Elucidating Differences in Early-Stage Centrosome Amplification in Primary and Immortalized Mouse Cells
Abstract
1. Introduction
2. Results
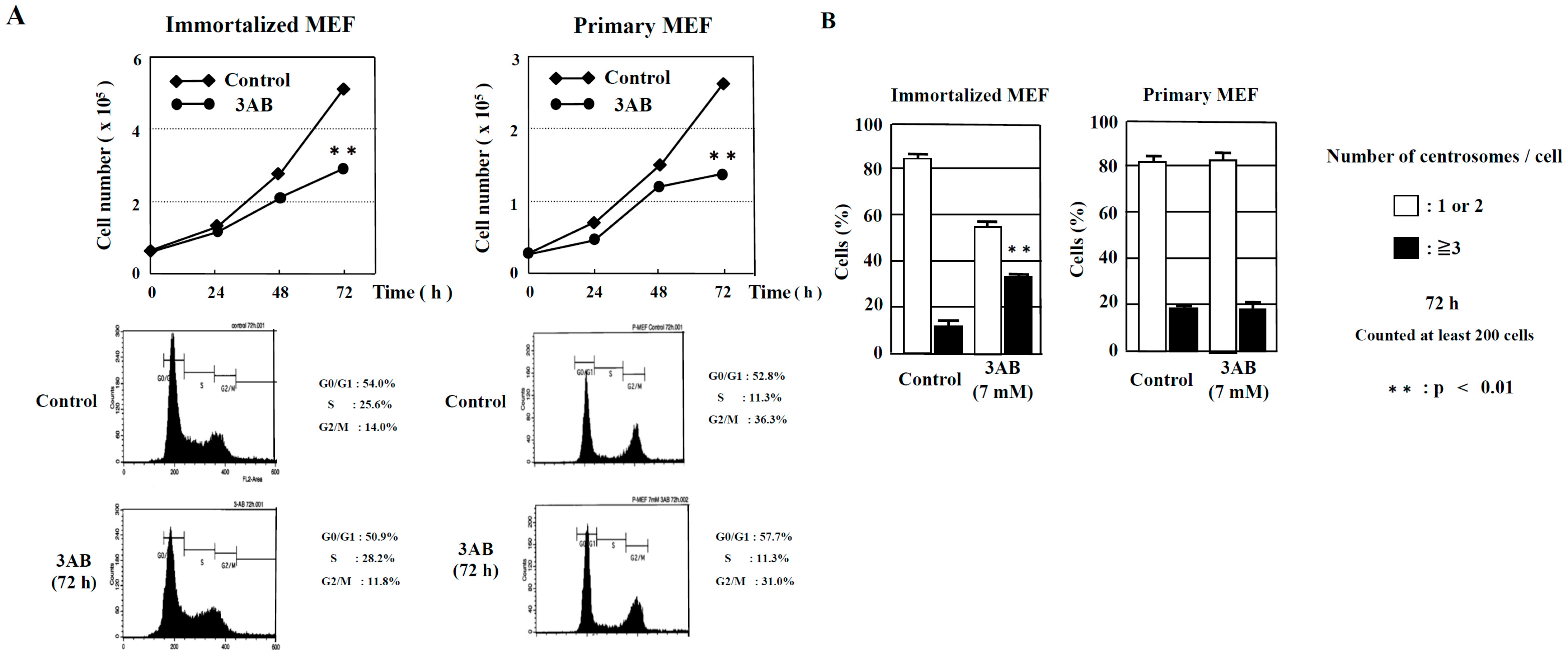
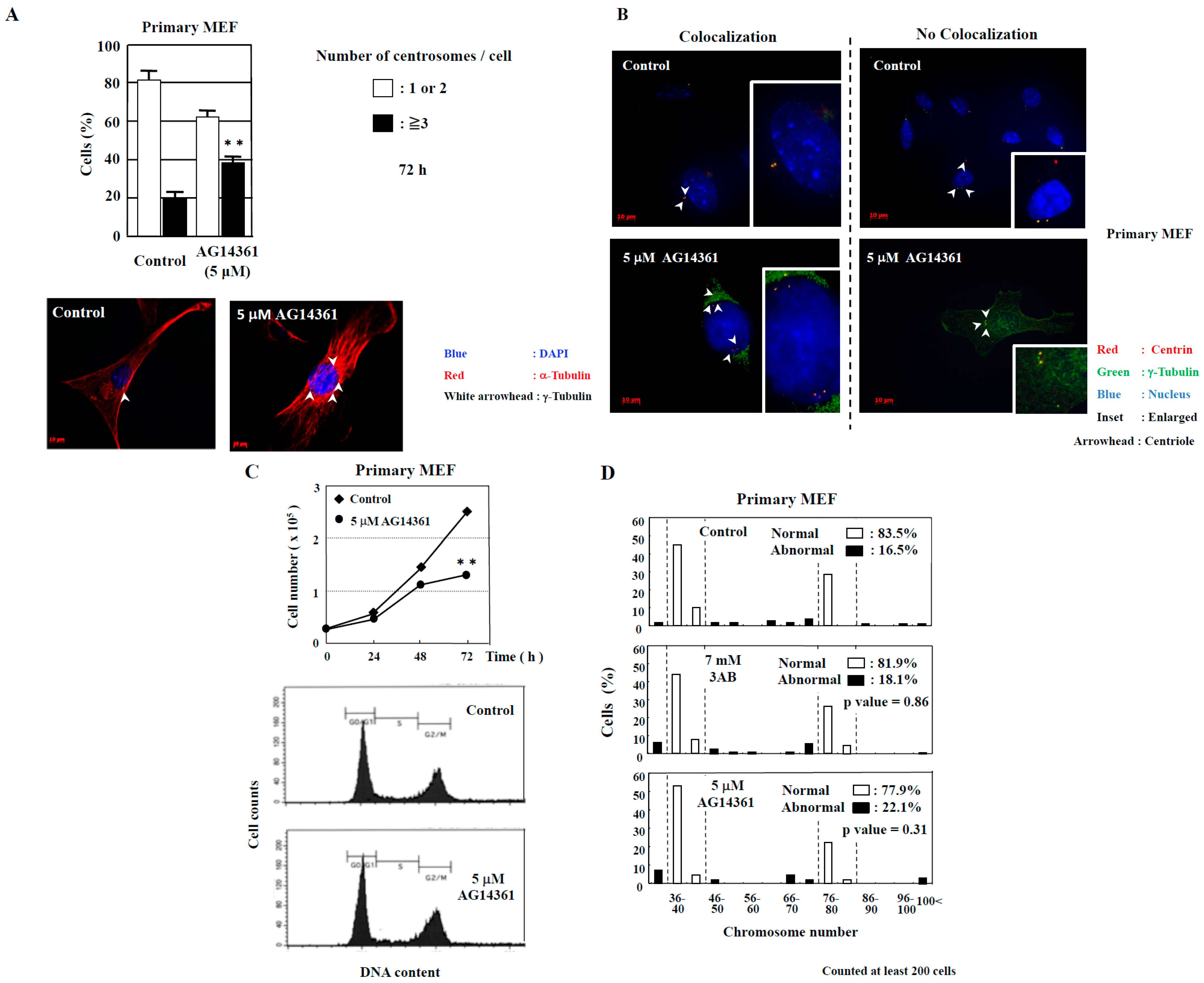
2.1. Evaluation of Centrosome Amplification after Treatment with PARP Inhibitors in Immortalized MEF and Primary MEF
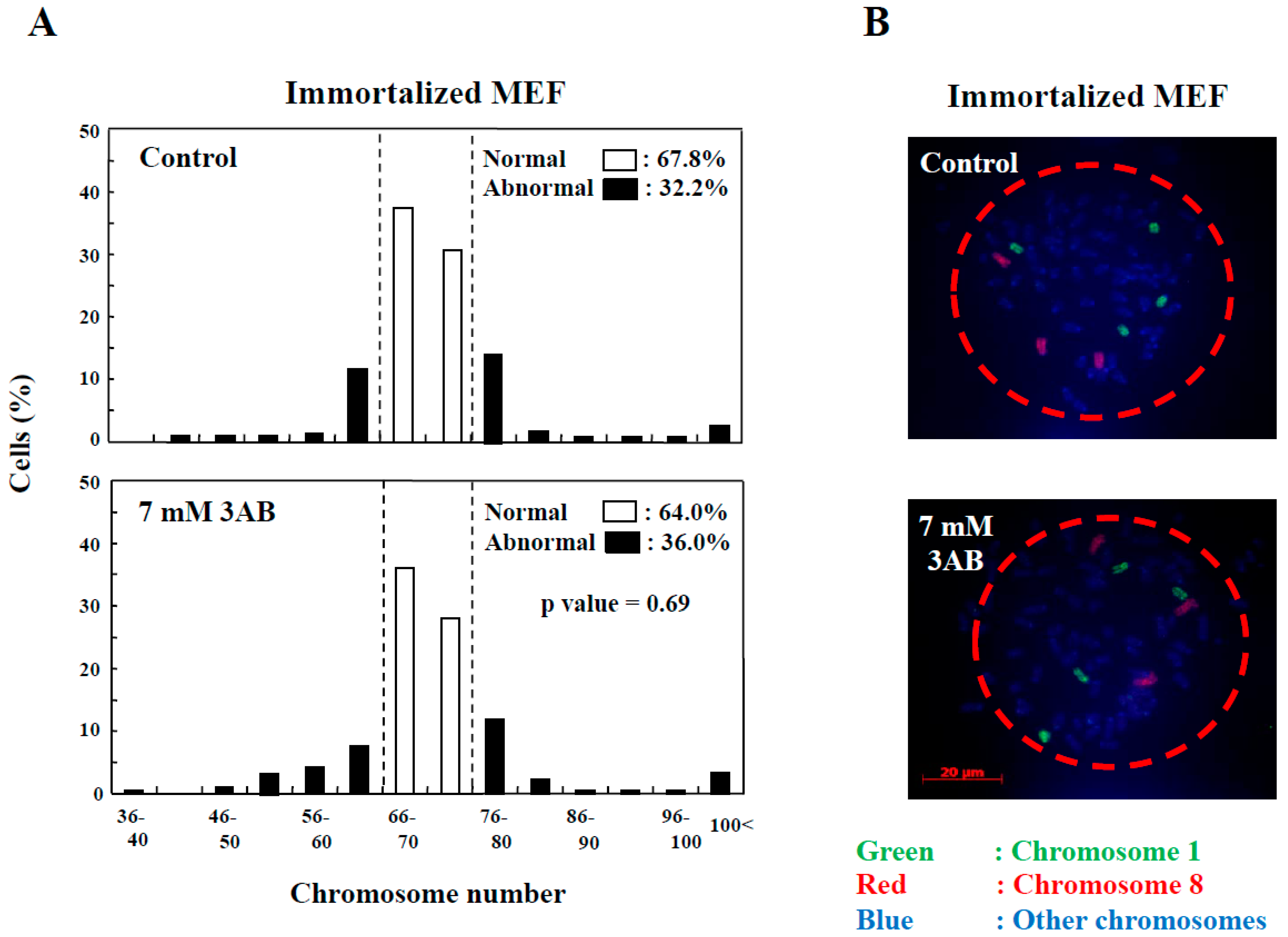
2.2. Examination of Chromosome Number Abnormality after PARP Inhibition
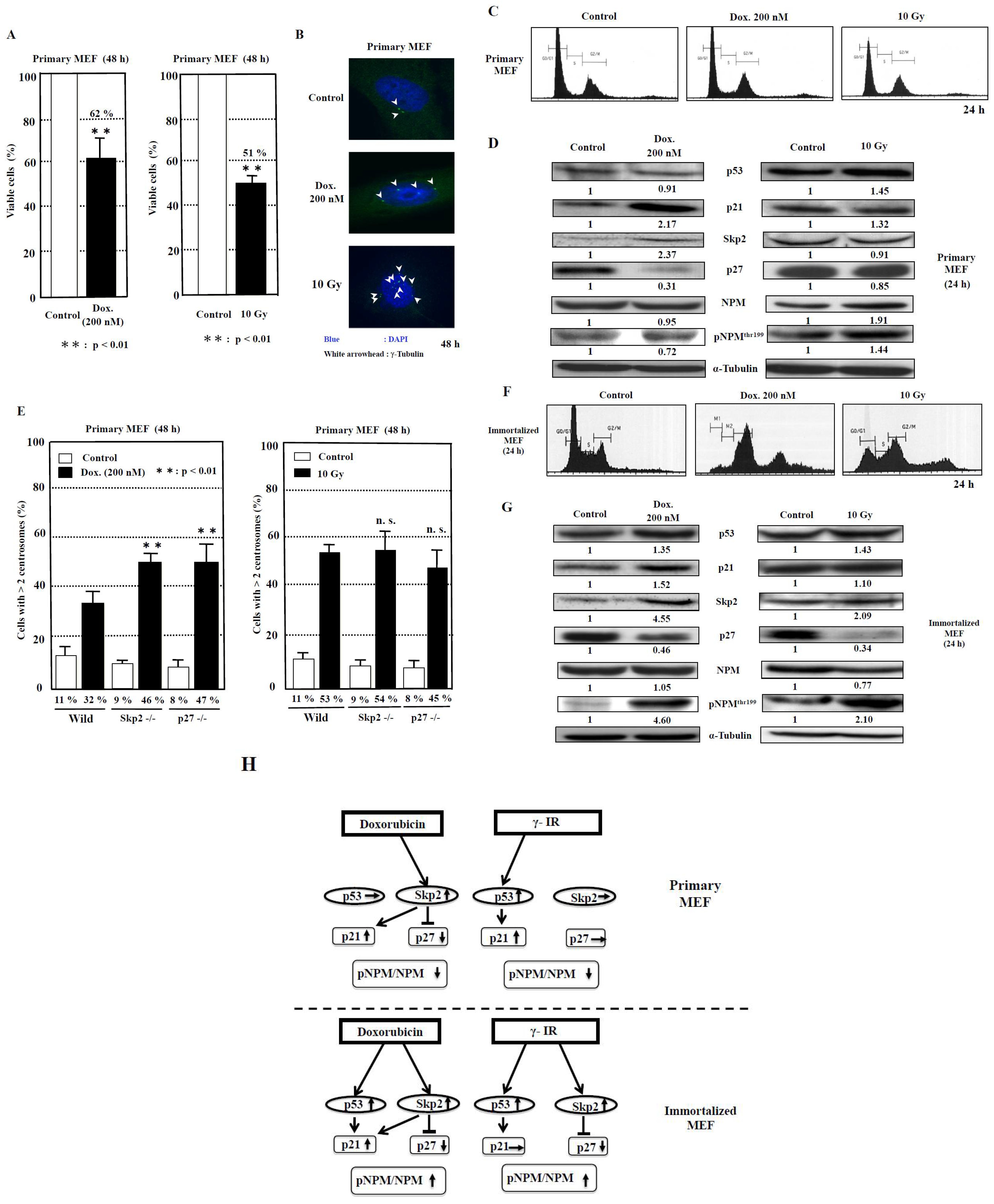
2.3. Exploring the Pathway of Centrosome Amplification by Doxorubicin and γ-Irradiation in Primary MEF
2.4. Exploring the Pathway of Centrosome Amplification by Doxorubicin and γ-Irradiation in Immortalized MEF
3. Discussion
4. Materials and Methods
4.1. Isolation of Mouse Embryonic Fibroblasts (MEF)
4.2. Cells
4.3. Reagents and Antibodies
4.4. Growth Inhibition Assays
4.5. Flow Cytometry
4.6. Indirect Immunofluorescence
4.7. Counting of Metaphase Spread Chromosomes
4.8. Fluorescence In Situ Hybridization (FISH) Analysis
4.9. γ-Irradiation
4.10. Western Blot Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3AB | 3-aminobenzamide |
| AG14361 | 1-(4-dimethylaminomethyl-phenyl)-8,9-dihydro-7H-2,7,9a-triaza-benzo[cd]azulen-6-one |
| Dox | doxorubicin |
| FACS | fluorescence-activated cell sorting |
| MEF | mouse embryonic fibroblasts |
| PARP | poly(ADP-ribose) polymerase |
References
