Acetyl-11-Keto-β-Boswellic Acid Accelerates the Repair of Spinal Cord Injury in Rats by Resisting Neuronal Pyroptosis with Nrf2
Abstract
:1. Introduction
2. Results
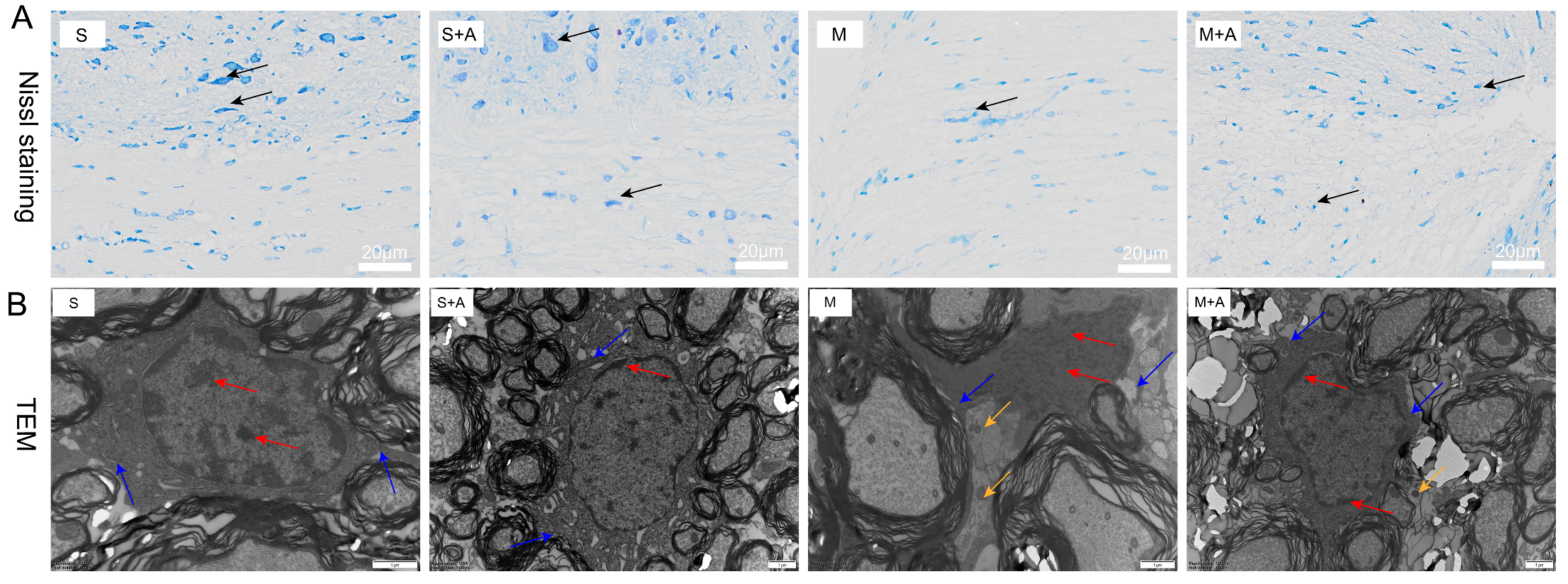
2.1. AKBA Intervention Alleviates Neuronal Pyroptosis Caused by Spinal Cord Injury
2.2. AKBA Alleviates Neuronal Pyroptosis-Related Pathways in Rat Spinal Cord Injury
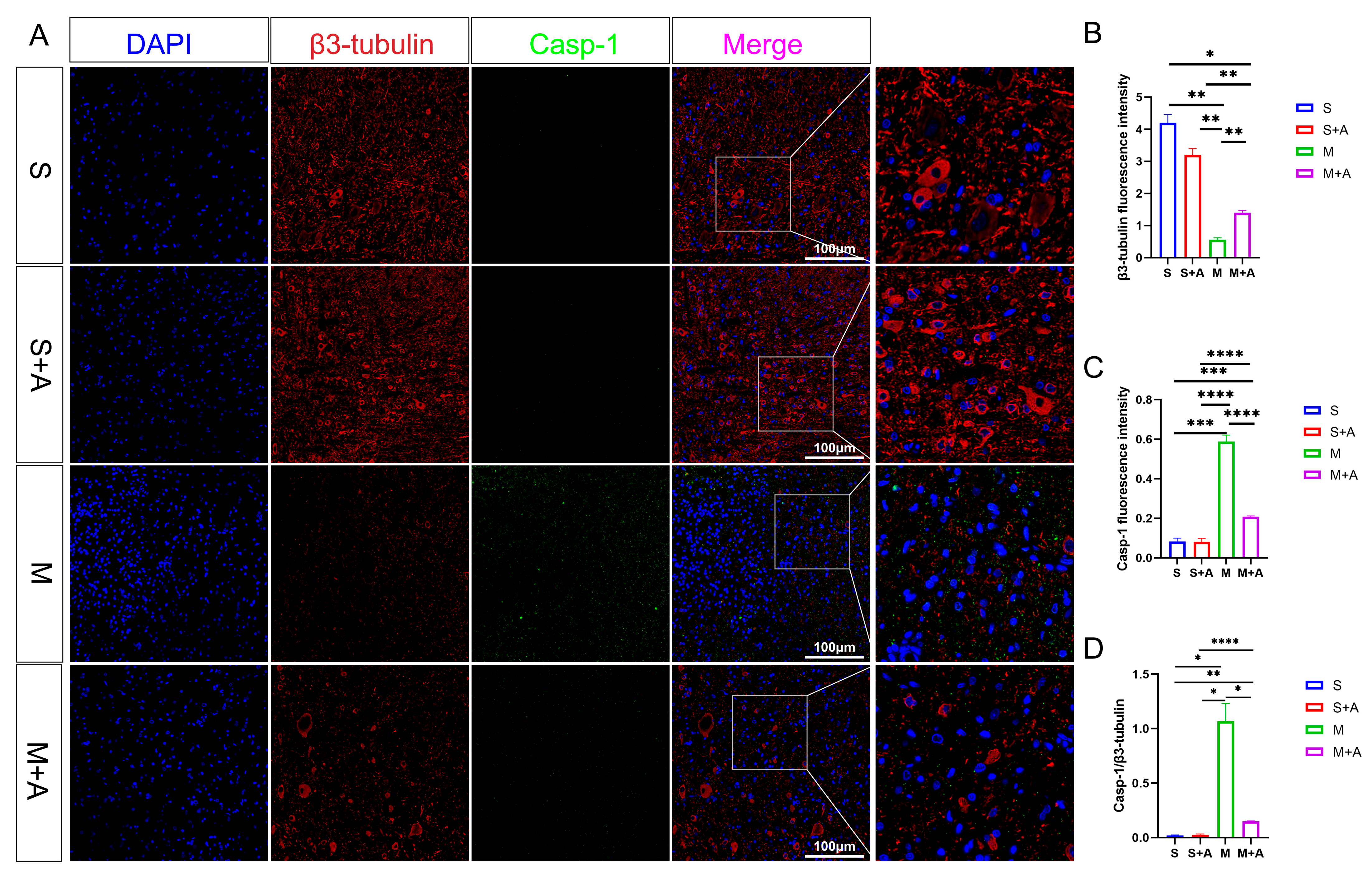
2.3. AKBA Mitigates Expression of Pyroptosis Initiation Protein
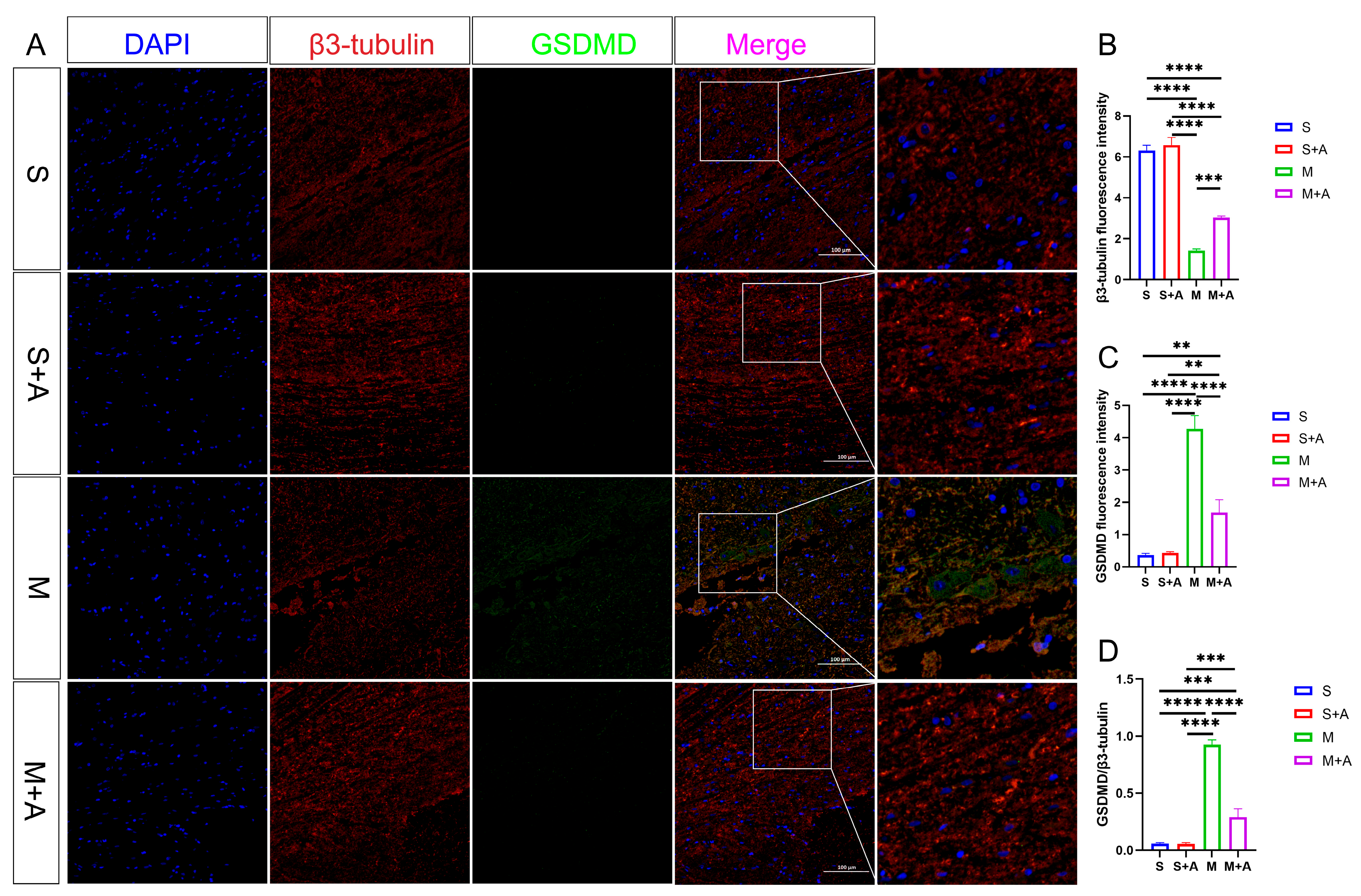
2.4. AKBA Attenuates Expression of Executory Pyroptotic Protein
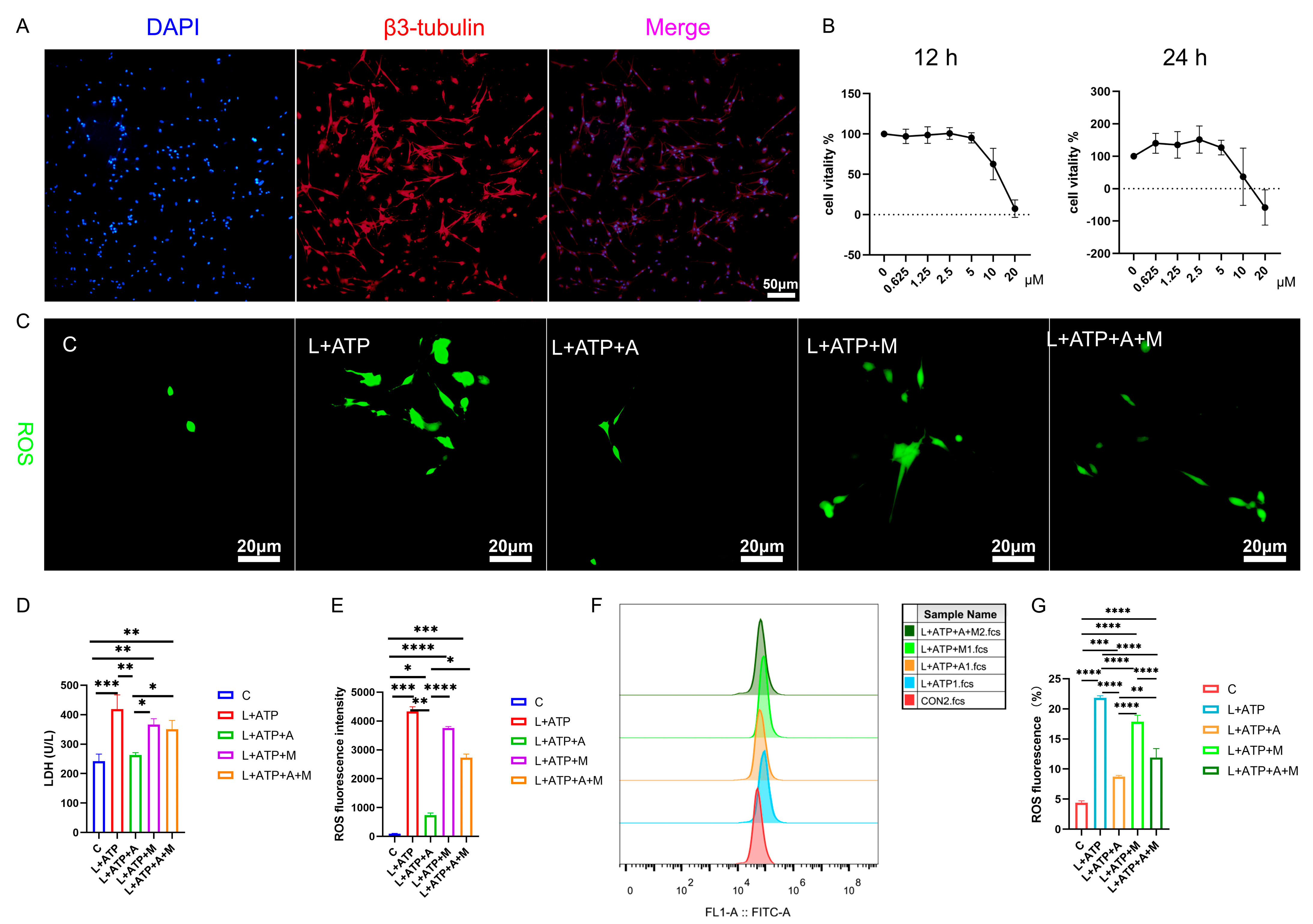
2.5. AKBA Attenuates LDH Release and ROS Generation in a Spinal Neuronal Pyroptosis Model
2.6. Impact of AKBA on Mitochondrial Membrane Potential in Rat Spinal Neurons
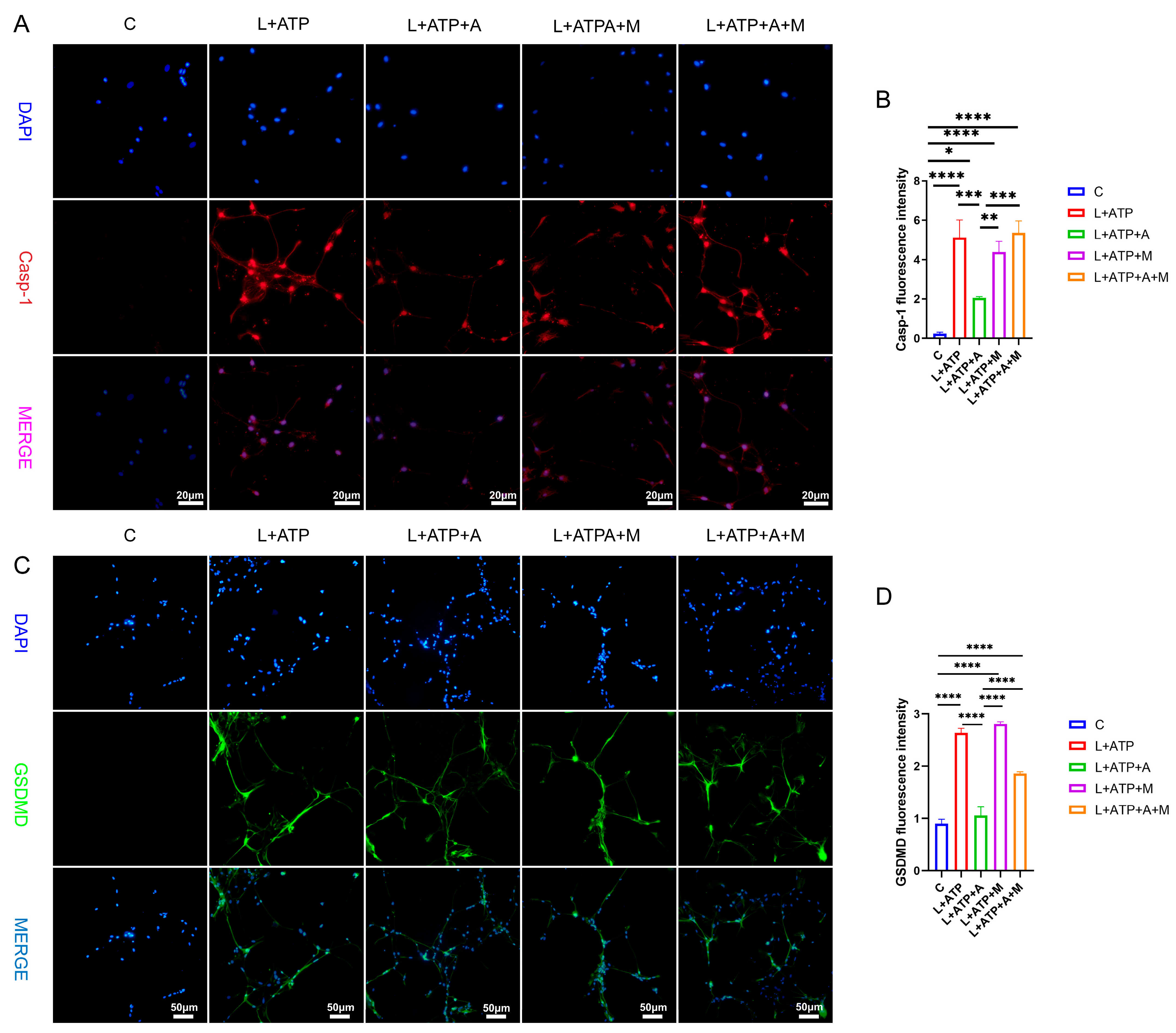
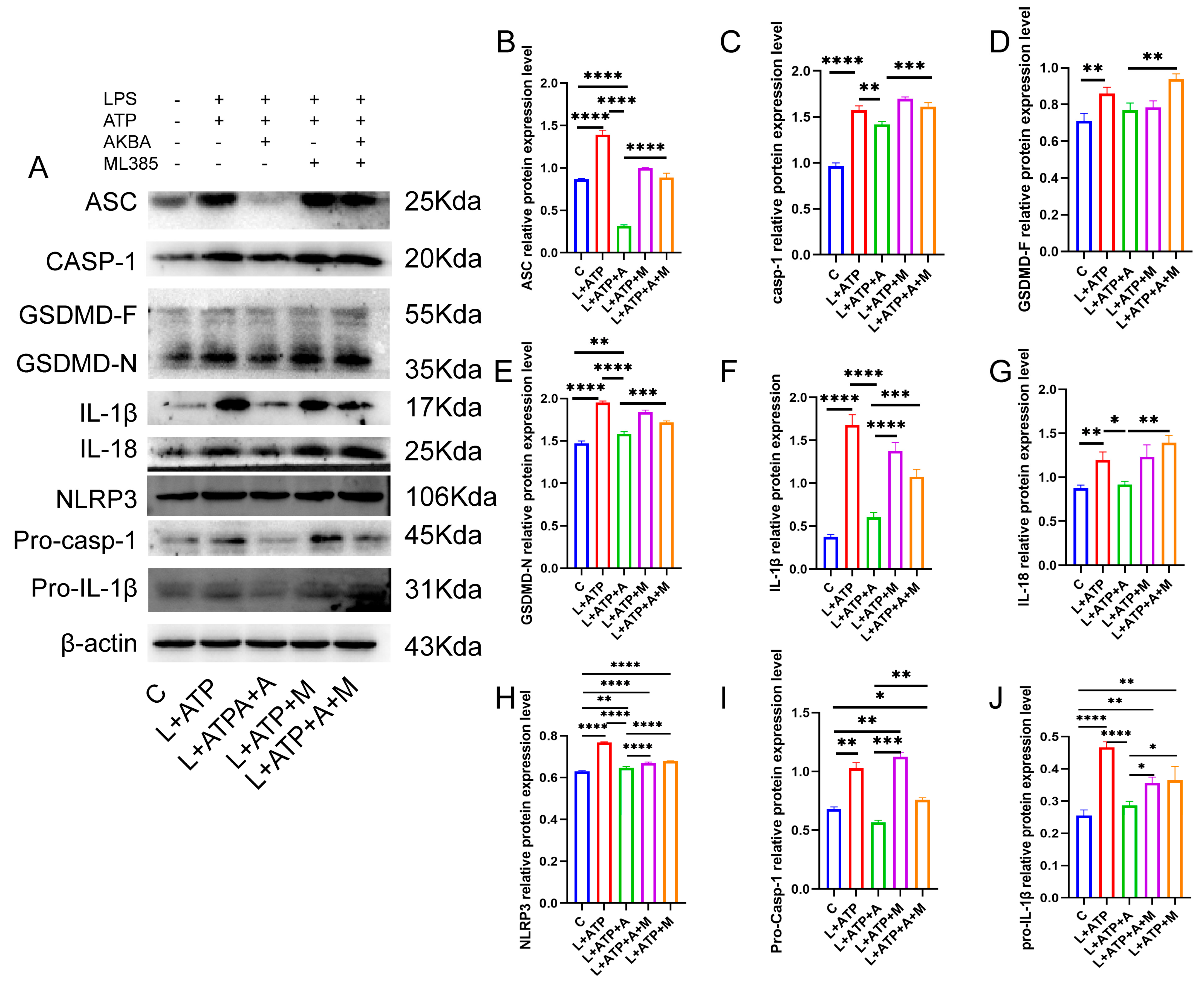
2.7. The Impact of AKBA on the Expression of Crucial Proteins Associated with Spinal Neuronal Pyroptosis
2.8. The Influence of AKBA on Spinal Neuronal Pyroptosis-Associated Proteins
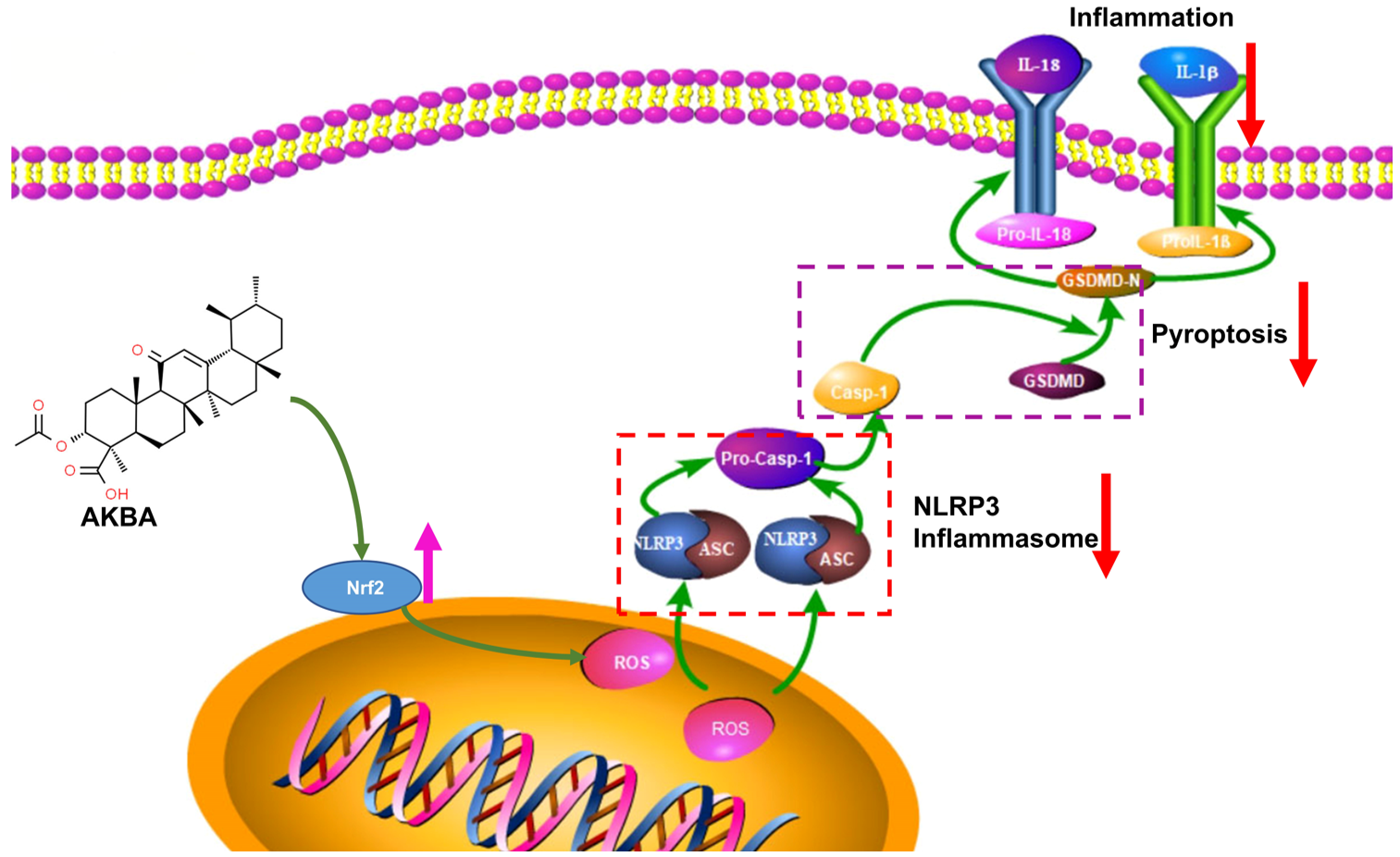
3. Discussion
4. Materials and Methods
4.1. Construction and Administration of Spinal Cord Injury Model
4.2. Nissl Staining for Neuronal Loss Observation
4.3. Ultrastructural Observation of Cellular Apoptosis
4.4. Immunofluorescence Analysis of Casp-1 and GSDMD Protein Expression
4.5. Lactate Dehydrogenase Release Assay
4.6. Western Blot of NLRP3 Pyroptotic Pathway-Associated Proteins
4.7. Quantitative Real-Time PCR for Analysis of NLRP3 Pyroptotic Pathway-Related Genes
4.8. Isolation of Spinal Cord Neurons from Rats
