Abstract
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a distinct subtype of T-cell non-Hodgkin lymphoma that arises in the context of prolonged exposure to textured breast implants. The intent of this manuscript is to explore whether the bacterial presence in biofilms on these implants is a mere incidental finding or plays a pivotal role in the pathogenesis of BIA-ALCL. Our goal is to delineate the extent of bacterial involvement, offering insights into potential underlying mechanisms, and establishing future research priorities aimed at resolving the remaining uncertainties surrounding this complex association. A comprehensive systematic review of several databases was performed. The search strategy was designed and conducted by an experienced librarian using controlled vocabulary with keywords. The electronic search identified 442 publications. After evaluation, six studies from 2015 to 2021 were included, encompassing 201 female patients aged 23 to 75. The diagnosis span post-implantation ranged from 53 to 135.6 months. Studies consistently found bacteria near breast implants in both BIA-ALCL cases and controls, with varied microbial findings. Both BIA-ALCL cases and controls exhibited the presence of specific bacteria, including Pseudomonas aeruginosa, Klebsiella oxytoca, Staphylococcus aureus, and Ralstonia spp., without any statistically significant differences between groups. The use of antiseptic and antimicrobial agents during implant insertion did not demonstrate any impact on reducing or altering the risk of developing BIA-ALCL. Our systematic review reveals that the current evidence is inadequate to link bacterial etiology as a central factor in the development of BIA-ALCL. The limitations in the existing data prevent a complete dismissal of the role of biofilms in its pathogenesis. The observed gap in knowledge underscores the need for more focused and comprehensive research, which should be structured in a multi-faceted approach. Initially, this involves the utilization of sophisticated genomic and proteomic methods. Following this, it is crucial to delve into the study of immunological reactions specifically induced by biofilms. Finally, this research should incorporate extended observational studies, meticulously tracking the evolution of biofilm development and its correlation with the emergence of BIA-ALCL. In light of the inconclusive nature of current findings, further investigation is not only justified but urgently needed to clarify these unresolved issues.
1. Introduction
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a type of T-cell non-Hodgkin lymphoma (NHL) that arises around breast implants, specifically in textured prostheses. This uncommon and emerging entity of NHL was first described by Keech and Creech in 1997 but it was not until 2016 that the World Health Organization (WHO) acknowledged it as a type of NHL [1,2].
According to the FDA as of April 2022, there is a prevalence of 1130 worldwide cases of BIA-ALCL with 59 deaths [3]. Of the total documented cases with a complete clinical history, by definition, 100% of cases involved prior exposure to textured surface implants or tissue expanders, with a median age of diagnosis of 53 years and a median time to diagnosis of 8 years [3]. Clinically, most recent statistics report that BIA-ALCL presents in 49% of cases with seroma formation, followed by non-specific symptoms and swelling in 32% and 23% and only presenting as a lump in 11% of the cases [3]. The risk of BIA-ALCL varies according to the manufactured risks but the American Society of Plastic Surgeons has estimated a 1:2207 to 1:86,029 lifetime risk of BIA-ALCL for women with textured implants based upon current confirmed cases and textured implant sales data over the past two decades [4,5].
The pathogenesis involved in the development of BIA-ALCL is still not completely understood. However, several possible mechanisms have been proposed. Among the most commonly cited hypotheses are genetic predisposition (i.e., DNMT3A, JAK-STAT3 pathway, and mutations in TP53), bacterial biofilm (BF), chronic inflammation, and digestion of particulate debris shed from textured breast implants [6].
The investigation into the role of BF in the development of BIA-ALCL continues to captivate a diverse array of stakeholders, including surgeons, regulatory authorities, manufacturers, and those involved in litigation efforts [7]. Existing research suggests that the textured surface of breast implants may facilitate biofilm formation, fostering bacterial growth and eliciting an enhanced T-cell response. This bacterial proliferation can lead to chronic inflammation, which, in individuals with certain genetic predispositions, may trigger a malignant transformation resulting in BIA-ALCL [7]. In light of these findings, the primary aim of this manuscript is to conduct a thorough examination of the existing evidence regarding the relationship between biofilm formation on breast implants and the onset of BIA-ALCL. By investigating these aspects, we seek to clarify the extent to which biofilms contribute to the pathogenesis of BIA-ALCL and to identify critical areas for future research in understanding and addressing this complex medical challenge.