- Nigg, E.A.; Stearns, T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat. Cell Biol. 2011, 13, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Bornens, M. The centrosome in cells and organisms. Science 2012, 335, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Godinho, S.A.; Kwon, M.; Pellman, D. Centrosomes and cancer: How cancer cells divide with too many centrosomes. Cancer Metastasis Rev. 2009, 28, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Godinho, S.A.; Chandhok, N.S.; Ganem, N.J.; Azioune, A.; Thery, M.; Pellman, D. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008, 22, 2189–2203. [Google Scholar] [CrossRef]
- Löffler, H.; Fechter, A.; Liu, F.Y.; Poppelreuther, S.; Krämer, A. DNA damage-induced centrosome amplification occurs via excessive formation of centriolar satellites. Oncogene 2013, 32, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Kuang, J.; Zhong, L.; Kuo, W.L.; Gray, J.W.; Sahin, A.; Brinkley, B.R.; Sen, S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 1998, 20, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Horejsí, Z.; Koed, K.; Krämer, A.; Tort, F.; Zieger, K.; Guldberg, P.; Sehested, M.; Nesland, J.M.; Lukas, C.; et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005, 434, 864–870. [Google Scholar] [CrossRef]
- Friedberg, E.C. DNA damage and repair. Nature 2003, 421, 436–440. [Google Scholar] [CrossRef]
- Rouse, J.; Jackson, S.P. Interfaces between the detection, signaling, and repair of DNA damage. Science 2002, 297, 547–551. [Google Scholar] [CrossRef]
- Fero, M.L.; Randel, E.; Gurley, K.E.; Roberts, J.M.; Kemp, C.J. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 1998, 396, 177–180. [Google Scholar] [CrossRef]