4.9. Establishment of Spinal Cord Neuronal Pyroptosis Model and Screening for Optimal AKBA Dosage
4.10. Immunofluorescence Detection of Caspase-1 and GSDMD Expression in Cells
4.11. ROS Release Analysis in the Spinal Cord Neuronal Necrosis Model
4.12. JC-1 Variation in the Spinal Cord Neuronal Necrosis Model
4.13. Protein Immunoblot Analysis of NLRP3 Pathway-Related Proteins
4.14. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, H.; Liu, Y.; Xiao, F.; Zhang, L.; Li, W.; Wang, B.; Weng, Z.; Liu, Y.; Chen, G. Research progress of hydrogels as delivery systems and scaffolds in the treatment of secondary spinal cord injury. Front. Bioeng. Biotechnol. 2023, 11, 1111882. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Quan, Z.X.; Wang, G.J.; He, T.; Chen, Z.Y.; Li, Q.C.; Yang, J.; Wang, Q. Elevated intraspinal pressure in traumatic spinal cord injury is a promising therapeutic target. Neural Regen. Res. 2022, 17, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Aschauer-Wallner, S.; Leis, S.; Bogdahn, U.; Johannesen, S.; Couillard-Despres, S.; Aigner, L. Granulocyte colony-stimulating factor in traumatic spinal cord injury. Drug Discov. Today 2021, 26, 1642–1655. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Song, N.; Li, T. Regulation of NLRP3 Inflammasome by Phosphorylation. Front. Immunol. 2018, 9, 2305. [Google Scholar] [CrossRef]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef]
- Groslambert, M.; Py, B.F. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 2018, 11, 359–374. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Al Mamun, A.; Wu, Y.; Monalisa, I.; Jia, C.; Zhou, K.; Munir, F.; Xiao, J. Role of pyroptosis in spinal cord injury and its therapeutic implications. J. Adv. Res. 2021, 28, 97–109. [Google Scholar] [CrossRef]
