substances toxic to the surrounding community provide the producer with a selective advantage in fighting for limited space, light, minerals, or nutrients [15]. Nowadays, the structures of bacterial secondary metabolites represent the finest result of interactions between different species [17].
The classical source of antibiotic-producing strains is soil samples. The main discoveries of the “golden era” of antibiotics are associated with soil streptomycetes [1]. Later, bacteria from marine sediments were turned into an important source of microbiota [18,19]. At the same time, marine invertebrates were considered to be rich sources of various medicinal compounds. For a number of compounds, long after their discovery, it was revealed that the real producers of active metabolites were symbiotic microorganisms [20,21].
Microbes inhabit the organs of animals and the surface of their bodies, and in some cases, specialized organs are formed in the host organisms to contain cultures of symbionts [22]. Despite the ubiquity of free-living forms of bacteria, fungi, and archaea, most associations of microorganisms with animals turn out to be established. Since their inception, multicellular organisms have evolved together with microbial communities. The hologenomic model of animal evolution is currently being considered, in which animals act as units of natural selection together with symbiotic microflora [17,22,23]. The host animal supplies the microbial community with nutrients and ensures the relative stability of the environmental conditions. Microorganisms compete with each other and influence the host. The microbiota participates in maintaining the metabolic and immune functions of the animal, providing additional protection against pathogen invasion [22].
This review focuses on antimicrobial agents discovered through the study of animal-associated microbial communities and the enormous potential of these microbiota to produce evolutionarily optimized active compounds (Figure 1).
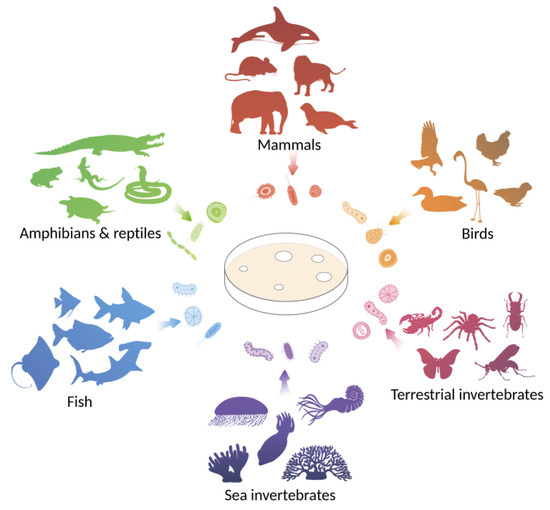
Figure 1.
Animals are considered as sources of unique communities of associated microorganisms that exhibit antagonistic activity against pathogenic strains. Sources of microorganisms considered in the review are marine invertebrates, terrestrial invertebrates, fish, amphibians and reptiles, birds, and mammals.
2. Marine Invertebrates
Historically, most natural bioactive compounds were found in terrestrial sources. This is explained by their greater availability compared to aquatic ones. However, improvements in sampling methods have revealed the huge diversity of marine biotopes [24]. The microbiome of marine invertebrates represents a highly competitive environment. The bacteria, archaea, and fungi that inhabit it are limited in nutrients, space, and light. High environmental pressure promotes the development of chemical defense strategies, including antibiotic production [24].
Most mature sponges are sessile animals that feed by filtration. The large surface of their bodies is covered with channels and pores that facilitate the accumulation of bacteria and fungi from the environment [24]. Sponges are hosts for diverse groups of bacteria and fungi, including both intracellular and extracellular symbionts. Microbiota make up 35–60% of an animal’s weight. Evolutionarily, sponges needed protection against pathogens. Symbionts able to secrete antimicrobial substances colonized healthy animals, coevolved with hosts, and gained competitive advantages [24].
Actinobacteria are classically considered to be the richest source of secondary metabolites. Their potential is well known from soil samples. Adaptation to the specific environment has made actinobacteria from marine sponges physiologically and genetically distinct from terrestrial species [13,25]. Streptomonospora sp. PA3 isolated from a sponge from the Persian Gulf showed inhibitory activity against P. aeruginosa and S. aureus [25]. Despite the smaller number of secondary metabolite biosynthesis clusters in the genome compared to terrestrial species, a novel antibiotic was identified among the strain’s metabolites—persiamycin A [25]. A wide range of biologically active compounds, including manzamines active against Mycobacterium tuberculosis and parasitic protists, have been obtained from Indonesian sponges [5]. Strains producing manzamines were described only later; for example, in the sponge Acanthostrongylophora ingens, the production of manzamines was provided by the actinomycete Micromonospora sp. [26,27]. Eight new antifungal compounds, antimycins I–P, were discovered in the culture of a sponge-associated strain of Streptomyces sp. NBU3104 [28]. Antimycin I showed high inhibitory activity against C. albicans and the phytopathogenic fungi Penicillium expansum, Penicillium citrinum, and Botrytis cinerea, while six rare acetylated antimycins and deformylated antimycin O are of interest from their structural point of view [28]. The application of novel cultivation methods, such as ichip, provides novel sponge-associated strains and compounds [29]. A new Alteromonas strain was derived from Xestospongia muta by employing the isolation chip into the sponge. The strain exhibited activity against Staphylococci and Enterococcus faecium mediated by a novel N-acyltyrosine [29].
In some sponges, the main contribution to the antibiotic activity of the symbiont community is made not by actinobacteria but by representatives of the genera Pseudovibrio, Vibrio, Bacillus, and others [24,30,31,32,33]. An unusual example of the main active metabolite produced by a bacterium of different genera is kocurin, a new member of the thiazolyl peptide family. Kocurin was isolated from the symbiont Kocuria palustris of the sponge genus Xestospongia. This substance was active against Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA), E. faecium, and B. subtilis [34,35]. In addition to K. palustris, kocurin was detected in isolates of the sponge symbionts K. marina and Micrococcus yunnanensis [34]. The beta-carboline alkaloid norharmane, previously discovered in land plants, was found to be produced by Pseudoalteromonas piscicida, a symbiont of the sponge Hymeniacidon perlevis [36].
The sponge-associated strain identified as Pseudomonas sp. (Pseudomonas fulva was the closest relative strain) produced three high-molecular-weight peptides and two first-discovered alpha-pyrones (I and II) [37]. The more active alpha-pyrone I inhibited the growth of B. subtilis, S. aureus (MRSA), M. catarrhalis, and E. faecium, selectively affecting the membrane transport function of Gram-positive bacteria [37]. Bacillus strains with antagonistic properties against human pathogens have been repeatedly isolated from sponges [30,31,32]. Moreover, in some cases, antimicrobial activity was mediated by new antibiotics. Previously unknown thiopeptides YM-266183 and YM-266184 were isolated from Bacillus cereus, which are bacteria from the sponge Halichondria japonica [30]. In other cases, the antibiotic properties were provided by a set of metabolites characteristic of terrestrial species. For example, B. pumilus and B. subtilis strains obtained from the sponge Aplysina aerophoba were able to inhibit the growth of S. aureus, E. coli, C. albicans, and S. epidermidis, and this activity was provided by surfactins, fengycins, iturins, and lanthipeptides, such as subtilin and mersacidin [31]. Aplysina aerophoba is also known for the red pigment heptyl prodigiosin, which has antibiotic activity against S. aureus. In the sponge microbiome, it was produced by the gamma-proteobacterium Pseudovibrio denitrificans [33].
Freshwater sponges could also be an interesting and yet insufficiently studied source of strains [35]. Metagenomic analysis revealed that freshwater sponge microbiota was specific to their hosts and shared a number of genomic similarities with marine-sponge-associated bacteria. These similarities included defensive protein genes. We propose that the microbiota of freshwater sponges participates in the host’s defense against infection and is a prominent source of antimicrobials [38].
Coral polyps [39], mollusks [40], and echinoderms [41] also represent rich sources of antimicrobials. Lobophorin K is a cytotoxic antibiotic from the coral polyp Lophelia pertusa showing moderate and selective activity against Gram-positive bacteria [39]. Three known and two new lobophorins, H and I, were produced by a strain of Streptomyces sp. 1053U.I.1a.3b associated with the mollusk Lienardia totopotens [40]. Lobophorin I effectively inhibited the growth of the Mycobacterium tuberculosis culture, providing more than threefold selectivity for antituberculosis activity compared to cytotoxicity. Lobophorin H is probably a precursor compound, which does not affect the M. tuberculosis growth [40]. The strain Streptomyces cavourensis SV 21 was obtained from the sea cucumber Stichopus vastus. S. cavourensis SV 21 was found to produce valinomycin, a known depsipeptide, and a new analogue of valinomycin, streptodepsipeptide SV21 [41].