2. Method
This study protocol was prospectively registered with PROSPERO (York, UK) (Study#: CRD42023424348) [8,9]. Completion of this study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines [8,9].
2.1. Eligibility Criteria
Criteria for included studies were defined as any studies investigating the relationship between bacteria or bacterial biofilm and the formation of BIA-ALCL in experimental or human settings. The full eligibility criteria are accessible at PROSPERO and are as follows:
Inclusion criteria:
- Female adult population 18 years and above
- Female with BIA-ALCL or suspicion of BIA-ALCL
- Animal, cadaveric, or experimental studies on BIA-ALCL
- Studies on BIA-ALCL investigating BF or bacteria
- Studies in English and studies translated to English
Exclusion criteria:
- Editorials, commentary reports, abstracts, and letters to the editors
- Studies conducted in patients with history of non-Hodgkin lymphoma or Hodgkin lymphoma
2.2. Search Strategy
A comprehensive research review using subject headings, controlled vocabulary, and keywords was conducted on 10 June 2023, on MEDLINE (in Ovid), Web of Science, and the Cochrane Central Register for studies published until June 2023. Our full-text search strategy is accessible at PROSPERO [8,9].
2.3. Study Selection
The search results were uploaded into the online systematic review program Covidence to conduct study selection [8,9]. Five independent reviewers performed a two-stage screening process for study selection. First, titles and abstracts were screened. A third reviewer then moderated if discordances were present and resolved any conflicts. Next, a full-text analysis was performed by the same five reviewers. If conflicts arose between reviewers, a third reviewer moderated a discussion to come to a joint decision.
2.4. Data Extraction/Synthesis
Data extraction was guided by a predetermined checklist: author, year of publication, type of study, total population, total samples, age of population, time till diagnosis, total number of BIA-ALCL cases, type of implants, method for biofilm analysis, and results.
2.5. Outcomes
These results of this systematic review focused on gathering the evidence in the literature for the biofilm induced theory of BIA-ALCL. This includes the type, colony forming unit, and other analysis performed in this area.
2.6. Quality Assessment
To assess the risk of bias, the National Institute of Health (NIH) quality assessment tool was utilized [8,9,10]. Each article was categorized as follows: “low risk”, “moderate risk”, or “high risk” of bias. All six studies were found to have a low risk of bias.
2.7. Statistical Analysis
Due to the heterogenicity of the topics covered in the studies constituting this systematic review, it was not possible to perform any analysis beyond a qualitative synthesis [11,12,13,14].
3. Results
The electronic search initially identified 442 related publications, which ultimately yielded 14 studies for full-text review (Figure 1). After thorough assessment and subsequent exclusion, six studies from 2015 to 2021 were included for qualitative analysis (Table 1). These consisted of 3 clinical studies, 2 experimental studies, and 1 mixed study. Figure 1 Prisma flow chart [7,15,16,17,18,19].
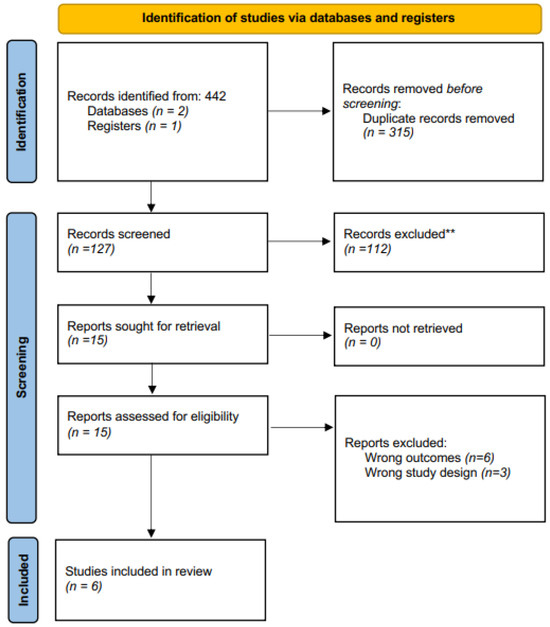
Figure 1.