- Hanashiro, K.; Kanai, M.; Geng, Y.; Sicinski, P.; Fukasawa, K. Roles of cyclins A and E in induction of centrosome amplification in p53-compromised cells. Oncogene 2008, 27, 5288–5302. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Ishida, N.; Shirane, M.; Inomata, A.; Inoue, T.; Shishido, N.; Horii, I.; Loh, D.Y.; Nakayama, K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 1996, 85, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Nagahama, H.; Minamishima, Y.A.; Miyake, S.; Ishida, N.; Hatakeyama, S.; Kitagawa, M.; Iemura, S.; Natsume, T.; Nakayama, K.I. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell 2004, 6, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Nagahama, H.; Minamishima, Y.A.; Matsumoto, M.; Nakamichi, I.; Kitagawa, K.; Shirane, M.; Tsunematsu, R.; Tsukiyama, T.; Ishida, N.; et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000, 19, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Tanaka, M.; Mushiake, M.; Takahashi, J.; Tanaka, K.; Watase, J.; Fujisawa, J.I.; Miwa, M. Novel pathway of centrosome amplification that does not require DNA lesions. Cancer Sci. 2012, 103, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Mushiake, M.; Takahashi, J.; Sasaki, Y.; Yamashita, S.; Ida, C.; Masutani, M.; Miwa, M. PARP Inhibitor Decreases Akt Phosphorylation and Induces Centrosome Amplification and Chromosomal Aneuploidy in CHO-K1 Cells. Int. J. Mol. Sci. 2022, 23, 3484. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.L. PARPs and ADP-Ribosylation: 50 Years and Counting. Mol. Cell 2015, 58, 902–910. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Curtin, N.J.; Szabo, C. Poly(ADP-ribose) polymerase inhibition: Past, present and future. Nat. Rev. Drug Discov. 2020, 19, 711–736. [Google Scholar] [CrossRef]
- Lindahl, T.; Andersson, A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry 1972, 11, 3618–3623. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.R.; Almassy, R.; Barton, S.; Batey, M.A.; Calvert, A.H.; Canan-Koch, S.; Durkacz, B.W.; Hostomsky, Z.; Kumpf, R.A.; Kyle, S.; et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J. Natl. Cancer Inst. 2004, 96, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Pihan, G.A.; Purohit, A.; Wallace, J.; Knecht, H.; Woda, B.; Quesenberry, P.; Doxsey, S.J. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 1998, 58, 3974–3985. [Google Scholar] [PubMed]
- Quintyne, N.J.; Reing, J.E.; Hoffelder, D.R.; Gollin, S.M.; Saunders, W.S. Spindle multipolarity is prevented by centrosomal clustering. Science 2005, 307, 127–129. [Google Scholar] [CrossRef] [PubMed]
- McIlrath, J.; Bouffler, S.D.; Samper, E.; Cuthbert, A.; Wojcik, A.; Szumiel, I.; Bryant, P.E.; Riches, A.C.; Thompson, A.; Blasco, M.A.; et al. Telomere length abnormalities in mammalian radiosensitive cells. Cancer Res. 2001, 61, 912–915. [Google Scholar]
- Timmer-Bosscha, H.; de Vries, E.G.; Meijer, C.; Oosterhuis, J.W.; Mulder, N.H. Differential effects of all-trans-retinoic acid, docosahexaenoic acid, and hexadecylphosphocholine on cisplatin-induced cytotoxicity and apoptosis in a cisplantin-sensitive and resistant human embryonal carcinoma cell line. Cancer Chemother. Pharmacol. 1998, 41, 469–476. [Google Scholar] [CrossRef]