Tunicates are another group of marine animals known to be rich sources of bioactive metabolites [42]. Most of these chordates are sessile filter feeders in their mature form. This ecological niche contributes to the accumulation of microbiota. Due to the close interaction between the host and symbiont bacteria in the case of ascidians, it often remains unknown which organism is the true producer of the biologically active compound. For approximately 8% of the known secondary metabolites obtained from ascidians, there is evidence of an entirely bacterial origin [42]. Lissoclimum patella is a colonial sea squirt of the family Didemnidae, which is widespread in the western Pacific Ocean. It is one of the best-studied examples of bacterial symbiont–host relationships among marine systems [42]. L. patella contains photosynthetic symbionts, such as the cyanobacterium Prochloron didemni. These organisms synthesize cyanobactins, which are highly modified ribosomally synthesized peptides. The cyanobactin biosynthetic pathway is characterized by high tolerance to changes in precursor molecule cassettes [42]. P. didemni, a free-living bacterium, comprises panmictic oceanic populations [42,43]. Representatives of phylogenetically distant populations of symbionts occur in a single L. patella organism [43]. However, the metabolome of L. patella symbionts depends on the subspecies of the host organism, not on the geographical location. Symbionts of phylogenetically related ascidians produce similar sets of secondary metabolites. Thus, ascidians are able to modulate the spectrum of secondary metabolites of symbiotic bacteria [43]. L. patella and P. didemni are a classic example of a symbiosis between ascidians and bacteria but not the only one. Ascidians are characterized by species-specific microbiomes with unique biosynthetic fingerprints [44]. In addition to cyanobactins, ascidian symbionts produce alkaloids, modified peptides, and polyketides, many of which are considered potential candidates for clinical medicine [45].
Among actively moving marine invertebrates, the Hawaiian bobtail squid, Euptymna scolopes, contains the bacterial symbiont Leisingera sp., which has a known role in the host life cycle [46]. Cephalopods are characterized by the presence of a microbial community in the nidamental gland, an organ of the female reproductive system. Leisingera sp. penetrates inside eggs and selectively inhibits the growth of cultures of the genus Vibrio through the secretion of an indigoidine pigment [46].
3. Terrestrial Invertebrates
3.1. Nematodes
Nematodes and symbiotic gammaproteobacteria are one of the best-studied animal–bacterium systems. Representatives of the genus Xenorhabdus form a close association with entomopathogenic nematodes of the family Steinernematidae. These bacteria produce a wide range of compounds that suppress the growth of other bacteria, fungi, and protozoa. Xenorhabdus strains also inhibit the development of insects and nematodes competing with the host [47]. A similar system consists of bacteria of the genus Photorhabdus and nematodes of the family Heterorhabditidae [48].
Biologically active substances produced by Xenorhabdus spp. include depsipeptides [49], xenocoumacins [50], and genus-specific antimicrobial peptides [51] as well as benzylidene acetone, indole derivatives, and bacteriocins, the most famous of which is xenorhabdicin [52]. The diversity of BGCs in Xenorhabdus genomes significantly exceeds known compounds, and the biosynthetic potential of these bacteria remains underestimated [47]. In the case of Streptomyces, alternative cultivation methods are used to induce secondary metabolite production. This approach could also be used to discover products of Xenorhabdus strains. For example, the detection of amicoumacins among the secondary metabolites of Xenorhabdus bovienii was made possible by the use of a medium simulating the content of amino acids in the circulating fluid of the wax moth [53]. Photorhabdus luminescens produces a number of bacteriocins, including photorhabdicins and luminescines [54], as well as the known antifungal compound 3,5-dihydroxy-4-isopropylstilbene [55]. From the Photorhabdus khanii strain HGB1456, a new antibiotic, darobactin, was obtained [56]. Darobactin is a modified heptapeptide and shows activity against Gram-negative bacteria, including multidrug-resistant (MDR) strains of E. coli and K. pneumoniae. Darobactin is effective against Gram-negative bacteria in a murine model of sepsis, making it a promising drug candidate [56].
3.2. Insects
Insects make up the most numerous class of animals. More than a million species have been described, and they make up just a fraction of the total diversity. Insects occupy a huge number of ecological niches, and different hosts provide different habitats for the microbiota. Insect symbionts can be found on the cuticle, in the digestive tract, or inside cells and tissues. In addition to symbionts, antimicrobials were isolated from insect metabolites, such as honey, and nests [57]. Most studies of the antimicrobial activity of microbiota have been carried out with Hymenoptera species. Particular attention is paid to ants that grow fungi. The microbiome of their garden nests selectively prevents the development of entomopathogenic bacteria and fungi [57].
As streptomycetes are traditionally considered to be the main producers of antibiotics, most studies have been devoted to this genus only. Analysis of metagenomic data established that more Streptomyces strains were found in insect microbiomes than in water and marine sediments, making them an important source of antibiotic-producing strains [58]. The streptomycete strain ISID311 isolated from the mycobiome of the fungi-farming ant Cyphomyrmex sp. produced a new antimycotic compound called cyphomycin [58]. Cyphomycin has strong inhibitory activity against Escovopsis sp. fungi, which are pathogenic for ants. That may indicate its ecological significance for host nest protection. Cyphomycin also has antifungal activity against human pathogens, such as triazole-resistant Aspergillus fumigatus 11628, echinocandin-resistant Candida glabrata 4720, and MDR strain Candida auris B11211. Cyphomycin was tested in vivo in a model of disseminated candidiasis in mice and is considered to be a promising antifungal drug [58]. New pentacyclic polyketides that inhibit the growth of B. subtilis, S. aureus (MRSA), and vancomycin-resistant Enterococcus faecium (VRE) were obtained from a strain of Streptomyces formicae associated with the African ant Tetraponera penzigi [59]. These compounds were more effective than the structurally related compounds, fasamycins, which were also found among the secondary metabolites of the producer strain. The BGC of formamycin was further identified, and its formation by horizontal gene transfer was suggested [56,57]. A new polyene polyketide, selvamicin, was discovered in actinobacteria of the genus Pseudonocardia associated with the nest of Apterostigma ants [60]. Selvamicin is similar to nystatin and amphotericin but differs from them in the presence of a second sugar, a truncated macrocyclic core, and the absence of carboxylate and ammonium groups. These differences provide different pharmacokinetic properties of the compound, and, presumably, selvamicin has a different mode of action [57,60]. Selvamicin exhibits activity against C. albicans and a number of other fungi [57,60].
Termites are also known for cultivating fungi in their nests. The Streptomyces sp. M56 strain was obtained from the nest of African termites, Macrotermes natalensis [61]. The strain was first shown to produce natalamycin A, a compound of the ansamycin family, which showed antifungal activity both against Pseudoxylaria sp. X802 and against termite-cultivated Termitomyces T112 [61]. Further metabolomic analysis of the same strain revealed the production of efomycin M and two novel compounds—elaiophylin derivatives named efomycins K and L. The high antifungal activity of the strain was mediated by the synergistic effect of simultaneously produced geldanamycins, efomycins, and elaiophylins [62]. Other new compounds isolated from a termite symbiont strain were ilicolinic acids C and D [61]. These substances were produced by the fungus Neonectria discophora, obtained from the termite mound of the insect species Nasutitermes corniger. Both compounds exhibited moderate activity against E. coli and moderate cytotoxicity but were significantly less active compared to a previously discovered closely related compound, ilylicicolinic acid A [57,63].
A number of antibiotics, including frontalamides A and B and mycangimycin, have been identified by studying the microbiota of the southern pine beetle (Dendroctonus frontalis) [57,64]. The southern pine beetle participates in a multilateral symbiosis with fungi. Entomocorticium sp. is required for feeding larvae and is necessary for beetles to survive. Ophiostoma minus is an Entomocorticium antagonist, replacing Entomocorticium and leading to the death of the host. The bacterial community participates in maintaining the balance of fungal cultures through the production of secondary metabolites. Among the products of streptomycetes, frontalamides are polycyclic tetramate macrolactams [64], and mycangimycin is a polyene peroxide [65]. These compounds inhibited the growth of O. minus. Mycangimycin was also active against human pathogenic C. albicans [64].
Actinobacteria are the most thoroughly studied, but microorganisms of other phyla associated with insects also have the ability to produce antimicrobial compounds [57,66,67,68]. A strain of Serracia marcescens was discovered in the microbiome of the malaria vector mosquito Anopheles stephensi that inhibited the growth of Plasmodium falciparum and B. subtilis. The activity was mediated by the production of stephensiolides, cyclic lipodepsipeptides representing a novel family [66]. The bacteria Burkholderia gladioli of the unculturable strain Lv-StB were found on the eggs and glands of female darkling beetles, Lagria villosa [67]. This strain produced the polyketide lagriamide isolated from beetle eggs. Lagriamide was shown to be active against the entomopathogenic fungus Purpureocillium lilacinum [67]. Two novel compounds acting on the cell wall called lenzimycins A and B were isolated from a bacterium of the genus Brevibacillus associated with the dung beetle Onthophagus lenzii. Both of the lenzimycins had the ability to inhibit the growth of the entomopathogenic strain of Bacillus thuringiensis as well as the human pathogens E. faecium and E. faecalis [68].
4. Fish
Researchers turned to vertebrates as sources of microbiota that produce antimicrobial substances relatively late. There are few reports of new compounds found in such strains at present. However, microbial communities in the guts of fish are estimated to be more diverse than those of mammals. The associated communities vary within animal species depending on environmental conditions, such as water salinity or host diet [69,70]. A fish organism represents a unique environment, and the diversity of strains in such biotopes differs significantly from that in the surrounding seawater [69]. Stomach and intestinal microbiomes of fish are unique communities that contain strains phylogenetically distinct from previously known culturable bacteria [71].