Prisma flow chart. ** Records excluded based on the abstract.

Table 1.
Study characteristics.
3.1. Results—Clinical
A total of 201 female patients were included. The age range was 23 to 75 years old. Among this cohort, 60 were confirmed to have BIA-ALCL. Among these patients, the time to diagnosis ranged from 53 to 135.6 months from breast implant implantation [7,15,16,17,18,19].
3.1.1. Summary of Method—Clinical
In the pursuit to elucidate the potential relationship between bacteria and the development of BIA-ALCL, several studies have charted a detailed methodological landscape. Central to the research efforts of Di Napoli et al., Hu et al. (2015 and 2016), and Walker et al. is the extraction and evaluation of bacterial DNA from breast implant samples, leveraging the 16S rRNA gene as a key indicator for microbiome sequencing [7,15,16,17,18,19].
Each investigation utilized a combination of proteinase K digestion and lysozyme treatments, paired with phenol/chloroform extraction for DNA isolation. Additionally, all methodologies incorporated real-time quantitative polymerase chain reaction (qPCR) techniques, albeit with subtle variances in reaction mixes and cycling conditions. This common methodology underscores a cohesive scientific strategy to probe the bacterial milieu potentially associated with BIA-ALCL [7,15,16,17,18,19].
Despite a shared objective, the investigative methodologies in the study of BF and their role in BIA-ALCL reveal nuanced differences. Di Napoli et al. primarily utilized culture-based techniques, employing a range of agar media to facilitate bacterial growth detection. In a marked contrast, Hu et al. (2015) adopted scanning electron microscopy and confocal imaging for direct visualization of biofilms. Their subsequent study in 2016 further expanded the scope by integrating pyrosequencing and fluorescence in situ hybridization (FISH) for a more comprehensive analysis of bacterial species. Walker et al., meanwhile, uniquely focused on the use of sonication, coupled with Sanger sequencing, to pinpoint specific bacterial colonies [7,15,16,17,18,19].
These studies, while converging towards a common goal, showcase a diversity of techniques—both overlapping and distinct. This variation underscores the complex and multifaceted nature of researching bacterial associations with BIA-ALCL in a clinical setting. However, it is important to acknowledge that such methodological disparities pose challenges in directly comparing results, thereby adding a layer of complexity to the interpretation and understanding of the findings (Table 2) [7,15,16,17,18,19].

Table 2.
Clinical studies methodology.
3.1.2. Summary of Results—Clinical
All five studies aimed to elucidate the relationship between bacteria and the onset of BIA-ALCL. They consistently found bacteria near the breast implants in BIA-ALCL cases but also in all controls. For instance, Di Napoli et al. identified pathogens in one mixed-type seroma and in four acute-type effusions. Hu et al. (2015) found that all periprosthetic capsules from their cohort were positive for biofilm bacteria, averaging 2.52 × 107 bacteria/mg of a capsule. In Hu et al. (2016), the average bacterial count stood at 4.7 × 106 bacteria/mg of tissue for BIA-ALCL and 4.9 × 106 for nontumor capsule specimens. However, results from this study were limited by variability in specimen handling and preservation methods such as fresh versus formalin-fixed samples, absence of data on patients’ concurrent antibiotic treatments, and the detection of Ralstonia spp. in both BIA-ALCL and non-BIA-ALCL capsules. The predominant microbiome in both tumor and non-tumor capsules was characterized by Staphylococcus spp. Walker et al. revealed the presence of Staphylococcus spp. in both BIA-ALCL and controls [15,16,17,18,19].
