Molecular Profile of Intrahepatic Cholangiocarcinoma
Abstract
:1. Introduction
2. Methods
2.1. Database Search
2.2. Study Selection
2.3. Outcomes
2.4. Data Extraction
2.5. Data Synthesis
3. Results
3.1. Molecular Subtypes in Intrahepatic Cholangiocarcinoma
3.2. Regional Variations in Molecular Patterns
3.3. Tumor Characteristics According to Molecular Subtypes
3.4. Prognostication
3.5. Clinical Applications in Precision Medicine
3.5.1. Predicting Treatment Response and Treatment Selection
3.5.2. Targeted Therapies
3.5.3. Immunotherapy
3.5.4. Monitoring Treatment Responses
4. Discussion
4.1. Current Literature Gaps
4.2. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Yang, T.; Wu, M.; Shen, F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016, 379, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Vithayathil, M.; Khan, S.A. Current epidemiology of cholangiocarcinoma in Western countries. J. Hepatol. 2022, 77, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Semeraro, R.; Torrice, A.; Gatto, M.; Napoli, C.; Bragazzi, M.C.; Gentile, R.; Alvaro, D. Intra-hepatic and extrahepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J. Gastrointest. Oncol. 2010, 2, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Lee, S.; Azad, N.S.; Borad, M.J.; Kate Kelley, R.; Sivaraman, S.; Teschemaker, A.; Chopra, I.; Janjan, N.; Parasuraman, S.; et al. Temporal Changes in Cholangiocarcinoma Incidence and Mortality in the United States from 2001 to 2017. Oncologist 2022, 27, 874–883. [Google Scholar] [CrossRef]
- Choi, B.I.; Han, J.K.; Hong, S.T.; Lee, K.H. Clonorchiasis and cholangiocarcinoma: Etiologic relationship and imaging diagnosis. Clin. Microbiol. Rev. 2004, 17, 540–552. [Google Scholar] [CrossRef]
- Kurathong, S.; Lerdverasirikul, P.; Wongpaitoon, V.; Pramoolsinsap, C.; Kanjanapitak, A.; Varavithya, W.; Phuapradit, P.; Bunyaratvej, S.; Upatham, E.S.; Brockelman, W.Y. Opisthorchis viverrini infection and cholangiocarcinoma. A prospective, case-controlled study. Gastroenterology 1985, 89, 151–156. [Google Scholar] [CrossRef]
- De Lorenzo, S.; Tovoli, F.; Mazzotta, A.; Vasuri, F.; Edeline, J.; Malvi, D.; Boudjema, K.; Renzulli, M.; Jeddou, H.; D’Errico, A.; et al. Non-Alcoholic Steatohepatitis as a Risk Factor for Intrahepatic Cholangiocarcinoma and Its Prognostic Role. Cancers 2020, 12, 3182. [Google Scholar] [CrossRef]
- Shaib, Y.H.; El-Serag, H.B.; Davila, J.A.; Morgan, R.; McGlynn, K.A. Risk factors of intrahepatic cholangiocarcinoma in the United States: A case-control study. Gastroenterology 2005, 128, 620–626. [Google Scholar] [CrossRef]