Putative obligate psychrophiles were found in the microbiota of fish living in temperate climates [71]. Vibriosis is one of the most common bacterial diseases of fish, both on farms and in the wild. Vibrios are characterized by the rapid growth of cultures. If these bacteria colonize the intestines, they quickly become the dominant population. Virulence factors secreted by vibrios cause necrosis of fish tissues, slow growth and body malformation, blindness, and mortality. Vibriosis is normally prevented by both the host immune system of the fish and its microbiota. The search for mechanisms of such protection can contribute to both the production of valuable probiotics for fish farms and the search for compounds active against Gram-negative bacteria.
Marine Actinobacteria strains exhibiting activity against both Gram-negative and Gram-positive bacteria were isolated by studying fish intestinal microbiota communities [71]. New species of the Paraoerskovia genus were subjected to detailed analysis. The secondary metabolite responsible for the activity was purified and identified as the lipid sebasthenoic acid. Sebasthenoic acid inhibited the growth of the Gram-positive bacteria B. subtilis, S. aureus, and E. faecium [71]. Several strains with proven probiotic activity for fish have currently been characterized. The Enterococcus durans F3 strain from the intestines of freshwater fish Catla catla demonstrated tolerance to bile acid and gastric juice. E. durans F3 had a bactericidal effect against S. aureus, E. coli, P. aeruginosa, and Salmonella typhi. The effect was mediated by enterocin A, a known bacteriocin with a molecular weight of approximately 6.5 kDa [72].
Bacterial strains obtained from fish intestinal microbiota are used in aquaculture. Rummeliibacillus stabekisii [73] and various Bacillus species, including Bacillus velezensis, Bacillus aryabhattai and Bacillus mojavensis, are considered to be probiotic [74,75]. The advantage of fish-derived strains is their ability to colonize the intestines of fish and provide a prolonged effect [74]. Probiotic strains inhibited the growth of Staphylococcus, Aeromonas, and Streptococcus species [73,74]. A common feature of probiotic Bacillus strains is the secretion of lipase, amylase, and protease enzymes as well as nonribosomal bioactive metabolites [74,75].
The analysis of rainbow trout microbiomes revealed that the greatest diversity of microorganisms was found on the skin of the fish, followed by olfactory, gill, and intestine microbiomes [76]. The composition of skin microbial communities was reported to be similar in different species of teleost fish, with Proteobacteria and Bacteroidetes being the most represented [76]. Bacteria penetrate the fish skin epithelium and localize next to goblet cells. These communities differ from the general skin microbiome and are characterized by the dominance of Firmicutes and Actinobacteria [76]. It is supposed that the skin of teleosts provides a more favorable environment for colonization compared to the skin of terrestrial vertebrates [76]. The colonization of fish skin is improved by living epithelial cells that are free from dead keratinized layers. The microbiota on fish skin provides host protection against pathogens. Arthrobacter spp. (A. stackebrandtii and A. psychrolactophilus) and Psychrobacter maritimus inhibited the growth of two different aquatic pathogenic fungi, Saprolegnia australis and Mucor hiemalis [76].
5. Amphibians and Reptiles
The host habitat largely determines the diversity of the microbiota colonizing amphibians and reptiles [77]. The composition of a microbiota differs greatly even between animals of closely related species inhabiting the same region [77]. Different species of frogs have been shown to have a diverse composition of skin microbiota. Producers of antimicrobial agents highly active against both Gram-positive and Gram-negative pathogens were identified among members of this unique community [77]. The diversity of the microbiota of amphibians and reptiles is formed under the strong pressure of innate immunity. The specific components of amphibian immunity include diverse antimicrobial peptides (AMPs), which exhibit high activity and are characteristic of these animals [78,79]. The specific microbiota of amphibians and reptiles often contains antibiotic-resistant and multidrug-resistant Enterobacteriaceae strains, representing a risk factor for pathogen transmission [80,81].
Bacteriocins were frequently identified in the microbiomes of amphibians and reptiles, including nisin Z produced by Lactococcus lactis [82,83]. Nisin Z inhibits the growth of the amphibian pathogens Citrobacter freundii and Listeria monocytogenes. Prodigiosin, violacein, and volatile organic compounds are produced by the skin microbiota of geographically distant amphibian species [84]. These metabolites inhibit the growth of pathogenic fungi of the genus Batrachochytrium that cause chytridiomycosis in amphibians [84]. The symbiotic strains of the bacteria Serratia marcescens produce prodigiosin. The antimicrobial effect of prodigiosin was mediated by intercellular contacts with the pathogen Staphylococcus aureus [85]. Strains of Bacillus atrophaeus were identified in the skin microbiota of the Iranian marsh frog (Rana ridibunda) [86]. Bacillus atrophaeus exhibited a wide range of antimicrobial and antiproliferative activities [86].
Pseudomonas aeruginosa strains were isolated from the Amboina box turtle (Cuora amboinensis) [87]. Pseudomonas aeruginosa demonstrated antibacterial activity against Gram-positive bacteria (B. cereus, Streptococcus pyogenes, and Staphylococcus aureus MRSA) and Gram-negative pathogens (E. coli K1, S. marcescens, P. aeruginosa, S. enterica, and K. pneumoniae). Novel N-acyl-homoserine lactones, 4-hydroxy-2-alkylquinolines, and rhamnolipids were identified as active metabolites of P. aeruginosa [87]. A similar profile of metabolites was shown for the microbiota of the saltwater crocodile (Crocodylus porosus) [88]. In this case, the activity was also associated with Pseudomonas strains.
6. Birds
Despite the wide distribution of birds, the diversity of the avian microbiome has been studied rather limitedly. This is largely due to the difficulties associated with the intravital selection of native biomaterials. Moreover, metatranscriptomic data indicate that antibiotics associated with environmental pollution have a high impact on biodiversity and antibiotic resistance in the bacterial communities of wild birds [89]. The antibiotic impact is particularly acute due to the large-scale use of antibiotics in poultry farming [90]. Probiotics provide a highly effective alternative to antibiotics in agriculture. The application of probiotic strains of Enterococcus faecium, Pediococcus acidilactici, Bacillus animalis, Lactobacillus salivarius, and Lactobacillus reuteri prevents the colonization of birds by pathogens [91]. However, it has been repeatedly shown that specific biodiversity and the stability of bacterial communities are extremely important for the normal physiology of birds [92]. There are numerous studies on the functionality of the chicken microbiota [93,94,95] despite limited knowledge of the antimicrobial potential of wild birds.
In most cases, it was reported that the molecular mechanisms underlying microbiome stability in birds are associated with the production of short-chain fatty acids by Lactobacillus, Clostridium, and Streptococcus strains [92,93,94]. Class II lanthipeptides, including FK22 [96], OR7 [97], L-1077 [98], and salivaricin SMXD51 [99], are the most studied antimicrobials produced by Lactobacillus [100]. Lactic acid bacteria (LAB) with potent antimicrobial activity were isolated from the microbiome of griffon vulture (Gyps fulvus subsp. fulvus) [101]. It was identified that LAB Enterococcus faecium M3K31 produces enterocin HF (EntHF), which is highly active against Listeria spp. [101]. Plumage [102] and coccygeal gland [103,104] microbiomes are also particularly important for birds.
Proteobacteria, Actinobacteria, and Bacteroidetes were reported to be the most abundant phyla in the passerine feather microbiota, while Alphaproteobacteria, Gammaproteobacteria, and Betaproteobacteria are the most abundant classes [102]. The exceptions were the sand swallow and the common redstart, which had a poorer microbiome of feathers and a predominance of representatives of the Firmicutes phylum and the Bacilli class. Streptococcus and Lactobacillus genera were the most represented. The number of potential bacteriocin producers was negatively correlated with the overall diversity of the feather microbial community. A positive correlation between the number of bacteria capable of destroying keratin and bacteriocin producers was detected [102]. Many Bacillus and Pseudomonas species have protective properties due to their ability to inhibit the growth of other microorganisms, although some of them are considered harmful to the host bird [102].
Enterococcus faecalis producing specific bacteriocins was isolated from the coccygeal gland of the hoopoe (Upupa epops) [104]. Hoopoes are characterized by a different composition of fatty secretion of the gland depending on the nesting phase. The liquid for feather lubrication becomes dark and foul smelling in nesting females and in chicks [103]. Enterococcus faecalis was found to be the dominant culture in the secretion of the coccygeal gland, and enterocins were identified as the main antimicrobials. The combination of enterocins MR10A and MR10B inhibited the growth of S. aureus, B. cereus, and E. faecalis strains, different from the producer strain [103]. A more recent study has shown that Enterococcus faecalis is the dominant strain in hoopoe coccygeal gland secretions regardless of the individual bird or nest, and strains with greater potential for producing enterocins are more common [104].