A shared finding across the board was the association between immune response and bacteria. Both Di Napoli et al. and Hu et al. (2015) discussed the presence of CD30+ atypical cells. Hu et al. (2015) also recorded significantly more T cells than B cells, with correlations between bacterial load and lymphocyte count (e.g., CD4, r = 0.83) [7,15,16,17,18,19].
The studies varied in their clinical focus and depths of microbial investigation. Di Napoli et al. presented clinical details, like the BIA-ALCL localization to the fibrous capsule and treatments such as surgery or adjuvant chemotherapy. They found that culture was positive for Pseudomonas aeruginosa and Klebsiella oxytoca in one mixed-type seroma, and Staphylococcus aureus and Serratia marcescens in acute-type effusions [7,15,16,17,18,19]. Hu et al. (2015) drew correlations between implant texture and bacterial colonization; for example, that polyurethane implants harbored significantly more bacteria than other textures (p < 0.005) [7,15,16,17,18,19]. Hu et al. (2016) introduced deeper microbial insights, with BIA-ALCL and contralateral breast specimens predominantly showing Ralstonia spp. (p < 0.05), whereas nontumor capsules had a higher presence of Staphylococcus spp. (p < 0.001). Furthermore, they were able to visualize bacteria in 10 out of 11 samples (one BIA-ALCL sample was absent bacteria) using fluorescent in situ hybridization [7,15,16,17,18,19]. It is noteworthy that Ralstonia pickettii, a spirochete often a contaminant in laboratory water sources, was identified in this study. The implications of this finding, particularly in relation to potential laboratory contamination or environmental influences, warrant further exploration and consideration in the context of this study’s conclusions.
Walker et al. took a broader approach by comparing BIA-ALCL to multiple control types, including contralateral and cosmetic controls. They identified Propionibacterium as a significant driver of variability and noted that Ralstonia was found at low abundance in eight samples and that it was primarily identified in non-BIA-ALCL controls [7,15,16,17,18,19].
In a prospective study reported by Decoster et al. involving BIA-ALCL, details of intraoperative techniques used during the original breast implant placement were examined in 24 patients [7,15,16,17,18,19]. Among these patients, 12 underwent betadine irrigation (at varying strengths: 6 at 50%, 4 at 25%, and 2 with “tea-colored” solution), and 7 received antibiotic irrigation (5 with a combination of bacitracin/cefazolin/gentamicin and 2 with polymyxin/bacitracin). Notably, these patients developed BIA-ALCL despite these antimicrobial precautions. BIA-ALCL still occurred despite stringent antimicrobial measures. Globally, Decoster reported 18 clusters of BIA-ALCL, each involving two or more cases attributed to the same surgeon [7,15,16,17,18,19].
This study revealed that in only five clusters, the surgeon involved was the one who originally implanted the device. In the remaining cases, surgeons were handling secondary referrals. Four of the surgeons had previously contributed to peer-reviewed literature on BIA-ALCL. These surgeons represent established implant practices known for rigorous follow-up protocols, a low threshold for CD30 screening of seromas, and where feasible, lifelong annual surveillance of patients [7,15,16,17,18,19].
To sum up, whereas all studies consistently identified bacteria in proximity to breast implants, they varied in their specific microbial findings, clinical details, and correlations. The findings broadly reveal that bacteria are present in both BIA-ALCL and non-BIA-ALCL cases, which raises questions regarding a direct causal link between biofilm and this malignancy. Nevertheless, the data also emphasize the significant role bacteria play in inflammatory processes, contributing to the complex landscape of potential causative factors for this condition. No operative technique involving bacterial control has been shown to reduce the risk of developing this disease (Table 3) [7,15,16,17,18,19].

Table 3.
Clinical studies summary of results.
3.2. Results—Experimental
In the realm of experimental research, Hu et al. (2015) meticulously employed porcine models to delve deeper into the intricate relationship between the textures of implants—specifically distinguishing between smooth and textured varieties—and the associated bacterial colonization in the context of BIA-ALCL. Mempin et al. demonstrated functional differences between BIA-ALCL patient-derived primary cells and three BIA-ALCL cell lines, compared to T cells isolated from patients’ PBMC and cutaneous ALCL cell lines. Based on their observations, Mempin et al. proposed a unique BIA-ALCL pathogenesis, in which bacterial lipopolysaccharide promotes BIA-ALCL cell proliferation via a toll-like receptor 4 [7,15,16,17,18,19].