- Gupta, A.; Dixon, E. Epidemiology and risk factors: Intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 101–104. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Morimoto, Y.; Tanaka, Y.; Ito, T.; Nakahara, M.; Nakaba, H.; Nishida, T.; Fujikawa, M.; Ito, T.; Yamamoto, S.; Kitagawa, T. Long-term survival and prognostic factors in the surgical treatment for intrahepatic cholangiocarcinoma. J. Hepatobiliary Pancreat. Surg. 2003, 10, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.V.; Zhang, S.; Chen, X.; Calvisi, D.F.; Andersen, J.B. Molecular profiling of intrahepatic cholangiocarcinoma: The search for new therapeutic targets. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Slatko, B.E.; Gardner, A.F.; Ausubel, F.M. Overview of Next-Generation Sequencing Technologies. Curr. Protoc. Mol. Biol. 2018, 122, e59. [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child. Educ. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [CrossRef]
- Weinberg, B.A.; Xiu, J.; Lindberg, M.R.; Shields, A.F.; Hwang, J.J.; Poorman, K.; Salem, M.E.; Pishvaian, M.J.; Holcombe, R.F.; Marshall, J.L.; et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J. Gastrointest. Oncol. 2019, 10, 652–662. [Google Scholar] [CrossRef]
- Tomczak, A.; Springfeld, C.; Dill, M.T.; Chang, D.H.; Kazdal, D.; Wagner, U.; Mehrabi, A.; Brockschmidt, A.; Luedde, T.; Naumann, P.; et al. Precision oncology for intrahepatic cholangiocarcinoma in clinical practice. Br. J. Cancer 2022, 127, 1701–1708. [Google Scholar] [CrossRef]
- Voss, J.S.; Holtegaard, L.M.; Kerr, S.E.; Fritcher, E.G.; Roberts, L.R.; Gores, G.J.; Zhang, J.; Highsmith, W.E.; Halling, K.C.; Kipp, B.R. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum. Pathol. 2013, 44, 1216–1222. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Z.; Yu, Y.; Chen, Y.; Liu, H.; Guo, Y.; Peng, Z.; Cai, G.; Hua, Z.; Han, X.; et al. TP53/KRAS Co-Mutations Create Divergent Prognosis Signatures in Intrahepatic Cholangiocarcinoma. Front. Genet. 2022, 13, 844800. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Kamgar, M.; Mahipal, A. Targeted Therapies in Advanced Biliary Tract Cancer: An Evolving Paradigm. Cancers 2020, 12, 2039. [Google Scholar] [CrossRef]
- Miyamoto, M.; Ojima, H.; Iwasaki, M.; Shimizu, H.; Kokubu, A.; Hiraoka, N.; Kosuge, T.; Yoshikawa, D.; Kono, T.; Furukawa, H.; et al. Prognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinoma. Br. J. Cancer 2011, 105, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kipp, B.R.; Voss, J.S.; Kerr, S.E.; Barr Fritcher, E.G.; Graham, R.P.; Zhang, L.; Highsmith, W.E.; Zhang, J.; Roberts, L.R.; Gores, G.J.; et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum. Pathol. 2012, 43, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Reitman, Z.J.; Yan, H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: Alterations at a crossroads of cellular metabolism. J. Natl. Cancer Inst. 2010, 102, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Borger, D.R.; Tanabe, K.K.; Fan, K.C.; Lopez, H.U.; Fantin, V.R.; Straley, K.S.; Schenkein, D.P.; Hezel, A.F.; Ancukiewicz, M.; Liebman, H.M.; et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012, 17, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Esposito Abate, R.; Rachiglio, A.M.; Maiello, M.R.; Esposito, C.; Schettino, C.; Izzo, F.; Nasti, G.; Normanno, N. FGFR Fusions in Cancer: From Diagnostic Approaches to Therapeutic Intervention. Int. J. Mol. Sci. 2020, 21, 6856. [Google Scholar] [CrossRef] [PubMed]