7. Mammals
Similarly to birds, the microbiome of wild mammals has been studied much more fragmentarily than the microbiome of farm animals. Another important similarity between the microbiome of birds and mammals is the high importance of LAB (Lactobacillus species, Bifidobacterium spp., and Bacillus spp.), which have antagonistic properties towards pathogens and are widely used as probiotics in animal husbandry [105]. Ligilactobacillus salivarius strains were isolated from the microbiome of calves [106]. L. salivarius exhibited antagonistic properties against pathogenic strains of E. coli, demonstrating a probiotic effect in a rat model in vivo. Streptococci are also widespread members of the bovine microbiome, well known for the production of lantipeptides of the bovicin family [107].
Marine mammals with global migration routes are exposed to dramatic changes in their environment. Migrating marine mammals have an increased diversity of microbiomes compared to nonmigratory species [108]. A strain of Micromonospora auratingra was isolated from the intestinal microbiota of harbor porpoise (Phocoena phocoena) [108]. The strain selectively inhibited the growth of Gram-positive bacteria, including the intestinal pathogen Clostridium difficile. The activity of M. auratingra was provided by a new glycosylated polyketide antibiotic called focoenamycin [108]. The antibiotic showed low cytotoxicity. Focoenamycin shares a number of common structural features with fidaxomicin, a known drug effective against C. difficile, but has a different mechanism of action [108].
The human microbiota is the most extensively studied object [109]. It exhibits a high number of bacteriocin-producing strains, which play an extremely important role in the ecology of the human microbiome [110,111,112,113]. A lantibiotic produced by the commensal bacterium Blautia producta prevented colonization by vancomycin-resistant strains of E. faecium (VRE), restoring the sensitivity of enterococci to vancomycin [114]. Bacteroidetocins represent class IIa Gram-positive bacteriocins, inhibiting the growth of Bacteroides, Parabacteroides, and Prevotella species [115].
Secondary bile acids represent a unique group of antimicrobial agents from the human microbiome. These compounds are products of human biosynthesis pathways modified by bacteria. A new form of lithocholic acid (isoallolithocholic acid) was demonstrated to have high activity against the pathogenic bacteria Clostridioides difficile and Enterococcus faecium [116]. Moreover, the human microbiota could serve as a source of classical antibiotics. Lugdunin is a product of nonribosomal peptide synthetases (NRPS) produced by the human microbiota commensal Staphylococcus lugdunensis [117]. The microbiota of wild animals could also be a source of antibiotics synthesized by polyketide synthase (PKS)/NRPS. It has been repeatedly observed for amicoumacin A, which is highly abundant in a variety of different animal species [118,119].
8. Discussion
The animal microbiome represents a unique reservoir for antibiotic discovery [77,110,113,118,119,120] (Table 1).

Table 1.
Examples of new antimicrobial compounds identified from animal-associated microorganisms.
Probiotic microorganisms were repeatedly isolated from natural microbiota sources [121,122], representing an extensive reservoir for antimicrobial agent research. Most probiotic strains and commensal bacteria have an indirect effect on the microbiome by influencing the immune system of the host [123] or producing functionally important enzymes [124]. However, direct killing of pathogens is characteristic of the most ubiquitous commensals. In addition to bacteria with probiotic effects, pathogens are also able to produce antimicrobial agents, playing a fundamental role in the interaction of bacteria in vivo. The vast majority of Pseudomonas aeruginosa strains show antagonism towards Staphylococcus aureus, mediated by the synergistic effects [7,125] of pyocyanin [126], phenazine-1-carboxylic acid [127], and 2-heptyl-4-hydroxyquinoline [128]. The same bacteria may play diametrically opposite roles in different animal hosts. Pseudomonas are common symbionts of reptiles and amphibians. At the same time, Pseudomonas are pathogens of mammals.
In contrast, “universal probiotic commensals” have also been reported [119]. Bacillus species have been identified as the normal microbiota of a wide repertoire of organisms, including marine invertebrates, insects, and mammals. These strains often share a common “fingerprint” of secondary metabolites, of which the antibiotic amicoumacin is the most active antimicrobial agent. Bacillus species have a rich arsenal of highly active secondary metabolites [129]. Bacillus species not only play an important role as a common component of the microbiota of wild animals but are also widely used in practice as a probiotic for poultry farming [130], pig farming [131], aquaculture [132], and human healthcare [133].
The contaminated environment itself can be a source of antimicrobial agents [120]. However, microbiomes are highly sensitive to the “quality” of the environment [77]. It creates a high risk of degeneration of the natural biodiversity, leading to the loss of antibiotic producers as a result of destructive human activity.
Despite the enormous amount of data accumulated from host–cell interactions in the wild [134], we are still far from having a detailed understanding of the entire landscape of molecular interactions in microbiomes. Moreover, unique antimicrobial agents may be found right in front of our nose [117]. Modern technologies will undoubtedly make a major contribution to the identification of new antibiotics. Genome mining [6,135] and ultrahigh-throughput microfluidic technologies [9,10,118,136,137] will allow us to detail the unique biodiversity of antibacterial agents associated with wildlife microbiomes.
Author Contributions
M.N.B. and S.S.T. designed the structure of the review; M.N.B. and E.A.P. drafted the initial manuscript; the final version of the manuscript was prepared by S.S.T., I.V.S. and A.G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Grant 19-14-00331 from the Russian Science Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article. Additional data are freely available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef]
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 2, 29–45. [Google Scholar] [CrossRef]
- Ling, L.L. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Nichols, D. Use of Ichip for High-Throughput In Situ Cultivation of ‘Uncultivable’ Microbial Species. Appl. Environ. Microbiol. 2020, 76, 2445. [Google Scholar] [CrossRef]
- Rutledge, P.J.; Challis, G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Bauman, K.D.; Butler, K.S.; Moore, B.S.; Chekan, J.R. Genome mining methods to discover bioactive natural products. Nat. Prod. Rep. 2021, 38, 2100–2129. [Google Scholar] [CrossRef]
- Terekhov, S.S.; Smirnov, I.V.; Stepanova, A.V.; Bobik, T.V.; Mokrushina, Y.A.; Ponomarenko, N.A.; Belogurov, A.A., Jr.; Rubtsova, M.P.; Kartseva, O.V.; Gomzikova, M.O.; et al. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc. Natl. Acad. Sci. USA 2017, 114, 2550–2555. [Google Scholar] [CrossRef]
- Scanlon, T.C.; Dostal, S.M.; Griswold, K.E. A High-Throughput Screen for Antibiotic Drug Discovery. Biotechnol. Bioeng. 2014, 111, 232. [Google Scholar] [CrossRef]