- Broz, P.; Pelegrín, P.; Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, F.; Xu, H.; Yang, H.; Shao, M.; Xu, S.; Lyu, F. TLR4 aggravates microglial pyroptosis by promoting DDX3X-mediated NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury. Clin. Transl. Med. 2022, 12, e894. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Zhuang, R.; Zhang, H.; Wang, Y.; Hu, X.; Xu, Y.; Li, J.; Li, Y.; Wang, X.; et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int. J. Biol. Sci. 2021, 17, 1138–1152. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Zhong, J.; Shao, M.; Jiang, J.; Song, J.; Zhu, W.; Zhang, F.; Xu, H.; Xu, G.; et al. CD73 alleviates GSDMD-mediated microglia pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling. Clin. Transl. Med. 2021, 11, e269. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, P.; Chen, Q.; Huang, Z.; Zou, D.; Zhang, J.; Gao, X.; Lin, Z. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 2019, 11, 1069–1082. [Google Scholar] [CrossRef]
- Weindel, C.G.; Martinez, E.L.; Zhao, X.; Mabry, C.J.; Bell, S.L.; Vail, K.J.; Coleman, A.K.; VanPortfliet, J.J.; Zhao, B.; Wagner, A.R.; et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell 2022, 185, 3214–3231.e3223. [Google Scholar] [CrossRef]
- Ma, X.; Hao, J.; Wu, J.; Li, Y.; Cai, X.; Zheng, Y. Prussian Blue Nanozyme as a Pyroptosis Inhibitor Alleviates Neurodegeneration. Adv. Mater. 2022, 34, e2106723. [Google Scholar] [CrossRef]