- Donehower, L.A.; Soussi, T.; Korkut, A.; Liu, Y.; Schultz, A.; Cardenas, M.; Li, X.; Babur, O.; Hsu, T.K.; Lichtarge, O.; et al. Integrated Analysis of TP53 Gene and Pathway Alterations in The Cancer Genome Atlas. Cell Rep. 2019, 28, 1370–1384.e5, Erratum in Cell Rep. 2019, 28, 3010. [Google Scholar] [CrossRef]
- Momand, J.; Zambetti, G.P.; Olson, D.C.; George, D.; Levine, A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992, 69, 1237–1245. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, B.; Yang, Z.Y.; Li, O.; Liu, S.L.; Pan, L.J.; Gong, W.; Dong, P.; Shu, Y.J. Inhibition of XPO1 impairs cholangiocarcinoma cell proliferation by triggering p53 intranuclear accumulation. Cancer Med. 2023, 12, 5751–5763. [Google Scholar] [CrossRef]
- Hill, M.A.; Alexander, W.B.; Guo, B.; Kato, Y.; Patra, K.; O’Dell, M.R.; McCall, M.N.; Whitney-Miller, C.L.; Bardeesy, N.; Hezel, A.F. Kras and Tp53 Mutations Cause Cholangiocyte- and Hepatocyte-Derived Cholangiocarcinoma. Cancer Res. 2018, 78, 4445–4451. [Google Scholar] [CrossRef]
- Gualdrini, F.; Esnault, C.; Horswell, S.; Stewart, A.; Matthews, N.; Treisman, R. SRF Co-factors Control the Balance between Cell Proliferation and Contractility. Mol. Cell 2016, 64, 1048–1061. [Google Scholar] [CrossRef]
- Liu, R.Y.; Zhang, Y.; Smolen, P.; Cleary, L.J.; Byrne, J.H. Role of p90 ribosomal S6 kinase in long-term synaptic facilitation and enhanced neuronal excitability. Sci. Rep. 2020, 10, 608. [Google Scholar] [CrossRef]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS mutation: From undruggable to druggable in cancer. Signal Transduct. Target. Ther. 2021, 6, 386. [Google Scholar] [CrossRef]
- Mullen, J.; Kato, S.; Sicklick, J.K.; Kurzrock, R. Targeting ARID1A mutations in cancer. Cancer Treat. Rev. 2021, 100, 102287. [Google Scholar] [CrossRef]
- Park, Y.; Chui, M.H.; Suryo Rahmanto, Y.; Yu, Z.C.; Shamanna, R.A.; Bellani, M.A.; Gaillard, S.; Ayhan, A.; Viswanathan, A.; Seidman, M.M.; et al. Loss of ARID1A in Tumor Cells Renders Selective Vulnerability to Combined Ionizing Radiation and PARP Inhibitor Therapy. Clin. Cancer Res. 2019, 25, 5584–5594. [Google Scholar] [CrossRef]
- Park, J.H.; Park, E.J.; Lee, H.S.; Kim, S.J.; Hur, S.K.; Imbalzano, A.N.; Kwon, J. Mammalian SWI/SNF complexes facilitate DNA double-strand break repair by promoting gamma-H2AX induction. EMBO J. 2006, 25, 3986–3997. [Google Scholar] [CrossRef]