- Mahler, L.; Niehs, S.P.; Martin, K.; Weber, T.; Scherlach, K.; Hertweck, C.; Roth, M.; Rosenbaum, M.A. Highly parallelized droplet cultivation and prioritization of antibiotic producers from natural microbial communities. Elife 2021, 10, e64774. [Google Scholar] [CrossRef]
- Nuti, N.; Rottmann, P.; Stucki, A.; Koch, P.; Panke, S.; Dittrich, P.S. A Multiplexed Cell-Free Assay to Screen for Antimicrobial Peptides in Double Emulsion Droplets. Angew. Chem. Int. Ed. Engl. 2022, 61, e202114632. [Google Scholar] [CrossRef]
- Zada, S.; Sajjad, W.; Rafiq, M.; Ali, S.; Hu, Z.; Wang, H.; Cai, R. Cave Microbes as a Potential Source of Drugs Development in the Modern Era. Microb. Ecol. 2022, 84, 676–687. [Google Scholar] [CrossRef]
- Jose, P.A.; Maharshi, A.; Jha, B. Actinobacteria in natural products research: Progress and prospects. Microbiol. Res. 2021, 246, 126708. [Google Scholar] [CrossRef]
- Xu, D.; Han, L.; Li, C.; Cao, Q.; Zhu, D.; Barrett, N.H.; Harmody, D.; Chen, J.; Zhu, H.; McCarthy, P.J.; et al. Bioprospecting Deep-Sea Actinobacteria for Novel Anti-infective Natural Products. Front. Microbiol. 2018, 30, 787. [Google Scholar] [CrossRef]
- Malard, L.A.; Guisan, A. Into the microbial niche. Trends Ecol. Evol. 2023, 38, 936–945. [Google Scholar] [CrossRef]
- Mullis, M.M.; Rambo, I.M.; Baker, B.J.; Reese, B.K. Diversity, Ecology, and Prevalence of Antimicrobials in Nature. Front. Microbiol. 2019, 10, 2518. [Google Scholar] [CrossRef]
- Baquero, F.; Coque, T.M.; Galán, J.C.; Martinez, J.L. The Origin of Niches and Species in the Bacterial World. Front. Microbiol. 2021, 12, 657986. [Google Scholar] [CrossRef]
- Esser, D.; Lange, J.; Marinos, G.; Sieber, M.; Best, L.; Prasse, D.; Bathia, J.; Rühlemann, M.C.; Boersch, K.; Jaspers, C.; et al. Functions of the Microbiota for the Physiology of Animal Metaorganisms. J. Innate Immun. 2019, 11, 393–404. [Google Scholar] [CrossRef]
- Buchanan, G.O.; Williams, P.G.; Feling, R.H.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Sporolides A and B: Structurally unprecedented halogenated macrolides from the marine actinomycete Salinispora tropica. Org. Lett. 2005, 7, 2731–2734. [Google Scholar] [CrossRef]
- Bech, P.K.; Lysdal, K.L.; Gram, L.; Bentzon-Tilia, M.; Strube, M.L. Marine Sediments Hold an Untapped Potential for Novel Taxonomic and Bioactive Bacterial Diversity. mSystems 2020, 5, e00782-20. [Google Scholar] [CrossRef]
- Macintyre, L.; Zhang, T.; Viegelmann, C.; Martinez, I.J.; Cheng, C.; Dowdells, C.; Abdelmohsen, U.R.; Gernert, C.; Hentschel, U.; Edrada-Ebel, R. Metabolomic tools for secondary metabolite discovery from marine microbial symbionts. Mar. Drugs 2014, 12, 3416–3448. [Google Scholar] [CrossRef]
- Thomas-Poulsen, M.; van Overbeek, L.; Newman, D.J. Predominately Uncultured Microbes as Sources of Bioactive Agents. Front. Microbiol. 2016, 18, 1832. [Google Scholar]
- Rosenberg, E.; Zilber-Rosenberg, I. Microbes Drive Evolution of Animals and Plants: The Hologenome Concept. mBio 2016, 7, e01395. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Theis, K.R. Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef]
- Remya, T.; Thomas, A.; Kavlekar, D.P.; Lokabharathi, P.A. Marine Drugs from Sponge-Microbe Association-A Review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar]
- Matroodi, S.; Siitonen, V.; Baral, B.; Yamada, K.; Akhgari, A.; Metsä-Ketelä, M. Genotyping-Guided Discovery of Persiamycin A From Sponge-Associated Halophilic Streptomonospora sp. PA3. Front. Microbiol. 2020, 11, 1237. [Google Scholar] [CrossRef]
- Rao, K.V.; Santarsiero, B.D.; Mesecar, A.D.; Schinazi, R.F.; Tekwani, B.L.; Hamann, M.T. New Manzamine Alkaloids with Activity against Infectious and Tropical Parasitic Diseases from an Indonesian Sponge. J. Nat. Prod. 2003, 66, 823–828. [Google Scholar] [CrossRef]
- Waters, A.L.; Peraud, O.; Kasanah, N.; Sims, J.W.; Kothalawala, N.; Anderson, M.A.; Abbas, S.H.; Rao, K.V.; Jupally, V.R.; Kelly, M.; et al. An analysis of the sponge Acanthostrongylophora igens’ microbiome yields an actinomycete that produces the natural product manzamine A. Front. Mar. Sci. 2014, 1, 54. [Google Scholar] [CrossRef]
- Li, W.; Ding, L.; Li, J.; Wen, H.; Liu, Y.; Tan, S.; Yan, X.; Shi, Y.; Lin, W.; Lin, H.-W.; et al. Novel Antimycin Analogues with Agricultural Antifungal Activities from the Sponge-Associated Actinomycete Streptomyces sp. NBU3104. J. Agric. Food Chem. 2022, 70, 8309–8316. [Google Scholar] [CrossRef]
- MacIntyre, L.W.; Charles, M.J.; Haltli, B.A.; Marchbank, D.H.; Kerr, R.G. An Ichip-Domesticated Sponge Bacterium Produces an N-Acyltyrosine Bearing an α-Methyl Substituent. Org. Lett. 2019, 21, 7768–7771. [Google Scholar] [CrossRef]
- Nagai, K.; Kamigiri, K.; Arao, N.; Suzumura, K.; Kawano, Y.; Yamaoka, M.; Zhang, H.; Watanabe, M.; Suzuki, K. YM-266183 and YM-266184, Novel Thiopeptide Antibiotics Produced by Bacillus cereus Isolated from a Marine Sponge, I. Taxonomy, Fermentation, Isolation, Physico-chemical Properties and Biological Properties. J. Antibiot. 2003, 56, 123–128. [Google Scholar] [CrossRef]
- Pabel, C.T.; Vater, J.; Wilde, C.; Franke, P.; Hofemeister, J.; Adler, B.; Bringmann, G.; Hacker, J.; Hentschel, U. Antimicrobial Activities and Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry of Bacillus Isolates from the Marine Sponge Aplysina aerophoba. Mar. Biotechnol. 2003, 5, 424–434. [Google Scholar] [CrossRef]
- Graça, A.P.; Bondoso, J.; Gaspar, H.; Xavier, J.R.; Monteiro, M.C.; de la Cruz, M.; Oves-Costales, D.; Vicente, F.; Lage, O.M. Antimicrobial Activity of Heterotrophic Bacterial Communities from the Marine Sponge Erylus discophorus (Astrophorida, Geodiidae). PLoS ONE 2013, 8, 78992. [Google Scholar] [CrossRef]
- Sertan-De Guzman, A.A.; Predicala, R.Z.; Bernardo, E.B.; Neilan, B.A.; Elardo, S.P.; Mangalindan, G.C.; Tasdemir, D.; Ireland, C.M.; Barraquio, W.L.; Concepcion, G.P. Pseudovibrio denitrificans strain Z143-1, a heptylprodigiosin-producing bacterium isolated from a Philippine tunicate. FEMS Microbiol. Lett. 2007, 277, 188–196. [Google Scholar] [CrossRef]
- Palomo, S.; González, I.; de la Cruz, M.; Martín, J.; Tormo, J.R.; Anderson, M.; Hill, R.T.; Vicente, F.; Reyes, F.; Genilloud, O. Sponge-Derived Kocuria and Micrococcus spp. as Sources of the New Thiazolyl Peptide Antibiotic Kocurin. Mar. Drugs 2013, 11, 1071. [Google Scholar] [CrossRef]
- Martí, J.; da S Sousa, T.; Crespo, G.; Palomo, S.; González, I.; Tormo, J.R.; de la Cruz, M.; Anderson, M.; Hill, R.T.; Vicente, F.; et al. Kocurin, the True Structure of PM181104, an Anti-Methicillin-Resistant Staphylococcus aureus (MRSA) Thiazolyl Peptide from the Marine-Derived Bacterium Kocuria palustris. Mar. Drugs 2013, 11, 387–398. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, H.; Han, X.; Lin, W.; Yan, X. Antimicrobial screening and active compound isolation from marine bacterium NJ6-3-1 associated with the sponge Hymeniacidon perleve. World J. Microbiol. Biotechnol. 2005, 21, 201–206. [Google Scholar] [CrossRef]
- Singh, M.P.; Kong, F.; Janso, J.E.; Arias, D.A.; Suarez, P.A.; Bernan, V.S.; Petersen, P.J.; Weiss, W.J.; Carter, G.; Greenstein, M. Novel α-Pyrones Produced by a Marine Pseudomonas sp. F92S91 Taxonomy and Biological Activities. J. Antibiot. 2003, 56, 1033–1044. [Google Scholar] [CrossRef]
- Sugden, S.; Holert, J.; Cardenas, E.; Mohn, W.W.; Stein, L.Y. Microbiome of the freshwater sponge Ephydatia muelleri shares compositional and functional similarities with those of marine sponges. ISME J. 2022, 16, 2503–2512. [Google Scholar] [CrossRef]