- Jiang, X.-W.; Zhang, B.-Q.; Qiao, L.; Liu, L.; Wang, X.-W.; Yu, W.-H. Acetyl-11-keto-β-boswellic acid extracted from Boswellia serrata promotes Schwann cell proliferation and sciatic nerve function recovery. Neural Regen. Res. 2018, 13, 484–491. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Z.-L.; Ma, X.-L.; Zhou, C.; Huo, M.-H.; Jiang, X.-W.; Yu, W.-H. Acetyl-11-keto-beta-boswellic acid promotes sciatic nerve repair after injury: Molecular mechanism. Neural Regen. Res. 2022, 17, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, Z.; Zhou, C.; Zhang, Q.; Liu, S.; Dong, S.; Jiang, X.; Yu, W. AKBA Promotes Axonal Regeneration via RhoA/Rictor to Repair Damaged Sciatic Nerve. Int. J. Mol. Sci. 2022, 23, 15903. [Google Scholar] [CrossRef] [PubMed]
- Schurr, R.; Mezer, A.A. The glial framework reveals white matter fiber architecture in human and primate brains. Science 2021, 374, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Q.; Liu, L.; Wu, H.; Huang, F.; Wang, C.; Lan, Y.; Zheng, F.; Xing, F.; Zhou, Q.; et al. Modafinil protects hippocampal neurons by suppressing excessive autophagy and apoptosis in mice with sleep deprivation. Br. J. Pharmacol. 2019, 176, 1282–1297. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhang, J.; Chen, H.; Zhuge, Y.; Chen, H.; Qian, F.; Zhou, K.; Niu, C.; Wang, F.; Qiu, H.; et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Zheng, L.; Hu, Y.W.; Wang, Q. Pyroptosis and its relationship to atherosclerosis. Clin. Chim. Acta 2018, 476, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Zhang, X.; Yuan, J.; Chen, Y.; Ding, H.; Cao, X.; Huang, A. Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury. Stem Cell Res. Ther. 2021, 12, 36. [Google Scholar] [CrossRef]