- Mandal, J.; Mandal, P.; Wang, T.L.; Shih, I.M. Treating ARID1A mutated cancers by harnessing synthetic lethality and DNA damage response. J. Biomed. Sci. 2022, 29, 71. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Namjan, A.; Techasen, A.; Loilome, W.; Sa-Ngaimwibool, P.; Jusakul, A. ARID1A alterations and their clinical significance in cholangiocarcinoma. PeerJ 2020, 8, e10464. [Google Scholar] [CrossRef]
- Zou, S.; Li, J.; Zhou, H.; Frech, C.; Jiang, X.; Chu, J.S.; Zhao, X.; Li, Y.; Li, Q.; Wang, H.; et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat. Commun. 2014, 5, 5696. [Google Scholar] [CrossRef]
- Riener, M.O.; Bawohl, M.; Clavien, P.A.; Jochum, W. Rare PIK3CA hotspot mutations in carcinomas of the biliary tract. Genes. Chromosomes Cancer 2008, 47, 363–367. [Google Scholar] [CrossRef]
- Xu, R.F.; Sun, J.P.; Zhang, S.R.; Zhu, G.S.; Li, L.B.; Liao, Y.L.; Xie, J.M.; Liao, W.J. KRAS and PIK3CA but not BRAF genes are frequently mutated in Chinese cholangiocarcinoma patients. Biomed. Pharmacother. 2011, 65, 22–26. [Google Scholar] [CrossRef]
- Galdy, S.; Lamarca, A.; McNamara, M.G.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017, 36, 141–157. [Google Scholar] [CrossRef]
- Sadot, E.; Simpson, A.L.; Do, R.K.; Gonen, M.; Shia, J.; Allen, P.J.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Jarnagin, W.R. Cholangiocarcinoma: Correlation between Molecular Profiling and Imaging Phenotypes. PLoS ONE 2015, 10, e0132953. [Google Scholar] [CrossRef]
- Goyal, L.; Govindan, A.; Sheth, R.A.; Nardi, V.; Blaszkowsky, L.S.; Faris, J.E.; Clark, J.W.; Ryan, D.P.; Kwak, E.L.; Allen, J.N.; et al. Prognosis and Clinicopathologic Features of Patients with Advanced Stage Isocitrate Dehydrogenase (IDH) Mutant and IDH Wild-Type Intrahepatic Cholangiocarcinoma. Oncologist 2015, 20, 1019–1027. [Google Scholar] [CrossRef]
- Boberg, K.M.; Schrumpf, E.; Bergquist, A.; Broomé, U.; Pares, A.; Remotti, H.; Schjölberg, A.; Spurkland, A.; Clausen, O.P. Cholangiocarcinoma in primary sclerosing cholangitis: K-ras mutations and Tp53 dysfunction are implicated in the neoplastic development. J. Hepatol. 2000, 32, 374–380. [Google Scholar] [CrossRef]
- Sasaki, M.; Sato, Y.; Nakanuma, Y. Cholangiolocellular Carcinoma with “Ductal Plate Malformation” Pattern May Be Characterized by ARID1A Genetic Alterations. Am. J. Surg. Pathol. 2019, 43, 352–360. [Google Scholar] [CrossRef]
- Pu, X.H.; Yue, S.; Wu, H.Y.; Yang, J.; Fan, X.S.; Fu, Y.; Ye, Q.; Chen, J. C-MET in intrahepatic cholangiocarcinoma: High-Frequency amplification predicts protein expression and a unique molecular subtype. Pathol. Res. Pract. 2020, 216, 152857. [Google Scholar] [CrossRef]
- Wattanawongdon, W.; Simawaranon Bartpho, T.; Tongtawee, T. Expression of CD44 and MDM2 in cholangiocarcinoma is correlated with poor clinicopathologic characteristics. Int. J. Clin. Exp. Pathol. 2019, 12, 3961–3967. [Google Scholar]