- Braña, A.F.; Sarmiento-Vizcaíno, A.; Osset, M.; Pérez-Victoria, I.; Martín, J.; de Pedro, N.; de la Cruz, M.; Díaz, C.; Vicente, F.; Reyes, F.; et al. Lobophorin K, a New Natural Product with Cytotoxic Activity Produced by Streptomyces sp. M-207 Associated with the Deep-Sea Coral Lophelia pertusa. Mar. Drugs 2017, 15, 144. [Google Scholar]
- Lin, Z.; Koch, M.; Pond, C.D.; Mabeza, G.; Seronay, R.A.; Concepcion, G.P.; Barrows, L.R.; Olivera, B.M.; Schmidt, E.W. Lobophorins with antimycobacterial activity from a turrid mollusk-associated Streptomyces sp. J. Antibiot. 2014, 67, 121. [Google Scholar] [CrossRef]
- Wibowo, J.T.; Kellermann, M.Y.; Köck, M.; Putra, M.Y.; Murniasih, T.; Mohr, K.I.; Wink, J.; Praditya, D.F.; Steinmann, E.; Schupp, P.J. Anti-Infective and Antiviral Activity of Valinomycin and Its Analogues from a Sea Cucumber-Associated Bacterium, Streptomyces sp. SV 21. Mar. Drugs 2021, 19, 81. [Google Scholar] [CrossRef]
- Schmidt, E.W. The secret to a successful relationship: Lasting chemistry between ascidians and their symbiotic bacteria. Invertebr. Biol. 2015, 134, 88–102. [Google Scholar] [CrossRef]
- Kwan, J.C.; Tianero, M.D.; Donia, M.S.; Wyche, T.P.; Bugni, T.S.; Schmidt, E.W. Host control of symbiont natural product chemistry in cryptic populations of the tunicate Lissoclinum patella. PLoS ONE 2014, 9, e95850. [Google Scholar] [CrossRef]
- Erwin, P.; Pineda, M.; Webster, N.; Turon, X.; López-Legentil, S. Down under the tunic: Bacterial biodiversity hotspots and widespread ammonia-oxidizing archaea in coral reef ascidians. ISME J. 2014, 8, 575–588. [Google Scholar] [CrossRef]
- Dou, X.; Dong, B. Origins and Bioactivities of Natural Compounds Derived from Marine Ascidians and Their Symbionts. Mar. Drugs 2019, 17, 670. [Google Scholar] [CrossRef]
- Gromek, S.M.; Suria, A.M.; Fullmer, M.S.; Garcia, J.L.; Gogarten, J.P.; Nyholm, S.V.; Balunas, M.J. Leisingera sp. JC1, a Bacterial Isolate from Hawaiian Bobtail Squid Eggs, Produces Indigoidine and Differentially Inhibits Vibrios. Front. Microbiol. 2016, 7, 1342. [Google Scholar] [CrossRef]
- Dreyer, J.; Malan, A.P.; Dicks, L.M.T. Bacteria of the Genus Xenorhabdus, a Novel Source of Bioactive Compounds. Front. Microbiol. 2018, 9, 3177. [Google Scholar] [CrossRef]
- Eleftherianos, I.G. Novel antibiotic compounds produced by the insect pathogenic bacterium Photorhabdus. Recent. Pat. Antiinfect. Drug Discov. 2009, 4, 81–89. [Google Scholar] [CrossRef]
- Zhou, Q.; Grundmann, F.; Kaiser, M.; Schiell, M.; Gaudriault, S.; Batzer, A.; Kurz, M.; Bode, H.B. Structure and biosynthesis of xenoamicins from entomopathogenic Xenorhabdus. Chemistry 2013, 19, 16772–16779. [Google Scholar] [CrossRef]
- McInerney, B.V.; Taylor, W.C.; Lacey, M.J.; Akhurst, R.J.; Gregson, R.P. Biologically Active Metabolites from Xenorhabdus spp., Part 2. Benzopyran-1-one Derivatives with Gastroprotective Activity. J. Nat. Prod. 1991, 54, 785–795. [Google Scholar] [CrossRef]
- Gualtieri, M.; Aumelas, A.; Thaler, J.O. Identification of a new antimicrobial lysine-rich cyclolipopeptide family from Xenorhabdus nematophila. J. Antibiot. 2009, 62, 295–302. [Google Scholar] [CrossRef]
- Thaler, J.O.; Baghdiguian, S.; Boemare, N. Purification and characterization of xenorhabdicin, a phage tail-like bacteriocin, from the lysogenic strain F1 of Xenorhabdus nematophilus. Appl. Environ. Microbiol. 1995, 61, 2049. [Google Scholar] [CrossRef]
- Park, H.B.; Perez, C.E.; Perry, E.K.; Crawford, J.M. Activating and Attenuating the Amicoumacin Antibiotics. Molecules 2016, 21, 824. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.; Waterfield, N.; Daborn, P.; Joyce, S.; Bennett, H.; Au, C.; Dowling, A.; Boundy, S.; Reynolds, S.; Clarke, D. Photorhabdus: Towards a functional genomic analysis of a symbiont and pathogen. FEMS Microbiol. Rev. 2003, 26, 433–456. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Wu, H.; Webster, J.M. Identification of two pigments and a hydroxystilbene antibiotic from Photorhabdus luminescens. Appl. Environ. Microbiol. 1995, 61, 4329. [Google Scholar] [CrossRef]
- Imai, Y.; Meyer, K.J.; Iinishi, A.; Favre-Godal, Q.; Green, R.; Manuse, S.; Caboni, M.; Mori, M.; Niles, S.; Ghiglieri, M.; et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 2019, 576, 459. [Google Scholar] [CrossRef]
- Van Moll, L.; De Smet, J.; Cos, P.; Van Campenhout, L. Microbial symbionts of insects as a source of new antimicrobials: A review. Crit. Rev. Microbiol. 2021, 47, 562–579. [Google Scholar] [CrossRef]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef]
- Qin, Z.; Munnoch, J.T.; Devine, R.; Holmes, N.A.; Seipke, R.F.; Wilkinson, K.A.; Wilkinson, B.; Hutchings, M.I. Formicamycins, antibacterial polyketides produced by Streptomyces formicae isolated from African Tetraponera plant-ants. Chem. Sci. 2017, 8, 3218–3227. [Google Scholar] [CrossRef]
- Van Arnam, E.B.; Ruzzini, A.C.; Sit, C.S.; Horn, H.; Pinto-Tomás, A.A.; Currie, C.R.; Clardy, J. Selvamicin, an atypical antifungal polyene from two alternative genomic contexts. Proc. Natl. Acad. Sci. USA 2016, 113, 12940–12945. [Google Scholar] [CrossRef]
- Kim, K.H.; Ramadhar, T.R.; Beemelmanns, C.; Cao, S.; Poulsen, M.; Currie, C.R.; Clardy, J. Natalamycin A, an ansamycin from a termite-associated Streptomyces sp. Chem. Sci. 2014, 5, 4333–4338. [Google Scholar] [CrossRef]
- Klassen, J.L.; Lee, S.R.; Poulsen, M.; Beemelmanns, C.; Kim, K.H. Efomycins K and L From a Termite-Associated Streptomyces sp. M56 and Their Putative Biosynthetic Origin. Front. Microbiol. 2019, 10, 1739. [Google Scholar] [CrossRef]
- Nirma, C.; Eparvier, V.; Stien, D. Antibacterial ilicicolinic acids C and D and ilicicolinal from Neonectria discophora SNB-CN63 isolated from a termite nest. J. Nat. Prod. 2015, 78, 159–162. [Google Scholar] [CrossRef]
- Scott, J.J.; Oh, D.C.; Yuceer, M.C.; Klepzig, K.D.; Clardy, J.; Currie, C.R. Bacterial protection of beetle-fungus mutualism. Science 2008, 322, 63. [Google Scholar] [CrossRef]
- Blodgett, J.A.V.; Oh, D.C.; Cao, S.; Currie, C.R.; Kolter, R.; Clardy, J. Common biosynthetic origins for polycyclic tetramate macrolactams from phylogenetically diverse bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 11692–11697. [Google Scholar] [CrossRef]
- Ganley, J.G.; Carr, G.; Ioerger, T.R.; Sacchettini, J.C.; Clardy, J.; Derbyshire, E.R. Discovery of Antimicrobial Lipodepsipeptides Produced by a Serratia sp. within Mosquito Microbiomes. ChemBioChem 2018, 19, 1590–1594. [Google Scholar] [CrossRef]
- Flórez, L.V.; Scherlach, K.; Miller, I.J.; Rodrigues, A.; Kwan, J.C.; Hertweck, C.; Kaltenpoth, M. An antifungal polyketide associated with horizontally acquired genes supports symbiont-mediated defense in Lagria villosa beetles. Nat. Commun. 2018, 9, 2478. [Google Scholar] [CrossRef]
- An, J.S.; Hong, S.H.; Somers, E.; Lee, J.; Kim, B.Y.; Woo, D.; Kim, S.W.; Hong, H.J.; Jo, S.I.; Shin, J.; et al. Lenzimycins A and B, Metabolites with Antibacterial Properties from Brevibacillus sp. Associated with the Dung Beetle Onthophagus lenzii. Front. Microbiol. 2020, 11, 2705. [Google Scholar] [CrossRef]
- Schmidt, V.T.; Smith, K.F.; Melvin, D.W.; Amaral-Zettler, L.A. Community assembly of a euryhaline fish microbiome during salinity acclimation. Mol. Ecol. 2015, 24, 2537–2550. [Google Scholar] [CrossRef]
- Schmidt, V.; Amaral-Zettler, L.; Davidson, J.; Summerfelt, S.; Good, C. Influence of Fishmeal-Free Diets on Microbial Communities in Atlantic Salmon (Salmo salar) Recirculation Aquaculture Systems. Appl. Environ. Microbiol. 2016, 82, 4470–4481. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Wong, W.R.; Riener, R.M.; Schulze, C.J.; Linington, R.G. Examining the fish microbiome: Vertebrate-derived bacteria as an environmental niche for the discovery of unique marine natural products. PLoS ONE 2012, 7, e35398. [Google Scholar] [CrossRef]