- Orr, M.B.; Gensel, J.C. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 2018, 15, 541–553. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Sun, B.; Jiang, W.; Liao, C.; Dai, X.; Chen, Y.; Chen, J.; Ding, R. Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Free Radic. Biol. Med. 2021, 168, 142–154. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Jiang, W.; Li, W.; Zhu, S.; Liao, C.; Zou, L.; Ding, R.; Chen, J. Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. J. Neuroinflamm. 2020, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Xu, X.; Lu, S.; Yang, D.; Xie, C.; Jia, M.; Zhang, W.; Jin, L.; Wang, X.; et al. SARM1 promotes neuroinflammation and inhibits neural regeneration after spinal cord injury through NF-κB signaling. Theranostics 2021, 11, 4187–4206. [Google Scholar] [CrossRef] [PubMed]
- Evavold, C.L.; Hafner-Bratkovič, I.; Devant, P.; D’Andrea, J.M.; Ngwa, E.M.; Boršić, E.; Doench, J.G.; LaFleur, M.W.; Sharpe, A.H.; Thiagarajah, J.R. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell 2021, 184, 4495–4511.e4419. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lin, X.; Li, H.; Zhou, X.; Fan, F.; Yang, J.; Luo, Y.; Liu, X. Acetyl-11-Keto-Beta Boswellic Acid (AKBA) Protects Lens Epithelial Cells Against H2O2-Induced Oxidative Injury and Attenuates Cataract Progression by Activating Keap1/Nrf2/HO-1 Signaling. Front. Pharmacol. 2022, 13, 927871. [Google Scholar] [CrossRef]
- Li, C.; He, Q.; Xu, Y.; Lou, H.; Fan, P. Synthesis of 3-O-Acetyl-11-keto-β-boswellic Acid (AKBA)-Derived Amides and Their Mitochondria-Targeted Antitumor Activities. ACS Omega 2022, 7, 9853–9866. [Google Scholar] [CrossRef]