- Yang, S.Z.; Wang, A.Q.; Du, J.; Wang, J.T.; Yu, W.W.; Liu, Q.; Wu, Y.F.; Chen, S.G. Low expression of ARID1A correlates with poor prognosis in intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2016, 22, 5814–5821. [Google Scholar] [CrossRef]
- Conci, S.; Ruzzenente, A.; Simbolo, M.; Bagante, F.; Rusev, B.; Isa, G.; Lawlor, R.T.; Pedrazzani, C.; Iacono, C.; Guglielmi, A.; et al. Multigene mutational profiling of biliary tract cancer is related to the pattern of recurrence in surgically resected patients. Updates Surg. 2020, 72, 119–128. [Google Scholar] [CrossRef]
- Bi, C.; Liu, M.; Rong, W.; Wu, F.; Zhang, Y.; Lin, S.; Liu, Y.; Wu, J.; Wang, L. High Beclin-1 and ARID1A expression corelates with poor survival and high recurrence in intrahepatic cholangiocarcinoma: A histopathological retrospective study. BMC Cancer 2019, 19, 213. [Google Scholar] [CrossRef]
- Robertson, S.; Hyder, O.; Dodson, R.; Nayar, S.K.; Poling, J.; Beierl, K.; Eshleman, J.R.; Lin, M.T.; Pawlik, T.M.; Anders, R.A. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum. Pathol. 2013, 44, 2768–2773. [Google Scholar] [CrossRef]
- Xin, H.Y.; Sun, R.Q.; Zou, J.X.; Wang, P.C.; Wang, J.Y.; Ye, Y.H.; Liu, K.X.; Hu, Z.Q.; Zhou, Z.J.; Fan, J.; et al. Association of BRAF Variants with Disease Characteristics, Prognosis, and Targeted Therapy Response in Intrahepatic Cholangiocarcinoma. JAMA Netw. Open. 2023, 6, e231476. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Bibeau, K.; Schultz, N.; Yaqubie, A.; Millang, B.; Ren, H.; Féliz, L. Effect of FGFR2 Alterations on Overall and Progression-Free Survival in Patients Receiving Systemic Therapy for Intrahepatic Cholangiocarcinoma. Target. Oncol. 2022, 17, 517–527. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Aschele, C.; Lonardi, S.; Monfardini, S. Thymidylate Synthase expression as a predictor of clinical response to fluoropyrimidine-based chemotherapy in advanced colorectal cancer. Cancer Treat. Rev. 2002, 28, 27–47. [Google Scholar] [CrossRef]
- Kato, T.; Ono, H.; Fujii, M.; Akahoshi, K.; Ogura, T.; Ogawa, K.; Ban, D.; Kudo, A.; Tanaka, S.; Tanabe, M. Cytoplasmic RRM1 activation as an acute response to gemcitabine treatment is involved in drug resistance of pancreatic cancer cells. PLoS ONE 2021, 16, e0252917. [Google Scholar] [CrossRef]
- Du, P.; Wang, Y.; Chen, L.; Gan, Y.; Wu, Q. High ERCC1 expression is associated with platinum-resistance, but not survival in patients with epithelial ovarian cancer. Oncol. Lett. 2016, 12, 857–862. [Google Scholar] [CrossRef]
- Fiorini, C.; Cordani, M.; Padroni, C.; Blandino, G.; Di Agostino, S.; Donadelli, M. Mutant p53 stimulates chemoresistance of pancreatic adenocarcinoma cells to Gemcitabine. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2015, 1853, 89–100. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, M.; Xu, Y.; Yu, L. The Development of p53-Targeted Therapies for Human Cancers. Cancers 2023, 15, 3560. [Google Scholar] [CrossRef]