- Alshammari, E.; Patel, M.; Sachidanandan, M.; Kumar, P.; Adnan, M. Potential Evaluation and Health Fostering Intrinsic Traits of Novel Probiotic Strain Enterococcus durans F3 Isolated from the Gut of Fresh Water Fish Catla catla. Food Sci. Anim. Resour. 2019, 39, 844. [Google Scholar] [CrossRef]
- Tan, H.Y.; Chen, S.W.; Hu, S.Y. Improvements in the growth performance, immunity, disease resistance, and gut microbiota by the probiotic Rummeliibacillus stabekisii in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 92, 265–275. [Google Scholar] [CrossRef]
- Elsadek, M.M.; Wang, S.; Wu, Z.; Wang, J.; Wang, X.; Zhang, Y.; Yu, M.; Guo, Z.; Wang, Q.; Wang, G.; et al. Characterization of Bacillus spp. isolated from the intestines of Rhynchocypris lagowskii as a potential probiotic and their effects on fish pathogens. Microb. Pathog. 2023, 180, 106163. [Google Scholar] [CrossRef]
- Khalid, F.; Khalid, A.; Fu, Y.; Hu, Q.; Zheng, Y.; Khan, S.; Wang, Z. Potential of Bacillus velezensis as a probiotic in animal feed: A review. J. Microbiol. 2021, 59, 627–633. [Google Scholar] [CrossRef]
- Lowrey, L.; Woodhams, D.C.; Tacchi, L.; Salinas, I. Topographical mapping of the rainbow trout (Oncorhynchus mykiss) microbiome reveals a diverse bacterial community with antifungal properties in the skin. Appl. Environ. Microbiol. 2015, 81, 6915–6925. [Google Scholar] [CrossRef]
- De Assis, A.B.; Barreto, C.C.; Navas, C.A. Skin microbiota in frogs from the Brazilian Atlantic Forest: Species, forest type, and potential against pathogens. PLoS ONE 2017, 12, e0179628. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef]
- Eibach, D.; Nagel, M.; Lorenzen, S.; Hogan, B.; Belmar Campos, C.; Aepfelbacher, M.; Sarpong, N.; May, J. Extended-spectrum β-lactamase-producing Enterobacteriaceae among geckos (Hemidactylus brookii) in a Ghanaian hospital. Clin. Microbiol. Infect. 2019, 25, 1048–1050. [Google Scholar] [CrossRef]
- Li, C.-F.; Tang, H.-L.; Chiou, C.-S.; Tung, K.-C.; Lu, M.-C.; Lai, Y.-C. Draft genome sequence of CTX-M-type β-lactamase-producing Klebsiella quasipneumoniae subsp. similipneumoniae isolated from a Box turtle. J. Glob. Antimicrob. Resist. 2018, 12, 235–236. [Google Scholar] [CrossRef]
- Quintana, G.; Niederle, M.V.; Minahk, C.J.; Picariello, G.; Nader-Macías, M.E.F.; Pasteris, S.E. Nisin Z produced by Lactococcus lactis from bullfrog hatchery is active against Citrobacter freundii, a red-leg syndrome related pathogen. World J. Microbiol. Biotechnol. 2017, 33, 186. [Google Scholar] [CrossRef]
- Pasteris, S.E.; Pingitore, E.V.e.r.a.; Ale, C.E.; Nader-Macías, M.E. Characterization of a bacteriocin produced by Lactococcus lactis subsp. lactis CRL 1584 isolated from a Lithobates catesbeianus hatchery. World J. Microbiol. Biotechnol. 2014, 30, 1053–1062. [Google Scholar] [CrossRef]
- Woodhams, D.C.; LaBumbard, B.C.; Barnhart, K.L.; Becker, M.H.; Bletz, M.C.; Escobar, L.A.; Flechas, S.V.; Forman, M.E.; Iannetta, A.A.; Joyce, M.D.; et al. Prodigiosin, Violacein, and Volatile Organic Compounds Produced by Widespread Cutaneous Bacteria of Amphibians Can Inhibit Two Batrachochytrium Fungal Pathogens. Microb. Ecol. 2018, 75, 1049–1062. [Google Scholar] [CrossRef]
- Lim, S.; Bhak, J.; Jeon, S.; Mun, W.; Bhak, J.; Choi, S.Y.; Mitchell, R.J. The Kiss of Death: Serratia marcescens Antibacterial Activities against Staphylococcus aureus Requires Both de novo Prodigiosin Synthesis and Direct Contact. Microbiol. Spectr. 2022, 10, e00607-22. [Google Scholar] [CrossRef]
- Asadi, S.; Soleimani, N.; Babadi, Z.K.; Ebrahimipour, G.H. Isolation and identification of the bacterium producing antitumor and antimicrobial compounds derived from Iranian swamp frog (Rana ridibunda) skin. Iran. J. Microbiol. 2021, 13, 372–380. [Google Scholar] [CrossRef]
- Akbar, N.; Khan, N.A.; Sagathevan, K.; Iqbal, M.; Tawab, A.; Siddiqui, R. Gut bacteria of Cuora amboinensis (turtle) produce broad-spectrum antibacterial molecules. Sci. Rep. 2019, 9, 17012. [Google Scholar] [CrossRef]
- Khan, N.A.; Soopramanien, M.; Maciver, S.K.; Anuar, T.S.; Sagathevan, K.; Siddiqui, R. Crocodylus porosus Gut Bacteria: A Possible Source of Novel Metabolites. Molecules 2021, 26, 4999. [Google Scholar] [CrossRef]
- Marcelino, V.R.; Wille, M.; Hurt, A.C.; González-Acuña, D.; Klaassen, M.; Schlub, T.E.; Eden, J.S.; Shi, M.; Iredell, J.R.; Sorrell, T.C.; et al. Meta-transcriptomics reveals a diverse antibiotic resistance gene pool in avian microbiomes. BMC Biol. 2019, 17, 31. [Google Scholar] [CrossRef]
- Zou, A.; Nadeau, K.; Xiong, X.; Wang, P.W.; Copeland, J.K.; Lee, J.Y.; Pierre, J.S.; Ty, M.; Taj, B.; Brumell, J.H.; et al. Systematic profiling of the chicken gut microbiome reveals dietary supplementation with antibiotics alters expression of multiple microbial pathways with minimal impact on community structure. Microbiome 2022, 10, 127. [Google Scholar] [CrossRef]
- Neveling, D.P.; Dicks, L.M.T. Probiotics: An Antibiotic Replacement Strategy for Healthy Broilers and Productive Rearing. Probiotics Antimicrob. Proteins 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Bodawatta, K.H.; Hird, S.M.; Grond, K.; Poulsen, M.; Jønsson, K.A. Avian gut microbiomes taking flight. Trends Microbiol. 2022, 30, 268–280. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef]
- Pilasombut, K.; Sakpuaram, T.; Wajjwalku, W.; Nitisinprasert, S.; Swetwiwathana, A.; Zendo, T.; Fujita, K.; Nakayama, J.; Sonomoto, K. Purification and amino acid sequence of a bacteriocins produced by Lactobacillus salivarius K7 isolated from chicken intestine. Songklanakarin J. Sci. Technol. 2006, 28, 121–131. [Google Scholar]
- Stern, N.J.; Svetoch, E.A.; Eruslanov, B.V.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Pokhilenko, V.D.; Levchuk, V.P.; Svetoch, O.E.; Seal, B.S. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob. Agents Chemother. 2006, 50, 3111–3116. [Google Scholar] [CrossRef]
- Svetoch, E.A.; Eruslanov, B.V.; Levchuk, V.P.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Stepanshin, J.; Dyatlov, I.; Seal, B.S.; Stern, N.J. Isolation of Lactobacillus salivarius 1077 (NRRL B-50053) and characterization of its bacteriocin, including the antimicrobial activity spectrum. Appl. Environ. Microbiol. 2011, 77, 2749–2754. [Google Scholar] [CrossRef]
- Messaoudi, S.; Kergourlay, G.; Rossero, A.; Ferchichi, M.; Prévost, H.; Drider, D.; Manai, M.; Dousset, X. Identification of lactobacilli residing in chicken ceca with antagonism against Campylobacter. Int. Microbiol. 2011, 14, 103–110. [Google Scholar]
- Messaoudi, S.; Manai, M.; Kergourlay, G.; Prévost, H.; Connil, N.; Chobert, J.M.; Dousset, X. Lactobacillus salivarius: Bacteriocin and probiotic activity. Food Microbiol. 2013, 36, 296–304. [Google Scholar] [CrossRef]
- Arbulu, S.; Jiménez, J.J.; Gútiez, L.; Campanero, C.; Del Campo, R.; Cintas, L.M.; Herranz, C.; Hernández, P.E. Evaluation of bacteriocinogenic activity, safety traits and biotechnological potential of fecal lactic acid bacteria (LAB), isolated from Griffon Vultures (Gyps fulvus subsp. fulvus). BMC Microbiol. 2016, 16, 228. [Google Scholar] [CrossRef]
- Javůrková, V.G.; Kreisinger, J.; Procházka, P.; Požgayová, M.; Ševčíková, K.; Brlík, V.; Adamík, P.; Heneberg, P.; Porkert, J. Unveiled feather microcosm: Feather microbiota of passerine birds is closely associated with host species identity and bacteriocin-producing bacteria. ISME J. 2019, 13, 2363–2376. [Google Scholar] [CrossRef]