- Lv, X.; Fan, C.; Jiang, Z.; Wang, W.; Qiu, X.; Ji, Q. Isoliquiritigenin alleviates P. gingivalis-LPS/ATP-induced pyroptosis by inhibiting NF-κB/NLRP3/GSDMD signals in human gingival fibroblasts. Int. Immunopharmacol. 2021, 101, 108338. [Google Scholar] [CrossRef]
- Sun, J.; Ge, X.; Wang, Y.; Niu, L.; Tang, L.; Pan, S. USF2 knockdown downregulates THBS1 to inhibit the TGF-β signaling pathway and reduce pyroptosis in sepsis-induced acute kidney injury. Pharmacol. Res. 2022, 176, 105962. [Google Scholar] [CrossRef]
- Shen, S.; He, F.; Cheng, C.; Xu, B.; Sheng, J. Uric acid aggravates myocardial ischemia-reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed. Pharmacother. 2021, 133, 110990. [Google Scholar] [CrossRef]
- Wang, Y.W.; Dong, H.Z.; Tan, Y.X.; Bao, X.; Su, Y.M.; Li, X.; Jiang, F.; Liang, J.; Huang, Z.C.; Ren, Y.L.; et al. HIF-1α-regulated lncRNA-TUG1 promotes mitochondrial dysfunction and pyroptosis by directly binding to FUS in myocardial infarction. Cell Death Discov. 2022, 8, 178. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, T.; Yi, L.; Zhou, X.; Mi, M. Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. Biofactors 2018, 44, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Campolo, M.; Casili, G.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Sodium Butyrate Exerts Neuroprotective Effects in Spinal Cord Injury. Mol. Neurobiol. 2019, 56, 3937–3947. [Google Scholar] [CrossRef] [PubMed]
- Banji, D.; Banji, O.J.F.; Rashida, S.; Alshahrani, S.; Alqahtani, S.S. Bioavailability, anti-inflammatory and anti-arthritic effect of Acetyl Keto Boswellic acid and its combination with methotrexate in an arthritic animal model. J. Ethnopharmacol. 2022, 292, 115200. [Google Scholar] [CrossRef]