- Shen, H.; Maki, C.G. Pharmacologic activation of p53 by small-molecule MDM2 antagonists. Curr. Pharm. Des. 2011, 17, 560–568. [Google Scholar] [CrossRef]
- Wang, S.; Sun, W.; Zhao, Y.; McEachern, D.; Meaux, I.; Barrière, C.; Stuckey, J.A.; Meagher, J.L.; Bai, L.; Liu, L.; et al. SAR405838: An optimized inhibitor of MDM2-p53 interaction that induces complete and durable tumor regression. Cancer Res. 2014, 74, 5855–5865. [Google Scholar] [CrossRef]
- Burris, H.A., 3rd; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with Gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef]
- Mohell, N.; Alfredsson, J.; Fransson, Å.; Uustalu, M.; Byström, S.; Gullbo, J.; Hallberg, A.; Bykov, V.J.; Björklund, U.; Wiman, K.G. APR-246 overcomes resistance to cisplatin and doxorubicin in ovarian cancer cells. Cell Death Dis. 2015, 6, e1794. [Google Scholar] [CrossRef]
- Sallman, D.A.; DeZern, A.E.; Garcia-Manero, G.; Steensma, D.P.; Roboz, G.J.; Sekeres, M.A.; Cluzeau, T.; Sweet, K.L.; McLemore, A.; McGraw, K.L.; et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 1584–1594. [Google Scholar] [CrossRef]
- Wang, H.; Chi, L.; Yu, F.; Dai, H.; Gao, C.; Si, X.; Wang, Z.; Liu, L.; Zheng, J.; Shan, L.; et al. Annual review of KRAS inhibitors in 2022. Eur. J. Med. Chem. 2023, 249, 115124. [Google Scholar] [CrossRef]
- Wilson, C.Y.; Tolias, P. Recent advances in cancer drug discovery targeting RAS. Drug Discov. Today 2016, 21, 1915–1919. [Google Scholar] [CrossRef]
- Dong, M.; Liu, X.; Evert, K.; Utpatel, K.; Peters, M.; Zhang, S.; Xu, Z.; Che, L.; Cigliano, A.; Ribback, S.; et al. Efficacy of MEK inhibition in a K-Ras-driven cholangiocarcinoma pre-clinical model. Cell Death Dis. 2018, 9, 31. [Google Scholar] [CrossRef]
- Williamson, C.T.; Miller, R.; Pemberton, H.N.; Jones, S.E.; Campbell, J.; Konde, A.; Badham, N.; Rafiq, R.; Brough, R.; Gulati, A.; et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat. Commun. 2016, 7, 13837. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807, Erratum in Lancet Oncol. 2020, 21, e462. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma with IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, P.; Gęca, K.; Rawicz-Pruszyński, K.; Polkowski, W.P.; Skórzewska, M. FGFR Inhibitors in Cholangiocarcinoma-A Novel Yet Primary Approach: Where Do We Stand Now and Where to Head Next in Targeting This Axis? Cells 2022, 11, 3929. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, H.; Yu, X.; Qin, J.J. Drug-resistant HER2-positive breast cancer: Molecular mechanisms and overcoming strategies. Front. Pharmacol. 2022, 13, 1012552. [Google Scholar] [CrossRef] [PubMed]
- Ayasun, R.; Ozer, M.; Sahin, I. The Role of HER2 Status in the Biliary Tract Cancers. Cancers 2023, 15, 2628. [Google Scholar] [CrossRef]