3.2.1. Summary of Method—Experimental
Both Hu et al. (2015) and Mempin et al. investigate bacterial interactions with cell populations within the BIA-ALCL framework. A striking similarity is their utilization of advanced laboratory methodologies. Specifically, Hu et al. employed real-time qPCR, targeting the eubacterial 16 rRNA gene, to quantify bacterial numbers and discern specific T and B cell populations using genes like CD3, CD4, CD8a, and CD79a. Their approach aimed to comprehend bacterial colonization on implants and used confocal microscopy with monoclonal antibodies to visualize lymphocytic infiltrates on breast implant surfaces [7,15,16,17,18,19].
Conversely, Mempin et al. centered their research on cellular reactions to bacterial-secreted elements, particularly stimulants like PHA, LPS, SEA, and TSST-1. Their emphasis was on delineating apoptotic events in cells. To this end, they employed advanced staining and gating techniques, notably Zombie UV and Annexin V-FITC, to categorize cells based on their apoptotic states [7,15,16,17,18,19].
In essence, whereas both papers pivot on the bacterial-cell interface in BIA-ALCL, their research angles differ. Hu et al. delve deeper into bacterial colonization, its interaction with tissues, and their visualization, whereas Mempin et al. prioritize the cellular apoptotic responses upon exposure to bacterial-derived factors (Table 4) [7,15,16,17,18,19].

Table 4.
Experimental studies methodology.
3.2.2. Summary of Results
Both the study by Hu et al. (2015) and the research from Mempin et al. predominantly revolved around the intricate links between bacterial presence and BIA-ALCL, yet they approached the issue with varying focuses and depths [7,15,16,17,18,19].
Hu et al. (2015) concentrated on discerning the impact of implant texture on bacterial colonization and immune response. Their analysis comprised 10 capsular specimens surrounding both smooth and textured implants. Although there was no significant difference in bacterial load per milligram between capsules surrounding different implant types, textured implants were found to harbor significantly more bacteria than smooth ones (p < 0.001). Moreover, textured implants exhibited a staggering 63-fold increase in lymphocytes on their surface, predominantly T cells, in comparison to their smooth counterparts. This observation was validated by scanning electron microscopy which showcased numerous activated lymphocytes on the surface of textured implants, revealing a clear association between bacterial colonization and heightened immune response [7,15,16,17,18,19].
In contrast, Mempin et al. delved deeper into the cellular response of BIA-ALCL tumor cells and related cell lines upon exposure to bacterial stimulants. Their findings underscored that these cells exhibited a pronounced response to LPS stimulation over other bacterial agents like SEA and TSST-1. Remarkably, whereas LPS stimulation did amplify the BIA-ALCL and TLBR live cell number (p < 0.05), it did not significantly influence cell viability or the onset of apoptosis. This underscores a nuanced interplay where bacterial agents like LPS can boost cell proliferation without impacting their survival rates or leading them toward apoptosis [7,15,16,17,18,19].
In essence, both papers focus on differing aspects—Hu et al. (2015) on implant textures and Mempin et al. on cellular responses to specific bacterial agents—yet collectively, their results showcase the impact of bacteria on the immune system, not directly on the pathogenesis of BIA-ALCL. Table 5, summary of results of the experimental studies [7,15,16,17,18,19].

Table 5.
Summary of the results of experimental studies.
4. Discussion
Our review indicates that bacteria are found in both control samples and BIA-ALCL cases, offering no compelling evidence to link bacterial contamination during breast implantation directly to BIA-ALCL. Although there appears to be a connection between biofilm formation, years after implantation and inflammatory responses, a direct relationship with BIA-ALCL remains unsubstantiated. No operative technique involving bacterial mitigation at the time of implantation has been shown to reduce the risk of developing this disease.