- Martín-Platero, A.M.; Valdivia, E.; Ruíz-Rodríguez, M.; Soler, J.J.; Martín-Vivaldi, M.; Maqueda, M.; Martínez-Bueno, M. Characterization of Antimicrobial Substances Produced by Enterococcus faecalis MRR 10-3, Isolated from the Uropygial Gland of the Hoopoe (Upupa epops). Appl. Environ. Microbiol. 2006, 72, 4245–4249. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, M.; Martínez-Bueno, M.; Martín-Vivaldi, M.; Valdivia, E.; Soler, J.J. Bacteriocins with a broader antimicrobial spectrum prevail in enterococcal symbionts isolated from the hoopoe’s uropygial gland. FEMS Microbiol. Ecol. 2013, 85, 495–502. [Google Scholar] [CrossRef]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The use of lactic Acid bacteria as a probiotic in Swine diets. Pathogens 2015, 4, 34–45. [Google Scholar] [CrossRef]
- Gupta, M.; Pattanaik, A.K.; Singh, A.; Sharma, S.; Jadhav, S.E.; Kumar, A.; Verma, A.K. Functional and probiotic characterization of Ligilactobacillus salivarius CPN60 isolated from calf faeces and its appraisal in rats. J. Biosci. Bioeng. 2021, 132, 575–584. [Google Scholar] [CrossRef]
- Garsa, A.K.; Choudhury, P.K.; Puniya, A.K.; Dhewa, T.; Malik, R.K.; Tomar, S.K. Bovicins: The Bacteriocins of Streptococci and Their Potential in Methane Mitigation. Probiotics Antimicrob. Proteins 2019, 11, 1403–1413. [Google Scholar] [CrossRef]
- Ochoa, J.L.; Sanchez, L.M.; Koo, B.M.; Doherty, J.S.; Rajendram, M.; Huang, K.C.; Gross, C.A.; Linington, R.G. Marine Mammal Microbiota Yields Novel Antibiotic with Potent Activity Against Clostridium difficile. ACS Infect. Dis. 2018, 4, 59–67. [Google Scholar] [CrossRef]
- Mousa, W.K.; Athar, B.; Merwin, N.J.; Magarvey, N.A. Antibiotics and specialized metabolites from the human microbiota. Nat. Prod. Rep. 2017, 34, 1302–1331. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2019, 10, 1–21. [Google Scholar] [CrossRef]
- Tagg, J.R. Streptococcal Bacteriocin-Like Inhibitory Substances: Some Personal Insights into the Bacteriocin-Like Activities Produced by Streptococci Good and Bad. Probiotics Antimicrob. Proteins 2009, 1, 60–66. [Google Scholar] [CrossRef]
- Santagati, M.; Scillato, M.; Patanè, F.; Aiello, C.; Stefani, S. Bacteriocin-producing oral streptococci and inhibition of respiratory pathogens. FEMS Immunol. Med. Microbiol. 2012, 65, 23–31. [Google Scholar] [CrossRef]
- O’Sullivan, J.N.; Rea, M.C.; O’Connor, P.M.; Hill, C.; Ross, R.P. Human skin microbiota is a rich source of bacteriocin-producing staphylococci that kill human pathogens. FEMS Microbiol. Ecol. 2018, 95, fiy241. [Google Scholar] [CrossRef]
- Kim, S.G.; Becattini, S.; Moody, T.U.; Shliaha, P.V.; Littmann, E.R.; Seok, R.; Gjonbalaj, M.; Eaton, V.; Fontana, E.; Amoretti, L.; et al. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nature 2019, 572, 665–669. [Google Scholar] [CrossRef]
- Coyne, M.J.; Béchon, N.; Matano, L.M.; McEneany, V.L.; Chatzidaki-Livanis, M.; Comstock, L.E. A family of anti-Bacteroidales peptide toxins wide-spread in the human gut microbiota. Nat. Commun. 2019, 10, 3460. [Google Scholar] [CrossRef]
- Sato, Y.; Atarashi, K.; Plichta, D.R.; Arai, Y.; Sasajima, S.; Kearney, S.M.; Suda, W.; Takeshita, K.; Sasaki, T.; Okamoto, S.; et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 2021, 599, 458–464. [Google Scholar] [CrossRef]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef]
- Terekhov, S.S.; Smirnov, I.V.; Malakhova, M.V.; Samoilov, A.E.; Manolov, A.I.; Nazarov, A.S.; Danilov, D.V.; Dubiley, S.A.; Osterman, I.A.; Rubtsova, M.P.; et al. Ultrahigh-throughput functional profiling of microbiota communities. Proc. Natl. Acad. Sci. USA 2018, 115, 9551–9556. [Google Scholar] [CrossRef]
- Baranova, M.N.; Kudzhaev, A.M.; Mokrushina, Y.A.; Babenko, V.V.; Kornienko, M.A.; Malakhova, M.V.; Yudin, V.G.; Rubtsova, M.P.; Zalevsky, A.; Belozerova, O.A.; et al. Deep Functional Profiling of Wild Animal Microbiomes Reveals Probiotic Bacillus pumilus Strains with a Common Biosynthetic Fingerprint. Int. J. Mol. Sci. 2022, 23, 1168. [Google Scholar] [CrossRef]
- Akbar, N.; Siddiqui, R.; Sagathevan, K.A.; Khan, N.A. Gut bacteria of animals/pests living in polluted environments are a potential source of antibacterials. Appl. Microbiol. Biotechnol. 2019, 103, 3955–3964. [Google Scholar] [CrossRef]
- Pereira, W.A.; Mendonça, C.M.N.; Urquiza, A.V.; Marteinsson, V.Þ.; LeBlanc, J.G.; Cotter, P.D.; Villalobos, E.F.; Romero, J.; Oliveira, R.P.S. Use of Probiotic Bacteria and Bacteriocins as an Alternative to Antibiotics in Aquaculture. Microorganisms 2022, 10, 1705. [Google Scholar] [CrossRef]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Liang, L.; Yang, C.; Liu, L.; Mai, G.; Li, H.; Wu, L.; Jin, M.; Chen, Y. Commensal bacteria-derived extracellular vesicles suppress ulcerative colitis through regulating the macrophages polarization and remodeling the gut microbiota. Microb. Cell Fact. 2022, 21, 88. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Moree, W.J.; Phelan, V.V.; Wu, C.H.; Bandeira, N.; Cornett, D.S.; Duggan, B.M.; Dorrestein, P.C. Interkingdom metabolic transformations captured by microbial imaging mass spectrometry. Proc. Natl. Acad. Sci. USA 2012, 109, 13811–13816. [Google Scholar] [CrossRef]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef]
- Schiessl, K.T.; Hu, F.; Jo, J.; Nazia, S.Z.; Wang, B.; Price-Whelan, A.; Min, W.; Dietrich, L.E.P. Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat. Commun. 2019, 10, 762. [Google Scholar] [CrossRef]
- Orazi, G.; Ruoff, K.L.; O’Toole, G.A. Pseudomonas aeruginosa Increases the Sensitivity of Biofilm-Grown Staphylococcus aureus to Membrane-Targeting Antiseptics and Antibiotics. mBio 2019, 10, e01501-19. [Google Scholar] [CrossRef]
- Tran, C.; Cock, I.E.; Chen, X.; Feng, Y. Antimicrobial Bacillus: Metabolites and Their Mode of Action. Antibiotics 2022, 11, 88. [Google Scholar] [CrossRef]
- Bilal, M.; Si, W.; Barbe, F.; Chevaux, E.; Sienkiewicz, O.; Zhao, X. Effects of novel probiotic strains of Bacillus pumilus and Bacillus subtilis on production, gut health, and immunity of broiler chickens raised under suboptimal conditions. Poult. Sci. 2021, 100, 100871. [Google Scholar] [CrossRef]
- Opriessnig, T.; Karuppannan, A.K.; Beckler, D.; Ait-Ali, T.; Cubas-Atienzar, A.; Halbur, P.G. Bacillus pumilus probiotic feed supplementation mitigates Lawsonia intracellularis shedding and lesions. Vet. Res. 2019, 50, 85. [Google Scholar] [CrossRef]
- Hlordzi, V.; Kuebutornye, F.K.A.; Afriyie, G.; Abarike, E.D.; Lu, Y.; Chi, S.; Anokyewaa, M.A. The use of Bacillus species in maintenance of water quality in aquaculture: A review. Aquac. Rep. 2020, 18, 100503. [Google Scholar] [CrossRef]
- Duc le, H.; Hong, H.A.; Barbosa, T.M.; Henriques, A.O.; Cutting, S.M. Characterization of Bacillus Probiotics Available for Human Use. Appl. Environ. Microbiol. 2004, 70, 2161–2171. [Google Scholar] [CrossRef]
- Adnani, N.; Rajski, S.R.; Bugni, T.S. Symbiosis-inspired approaches to antibiotic discovery. Nat. Prod. Rep. 2017, 34, 784–814. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Terekhov, S.S.; Osterman, I.A.; Smirnov, I.V. High-Throughput Screening of Biodiversity for Antibiotic Discovery. Acta Naturae 2018, 10, 23–29. [Google Scholar] [CrossRef]
- Agresti, J.J.; Antipov, E.; Abate, A.R.; Ahn, K.; Rowat, A.C.; Baret, J.C.; Marquez, M.; Klibanov, A.M.; Griffiths, A.D.; Weitz, D.A. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 4004–4009. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).