- Huper, G.; Marks, J.R. Isogenic normal basal and luminal mammary epithelial isolated by a novel method show a differential response to ionizing radiation. Cancer Res. 2007, 67, 2990–3001. [Google Scholar] [CrossRef]
- Normand, G.; King, R.W. Understanding cytokinesis failure. Adv. Exp. Med. Biol. 2010, 676, 27–55. [Google Scholar]
- Okuda, M.; Horn, H.F.; Tarapore, P.; Tokuyama, Y.; Smulian, A.G.; Chan, P.K.; Knudsen, E.S.; Hofmann, I.A.; Snyder, J.D.; Bove, K.E.; et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 2000, 103, 127–140. [Google Scholar] [CrossRef]
- Ishikawa, T.; Zhang, S.S.; Qin, X.; Qin, X.; Takahashi, Y.; Oda, H.; Nakatsuru, Y.; Ide, F. DNA repair and cancer: Lessons from mutant mouse models. Cancer Sci. 2004, 95, 112–117. [Google Scholar] [CrossRef]
- Sugihara, E.; Kanai, M.; Saito, S.; Nitta, T.; Toyoshima, H.; Nakayama, K.; Nakayama, K.; Fukasawa, K.; Schwab, M.; Saya, H.; et al. Suppression of centrosome amplification after DNA damage depends on p27 accumulation. Cancer Res. 2006, 66, 4020–4029. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, Z.; Moten, A.; Peng, D.; Hsu, C.C.; Pan, B.S.; Manne, R.; Li, H.Y.; Lin, H.K. The skp2 pathway: A critical target for cancer therapy. Semin. Cancer Biol. 2020, 67, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, S.; Ben-Izhak, O.; Shapira, M.; Futerman, B.; Hershko, D.D. Over-expression of skp2 is associated with resistance to preoperative doxorubicin-based chemotherapy in primary breast cancer. Brest. Cancer Res. 2008, 10, R63. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Qu, X.; Li, H.; Xu, C.; Wei, M.; Wang, Q.; Ru, Y.; Liu, B.; Xu, Y.; Li, K.; et al. NDRG2 facilitates colorectal cancer differentiation through the regulation of Skp2-p21/p27 axis. Oncogene 2018, 37, 1759–1774. [Google Scholar] [CrossRef]
- Kanai, M.; Tong, W.M.; Sugihara, E.; Wang, Z.Q.; Fukasawa, K.; Miwa, M. Involvement of poly(ADP-ribose) polymerase 1 and poly(ADP-ribosyl)ation in regulation of centrosome function. Mol. Cell Biol. 2003, 23, 2451–2462. [Google Scholar] [CrossRef]
- Hu, T.; Miller, C.M.; Ridder, G.M.; Aardema, M.J. Characterization of p53 in Chinese hamster cell lines CHO-K1, CHO-WBL, and CHL: Implications for genotoxicity testing. Mutat. Res. 1999, 426, 51–62. [Google Scholar] [CrossRef]
| Treatment | No. Cells with 1 or 2γ-Tubulin Spots | No. Cells with Colocalization (%) | No. Cells without Colocalization (%) | No. Cells with >2 γ- Tubulin Spots | No. Cells with Colocalization (%) | No. Cells without Colocalization (%) |
|---|---|---|---|---|---|---|
| Control | 331 | 331 (100) | 0 (0) | 107 | 105 (98.1) | 2 (1.9) |
| 5 μM AG14361 | 296 | 293 (99) | 3 (1) | 156 | 152 (97.4) | 4 (2.6) |
| DNA—Damaging Agents | Primary MEF | Cell Line MEF | |
|---|---|---|---|
| p53 | Dox. | No | + |
| γ-IR | + | + | |
| p21 | Dox. | + | + |
| γ-IR | + | No | |
| Skp2 | Dox. | + | + |
| γ-IR | No | + | |
| p27 | Dox. | − | − |