The bacterial species and presence of bacterial toxins produced in the microenvironment surrounding breast implant has been hypothesized to be an important factor in BIA-ALCL development. Interestingly, Walker et al., as opposed to Hu et al., did not find a gram-negative shift in BIA-ALCL samples, with especially greater amounts of Ralstonia spp. found in control as opposed to BIA-ALCL samples [16,17,19]. This diverges from certain hypotheses previously referred to in the literature, which have posited a higher abundance of Gram-negative bacteria in BIA-ALCL cases [16,17,20,21]. Mempin et al. did indeed find an association between gram-negative bacteria and a dysregulation of immunomodulation leading to the proliferation of malignant lymphocytes [18]. In their 16 patients with BIA-ALCL, lipopolysaccharide (LPS) stimulation significantly increased BIA-ALCL tumor cell proliferation (n = 11), and this response was unique to LPS. When compared with exposure to Staphylococcal superantigens SEA and TSST-1 or to PHA, patient-derived BIA-ALCL had significantly more response to LPS (p < 0.001, p < 0.01). This response was also unique to the tumor cells themselves, and the same proliferative reaction to LPS was not seen in the peripheral blood mononuclear cells from BIA-ALCL patients. These results from Mempin et al. suggest that Gram-negative bacterial strains, and specifically the LPS coating their outer surface, may stimulate a local tumor response but not a general systemic response which was notably abrogated in the presence of a Toll-like receptor 4 (TLR4) inhibitor peptide. The findings underscored a novel pathway through which LPS could propel BIA-ALCL cell proliferation via TLR4 receptors [16,17,18]. Although one might infer from Mempin et al. that Gram-negative bacteria have a tumorigenic effect, and even a stimulatory effect in the presence of BIA-ALCL tumor cells, the absence of a Gram-negative predominance in clinical BIA-ALCL samples calls into question the direct involvement of this tumorigenic pathway in the genesis of BIA-ALCL [22]. Additional studies demonstrating a Gram-negative predominance in the native biofilm milieu of BIA-ALCL samples would be required to corroborate this as a relevant clinical pathogenic pathway.
To further analyze the effect of bacterial exposure in the breast implant environment on BIA-ALCL, the Walker et al. study helped show that the relative abundance of Gram-negative bacteria had no apparent role on BIA-ALCL [19]. They also showed that diversity in the microbiota of the skin, breast, implant, and capsule had no identifiable correlation with BIA-ALCL samples versus controls. Unlike Hu et al., they failed to see significantly elevated Ralstonia spp. in the breast implant microenvironment after using 16S rRNA sequencing between control and BIA-ALCL. Another layer of complexity pooling on the relatively low evidence for and against the part that biofilm plays in BIA-ALCL is that Ralstonia spp. are well-known contaminants that have been found in molecular grade water and PCR reagents [23,24]. This suggests that determining the predominance or lack thereof of gram-negative bacteria based on the species Ralstonia spp. as a representative may be flawed. More research is needed into the question of whether there are specific strains associated with the development of BIA-ALCL, which should focus on utilizing another Gram-negative bacterial species instead of Ralstonia spp.
In alignment with the guidelines from the American Society of Plastic Surgeons (ASPS) and numerous other organizations, the meticulous irrigation utilizing solutions such as Triple antibiotic solution, Povidone-iodine, Betadine in varying concentrations, or hypochlorous acid, has been emphasized to avert infections in the context of breast augmentation or alloplastic breast reconstruction. Although this does not preclude the theory that biofilm occurs due to contamination during implantation, it makes it rather unlikely [25,26,27,28,29,30,31]. It is more probable that the propensity toward biofilm formation is orchestrated via bacterial seeding, through bacteremia or other mechanisms, of the implant over time within the patients, a scenario mirroring those in other pathologies linked with closed cavities [32,33,34,35]. Within the domain of plastic surgery, if the hypothesis of a strong bacterial linkage to BIA-ALCL was validated, it could markedly shape breast implant safety protocols. The existing practice of submitting seroma fluid for culture in implant scenarios is a judicious measure that is in line with the standard of care and recommendation for suspicion of BIA-ALCL [36,37]. If a bacterial-derived BIA-ALCL pathway was identified, we could amplify this practice of seroma evaluation and integrate capsule biopsy and culture during implant or expander exchange. The identification of particular bacterial species in these instances could help stratify patients as having an escalated risk for BIA-ALCL. The magnitude of the association between bacterial existence and BIA-ALCL could, in turn, guide the cadence and thoroughness of monitoring regimes. Moreover, the insights procured from such endeavors could potentially delineate the prerequisites for prophylactic capsulectomy, thereby forging a more individualized and evidence-informed approach to augmenting patient safety and diminishing BIA-ALCL risks.