- Xiong, Z.-L.; Wang, Y.; Ma, X.-L.; Zhou, C.; Jiang, X.-W.; Yu, W.-H. Based on proteomics to explore the mechanism of mecobalamin promoting the repair of injured peripheral nerves. Can. J. Physiol. Pharmacol. 2022, 100, 562–572. [Google Scholar] [CrossRef]
- Chi, Q.; Zhang, Q.; Lu, Y.; Zhang, Y.; Xu, S.; Li, S. Roles of selenoprotein S in reactive oxygen species-dependent neutrophil extracellular trap formation induced by selenium-deficient arteritis. Redox Biol. 2021, 44, 102003. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Diao, J.; Yi, H.; Xu, L.; Xu, J.; Xu, W. Signaling pathways involved in HSP32 induction by hyperbaric oxygen in rat spinal neurons. Redox Biol. 2016, 10, 108–118. [Google Scholar] [CrossRef]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020, 177, 3617–3624. [Google Scholar] [CrossRef]


| Antibodies | Concentration | Species | Catalog No. | Supplier |
|---|---|---|---|---|
| IL-1β | 1:500 | Rabbit | WLH3903 | Wanleibio (Shenyang, China) |
| β-Actin | 1:1000 | Rabbit | bs-0061R | Bioss (Beijing, China) |
| NLRP3 | 1:1000 | Rabbit | WL02635 | Wanleibio (Shenyang, China) |
| Pro-IL-1β | 1:500 | Rabbit | WL02257 | Wanleibio (Shenyang, China) |
| Caspase-1 | 1:500 | Rabbit | WLH4550 | Wanleibio (Shenyang, China) |
| GSDMD | 1:1000 | Rabbit | DF12275 | Affinity (Liyang, China) |
| ASC | 1:500 | Rabbit | WL02462 | Wanleibio (Shenyang, China) |
| IL-18 | 1:500 | Rabbit | WL01127 | Wanleibio (Shenyang, China) |
| Genes | Primer Sequence (5′-3′) |
|---|---|