- Javle, M.M.; Oh, D.Y.; Ikeda, M.; Yong, W.P.; Hsu, K.; Lindmark, B.; McIntyre, N.; Firth, C. Varlitinib plus Capecitabine in second-line advanced biliary tract cancer: A randomized, phase II study (TreeTopp). ESMO Open 2022, 7, 100314. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Rodon, J.; Argilés, G.; Connolly, R.M.; Vaishampayan, U.; de Jonge, M.; Garralda, E.; Giannakis, M.; Smith, D.C.; Dobson, J.R.; McLaughlin, M.E.; et al. Phase 1 study of single-agent WNT974, a first-in-class Porcupine inhibitor, in patients with advanced solid tumours. Br. J. Cancer 2021, 125, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Wang, C.; Wang, J.; Zhang, S.; Bo, X.; Wang, Y.; Liu, H. ARID1A Downregulation Predicts High PD-L1 Expression and Worse Clinical Outcome in Patients with Gallbladder Cancer. Front. Oncol. 2022, 12, 787897. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. KEYNOTE-966 Investigators. Pembrolizumab in combination with Gemcitabine and cisplatin compared with Gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865, Erratum in Lancet 2023, 402, 964. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.; Wang, H.; DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Watts, J.M.; Pollyea, D.A.; et al. Molecular mechanisms mediating relapse following ivosidenib monotherapy in IDH1-mutant relapsed or refractory AML. Blood Adv. 2020, 4, 1894–1905. [Google Scholar] [CrossRef]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov. 2017, 7, 252–263. [Google Scholar] [CrossRef]
- Xue, R.; Chen, L.; Zhang, C.; Fujita, M.; Li, R.; Yan, S.M.; Ong, C.K.; Liao, X.; Gao, Q.; Sasagawa, S.; et al. Genomic and Transcriptomic Profiling of Combined Hepatocellular and Intrahepatic Cholangiocarcinoma Reveals Distinct Molecular Subtypes. Cancer Cell 2019, 35, 932–947.e8. [Google Scholar] [CrossRef]
- Farshidfar, F.; Zheng, S.; Gingras, M.C.; Newton, Y.; Shih, J.; Robertson, A.G.; Hinoue, T.; Hoadley, K.A.; Gibb, E.A.; Roszik, J.; et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell Rep. 2017, 18, 2780–2794, Erratum in Cell Rep. 2017, 19, 2878–2880. [Google Scholar] [CrossRef]
- Jiao, Y.; Pawlik, T.M.; Anders, R.A.; Selaru, F.M.; Streppel, M.M.; Lucas, D.J.; Niknafs, N.; Guthrie, V.B.; Maitra, A.; Argani, P.; et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat. Genet. 2013, 45, 1470–1473. [Google Scholar] [CrossRef]
- Jolissaint, J.S.; Soares, K.C.; Seier, K.P.; Kundra, R.; Gönen, M.; Shin, P.J.; Boerner, T.; Sigel, C.; Madupuri, R.; Vakiani, E.; et al. Intrahepatic Cholangiocarcinoma with Lymph Node Metastasis: Treatment-Related Outcomes and the Role of Tumor Genomics in Patient Selection. Clin. Cancer Res. 2021, 27, 4101–4108. [Google Scholar] [CrossRef]
- Boerner, T.; Drill, E.; Pak, L.M.; Nguyen, B.; Sigel, C.S.; Doussot, A.; Shin, P.; Goldman, D.A.; Gonen, M.; Allen, P.J.; et al. Genetic Determinants of Outcome in Intrahepatic Cholangiocarcinoma. Hepatology 2021, 74, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