Our systematic review on the association between BIA-ALCL and bacterial biofilm as a potential contributor is not without its limitations. A primary challenge encountered during this review was the limited number of studies that undertook bacterial sampling or testing within the available literature addressing BIA-ALCL. Given this scarcity, drawing a comprehensive and definitive correlation between BIA-ALCL and bacterial biofilms becomes inherently challenging. However, by aggregating the existing literature and evidence on this topic, our review has provided the most exhaustive insight on the subject to date. Additionally, the very nature of BIA-ALCL, and the extended latency period before the malignancy manifests, further underscores the need for better modeling in future research. As with any systematic review, there exists inherent heterogeneity between studies which, in this case, was further accentuated by the specificity of bacterial testing in relation to BIA-ALCL. This variability prevented a more unified and meta-analytic approach to the results. Despite these limitations, our review has shed critical light on the relationship between bacterial biofilms and BIA-ALCL. Additionally, the scope of this systematic review was confined to three medical databases. Although these databases are extensive, this limitation may have led to the exclusion of certain relevant data.
5. Conclusions
Our systematic review reveals an absence of compelling evidence to substantiate a direct link between bacterial biofilms and the pathogenesis of BIA-ALCL within the milieu of breast implants. Although current data intimate a potential contributory role for biofilms, these assertions remain uncorroborated. This observed data scarcity necessitates further targeted, rigorous scientific inquiry to either validate or refute the role of biofilms in the complex etiological framework of BIA-ALCL. Such forthcoming research could serve as a critical juncture in not only clarifying this enigmatic relationship but also in advancing our collective understanding of the disease, with implications for enhanced screening protocols and pre-surgical guidelines. Given the inconclusive nature of extant findings, further empirical scrutiny is both warranted and exigent. Given the current data limitations and inconclusive findings, continued exploration is both imperative and timely.
Author Contributions
J.A.F. and O.R. conceptualized and initiated this study. J.B. and M.J.E.-D. assisted in drafting the research protocol. J.A.F., I.T., A.H.A., D.L. and D.A. were responsible for the screening process. M.W.C. and P.K. served as subject matter experts and contributed significantly to both the initial draft and subsequent revisions. K.A.S. actively participated in refining the research methodology and literature review. S.J.L. provided overarching project oversight. All authors were involved in the revision process and reviewed and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable. No new data were created or analyzed in this study. Data sharing is not applicable to this article. All information relevant to this systematic review is part of the manuscript, figures, tables, and/or digital supplemental content. Additional information can be found within the publicly available PROSPERO protocol for this study. If any further information is required, the reader may contact the corresponding author for clarifications.
Acknowledgments
We sincerely thank Victor Joao, affiliated with the Universidad De La Republica Uruguay, for his help as the active librarian for this project. He helped with creating search strategies and gathering the search results for this manuscript.
Conflicts of Interest
All authors declared that there are no conflict of interest.
References
- Keech, J.A., Jr.; Creech, B.J. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast. Reconstr. Surg. 1997, 100, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Medical Device Reports of Breast Implant-Associated Anaplastic Large Cell Lymphoma. 2022. Available online: https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma (accessed on 4 December 2023).