| IL-1β | (F) 5′-TTGAGTCTGCACAGTTCCCC-3′ (R) 3′-GTCCTGGGGAAGGCATTAGG-5′ |
| NLRP3 | (F) 5′-TGCATGCCGTATCTGGTTGT-3′ (R) 3′-ACCTCTTGCGAGGGTCTTTG-5′ |
| IL-18 | (F) 5′-AGGGCACAGCCTCTCAGTT-3′ (R) 3′-ACTCATCGTTGTGGGGACAG-5′ |
| Casp-1 | (F) 5′-CTGACAAGATCCTGAGGGCA-3′ (R) 3′-AACTTGAGGGAACCACTCGG-5′ |
| β-actin | (F) 5′-AGGCATCCTGACCCTGAAGTAC-3′ (R) 3′-GAGGCATACAGGGACAACACAG-5′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xiong, Z.; Zhang, Q.; Liu, M.; Zhang, J.; Qi, X.; Jiang, X.; Yu, W. Acetyl-11-Keto-β-Boswellic Acid Accelerates the Repair of Spinal Cord Injury in Rats by Resisting Neuronal Pyroptosis with Nrf2. Int. J. Mol. Sci. 2024, 25, 358. https://doi.org/10.3390/ijms25010358
Wang Y, Xiong Z, Zhang Q, Liu M, Zhang J, Qi X, Jiang X, Yu W. Acetyl-11-Keto-β-Boswellic Acid Accelerates the Repair of Spinal Cord Injury in Rats by Resisting Neuronal Pyroptosis with Nrf2. International Journal of Molecular Sciences. 2024; 25(1):358. https://doi.org/10.3390/ijms25010358
Chicago/Turabian StyleWang, Yao, Zongliang Xiong, Qiyuan Zhang, Mengmeng Liu, Jingjing Zhang, Xinyue Qi, Xiaowen Jiang, and Wenhui Yu. 2024. "Acetyl-11-Keto-β-Boswellic Acid Accelerates the Repair of Spinal Cord Injury in Rats by Resisting Neuronal Pyroptosis with Nrf2" International Journal of Molecular Sciences 25, no. 1: 358. https://doi.org/10.3390/ijms25010358
APA StyleWang, Y., Xiong, Z., Zhang, Q., Liu, M., Zhang, J., Qi, X., Jiang, X., & Yu, W. (2024). Acetyl-11-Keto-β-Boswellic Acid Accelerates the Repair of Spinal Cord Injury in Rats by Resisting Neuronal Pyroptosis with Nrf2. International Journal of Molecular Sciences, 25(1), 358. https://doi.org/10.3390/ijms25010358