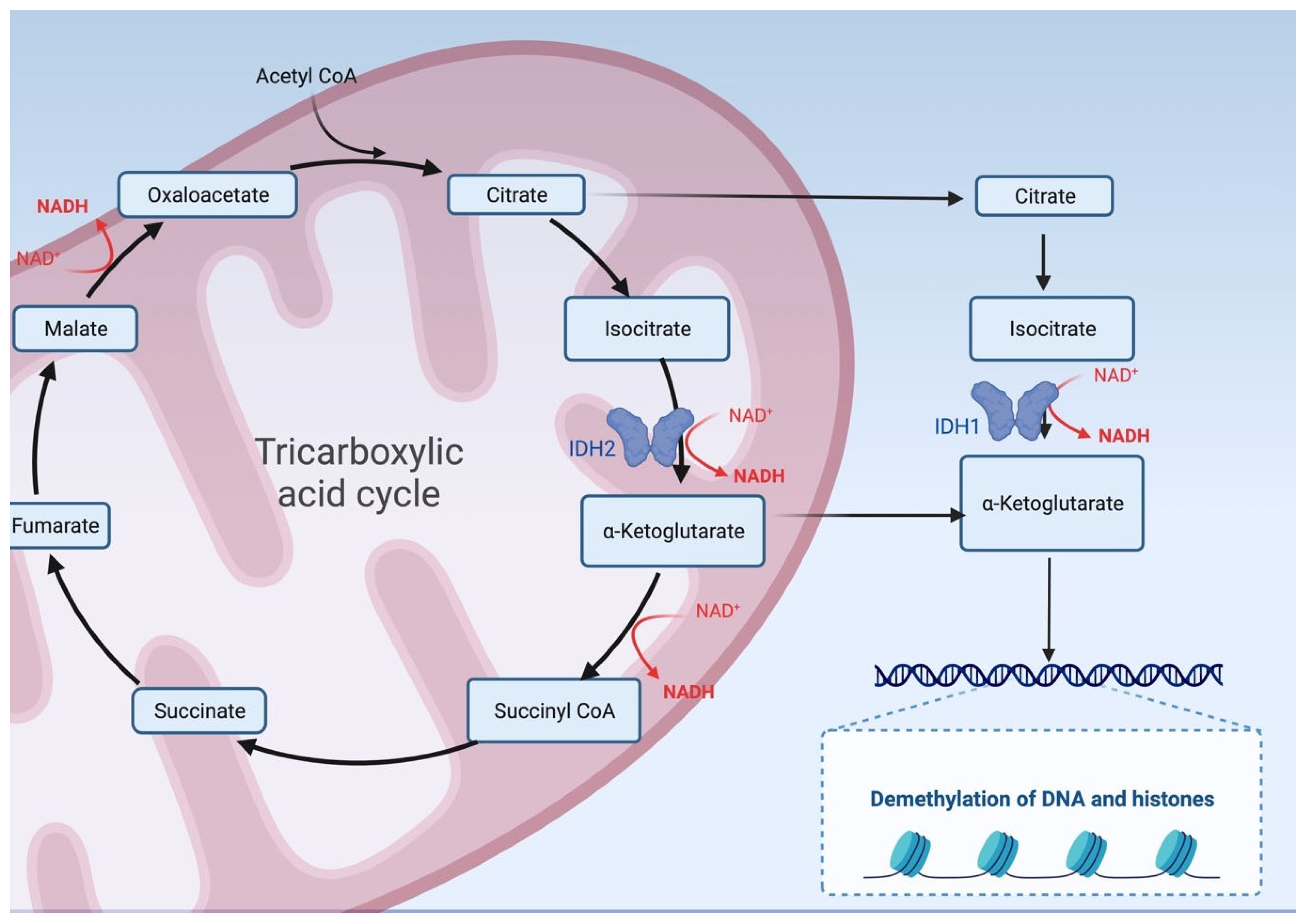
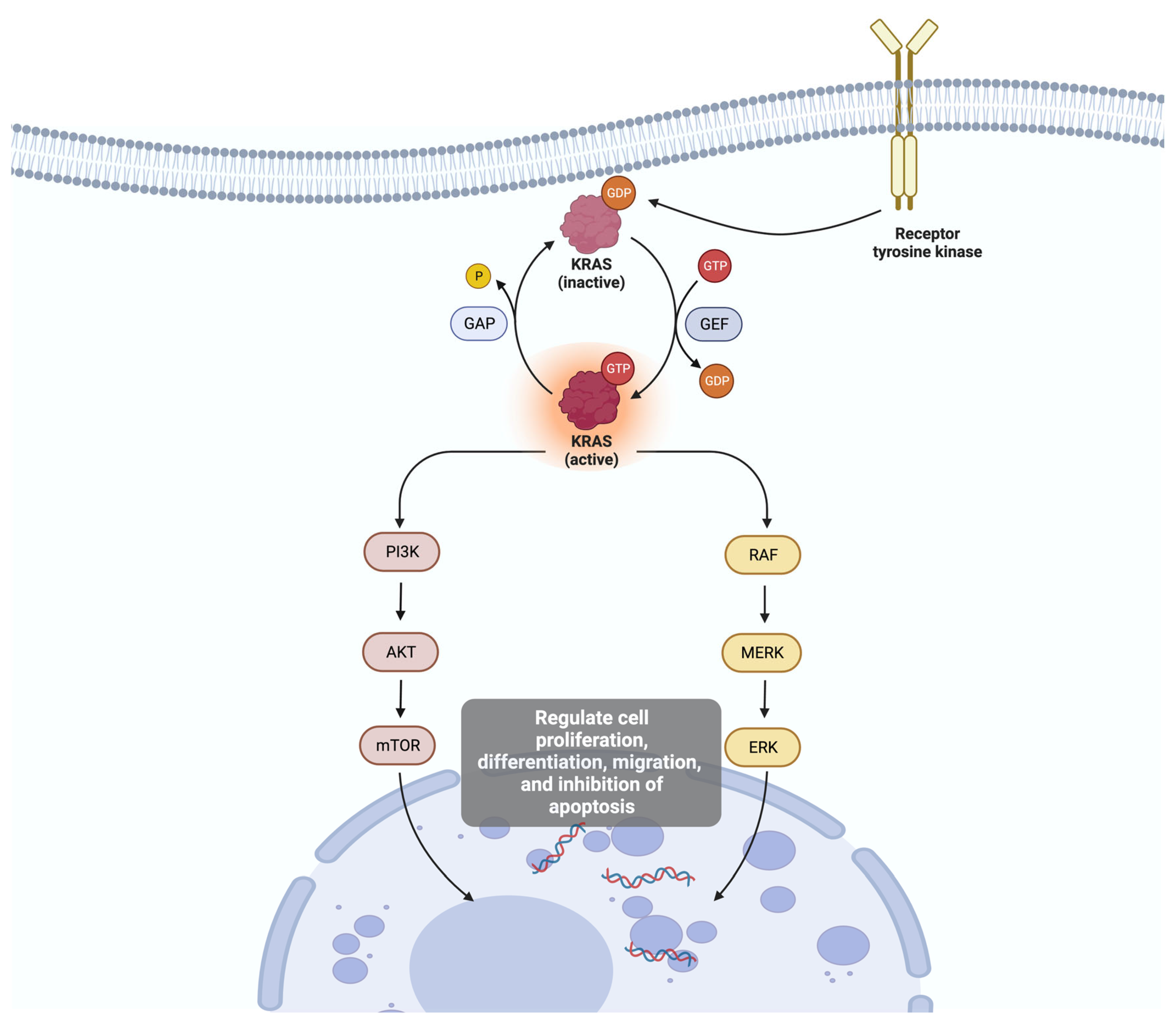
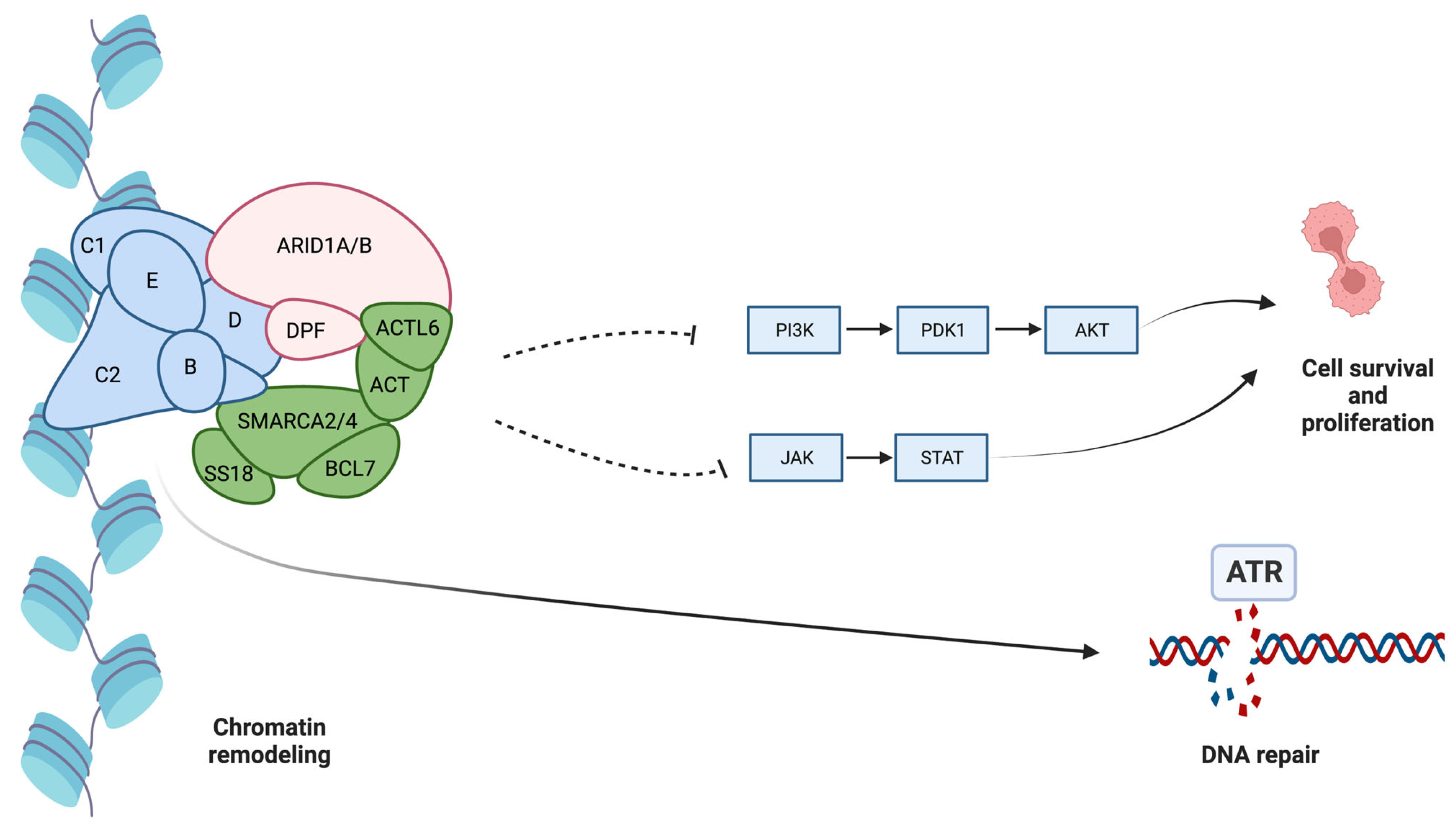
| Mutation | Frequency (%) * | Potential Target-Therapies |
|---|---|---|
| TP53 | 18–43 | Idasanutlin, Alrizomadlin, Eprenetapopt, SAR405838, Milademetan |
| IDH1 | 22 | Ivosidenib |
| IDH2 | 5 | Enasidenib |
| KRAS | 10–18 | Sotorasib, Adagrasib, Selumetinib, Trametinib |
| ARID1A | 19–22 | Olaparib, Niraparib, Rucaparib, Veliparib, Ceralasertib, Berzosertib |
| BAP1 | 9–19 | Olaparib |
| BRAF | 7 | Dabrafenib, Trametinib, Vemurafenib, Encorafenib |
| FGFR2 | 5 | Derazantinib, Infigratinib, Pemigatinib, Futibatinib, Ponatinib, Erdafitinib, Debio 1347 |
| Her2 | 1 | Trastuzumab, Pertuzumab, Lapatinib, Tucatinib, Neratinib, Varlitinib, Taselisib |
| Mutation | Prognostic Implications |
|---|---|
| TP53 | Advanced stages, increased tumor mutational burden, and poor survival rates |
| IDH1 | No significant association with either lymph node dissemination or overall or recurrence-free survival |
| IDH2 | |
| KRAS | Increased tumor mutational burden and poor survival rates |
| ARID1A | Poor survival, higher risk for vein invasion, and higher risk for recurrence (systemic or local) |
| C-MET | Advanced oncological stage, mainly the T stage |
| BRAF | Higher oncologic stage, resistance to systemic chemotherapy, and lower survival rate |
| FGFR2 | Better overall and progression-free survival |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andraus, W.; Tustumi, F.; de Meira Junior, J.D.; Pinheiro, R.S.N.; Waisberg, D.R.; Lopes, L.D.; Arantes, R.M.; Rocha Santos, V.; de Martino, R.B.; Carneiro D’Albuquerque, L.A. Molecular Profile of Intrahepatic Cholangiocarcinoma. Int. J. Mol. Sci. 2024, 25, 461. https://doi.org/10.3390/ijms25010461
Andraus W, Tustumi F, de Meira Junior JD, Pinheiro RSN, Waisberg DR, Lopes LD, Arantes RM, Rocha Santos V, de Martino RB, Carneiro D’Albuquerque LA. Molecular Profile of Intrahepatic Cholangiocarcinoma. International Journal of Molecular Sciences. 2024; 25(1):461. https://doi.org/10.3390/ijms25010461
Chicago/Turabian StyleAndraus, Wellington, Francisco Tustumi, José Donizeti de Meira Junior, Rafael Soares Nunes Pinheiro, Daniel Reis Waisberg, Liliana Ducatti Lopes, Rubens Macedo Arantes, Vinicius Rocha Santos, Rodrigo Bronze de Martino, and Luiz Augusto Carneiro D’Albuquerque. 2024. "Molecular Profile of Intrahepatic Cholangiocarcinoma" International Journal of Molecular Sciences 25, no. 1: 461. https://doi.org/10.3390/ijms25010461
APA StyleAndraus, W., Tustumi, F., de Meira Junior, J. D., Pinheiro, R. S. N., Waisberg, D. R., Lopes, L. D., Arantes, R. M., Rocha Santos, V., de Martino, R. B., & Carneiro D’Albuquerque, L. A. (2024). Molecular Profile of Intrahepatic Cholangiocarcinoma. International Journal of Molecular Sciences, 25(1), 461. https://doi.org/10.3390/ijms25010461