| γ-IR | No | − | |
| NPM | Dox. | No | No |
| γ-IR | + | − | |
| pNPM (Thr 199) | Dox. | − | + |
| γ-IR | + | + | |
| pNPM/NPM | Dox. | + | + |
| γ-IR | + | + | |
| Centrosome amplification | Dox. | + | + |
| γ-IR | + | + |
| Cell | PARP Inhibitor | Centrosome Abnormality | Chromosomal Aneuploidy |
|---|---|---|---|
| Primary MEF | AG14361 | + | − |
| 3AB | − | − | |
| Cell line MEF | 3AB | + | − |
| CHO–K1 | 3AB | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Yamada, M.; Mushiake, M.; Tsuda, M.; Miwa, M. Elucidating Differences in Early-Stage Centrosome Amplification in Primary and Immortalized Mouse Cells. Int. J. Mol. Sci. 2024, 25, 383. https://doi.org/10.3390/ijms25010383
Tanaka M, Yamada M, Mushiake M, Tsuda M, Miwa M. Elucidating Differences in Early-Stage Centrosome Amplification in Primary and Immortalized Mouse Cells. International Journal of Molecular Sciences. 2024; 25(1):383. https://doi.org/10.3390/ijms25010383
Chicago/Turabian StyleTanaka, Masakazu, Masaki Yamada, Masatoshi Mushiake, Masataka Tsuda, and Masanao Miwa. 2024. "Elucidating Differences in Early-Stage Centrosome Amplification in Primary and Immortalized Mouse Cells" International Journal of Molecular Sciences 25, no. 1: 383. https://doi.org/10.3390/ijms25010383
APA StyleTanaka, M., Yamada, M., Mushiake, M., Tsuda, M., & Miwa, M. (2024). Elucidating Differences in Early-Stage Centrosome Amplification in Primary and Immortalized Mouse Cells. International Journal of Molecular Sciences, 25(1), 383. https://doi.org/10.3390/ijms25010383