- Alessandri-Bonetti, M.; Jeong, T.; Vaienti, L.; De La Cruz, C.; Gimbel, M.L.; Nguyen, V.T.; Egro, F.M. The Role of Microorganisms in the Development of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Pathogens 2023, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). 2023. Available online: https://www.plasticsurgery.org/patient-safety/breast-implant-safety/bia-alcl-summary (accessed on 4 December 2023).
- Wang, G.; Zhao, R.; Bi, R.; Xie, H. Subcutaneous Face and Neck lift: A Traditional Method with Definite Effects Among Asians. Aesthetic Surg. J. 2021, 41, NP1890–NP1903. [Google Scholar] [CrossRef] [PubMed]
- DeCoster, R.C.; Lynch, E.B.; Bonaroti, A.R.; Miranda, R.N.; Hunt, K.K.; Clemens, M.W. Breast Implant–Associated Anaplastic Large Cell Lymphoma. Clin. Plast. Surg. 2021, 48, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Quatman, C.E.; Manring, M.; Siston, R.A.; Flanigan, D.C. How to write a systematic review. Am. J. Sports Med. 2013, 42, 2761–2768. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung and Blood Institute. Study Quality Assessment Tools. 2019. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 4 December 2023).
- Eysenck, H.J. Systematic Reviews: Meta-analysis and its problems. BMJ 1994, 309, 789–792. [Google Scholar] [CrossRef]
- Greco, T.; Zangrillo, A.; Biondi-Zoccai, G.; Landoni, G. Meta-analysis: Pitfalls and hints. Heart Lung Vessel. 2013, 5, 219–225. [Google Scholar]
- Nordmann, A.J.; Kasenda, B.; Briel, M. Meta-analyses: What they can and cannot do. Swiss Med. Wkly. 2012, 142, w13518. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Patsopoulos, N.A.; Rothstein, H.R. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ 2008, 336, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, A.; Pepe, G.; Giarnieri, E.; Cippitelli, C.; Bonifacino, A.; Mattei, M.; Martelli, M.; Falasca, C.; Cox, M.C.; Santino, I.; et al. Cytological diagnostic features of late breast implant seromas: From reactive to anaplastic large cell lymphoma. PLoS ONE 2017, 12, e0181097. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Jacombs, A.; Vickery, K.; Merten, S.L.; Pennington, D.G.; Deva, A.K. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: Implications for breast implant–associated lymphoma. Plast. Reconstr. Surg. 2015, 135, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Johani, K.; Almatroudi, A.; Vickery, K.; Van Natta, B.; Kadin, M.E.; Brody, G.; Clemens, M.; Cheah, C.Y.; Lade, S.; et al. Bacterial Biofilm Infection Detected in Breast Implant—Associated Anaplastic Large-Cell Lymphoma. Plast. Reconstr. Surg. 2016, 137, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Mempin, M.; Hu, H.; Vickery, K.; Kadin, M.E.; Prince, H.M.; Kouttab, N.; Morgan, J.W.; Adams, W.P.; Deva, A.K. Gram-Negative Bacterial Lipopolysaccharide Promotes Tumor Cell Proliferation in Breast Implant-Associated Anaplastic Large-Cell Lymphoma. Cancers 2021, 13, 5298. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.N.; Hanson, B.M.; Pinkner, C.L.; Simar, S.R.; Pinkner, J.S.; Parikh, R.; Clemens, M.W.; Hultgren, S.J.; Myckatyn, T.M. Insights into the Microbiome of Breast Implants and Periprosthetic Tissue in Breast Implant-Associated Anaplastic Large Cell Lymphoma. Sci. Rep. 2019, 9, 10393. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Deva, A.K.; Prince, H.M. Breast Implant-Associated Anaplastic Large Cell Lymphoma. Curr. Hematol. Malign. Rep. 2018, 13, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Lajevardi, S.S.; Rastogi, P.; Isacson, D.; Deva, A.K. What are the likely causes of breast implant associated anaplastic large cell lymphoma (BIA-ALCL)? JPRAS Open 2022, 32, 34–42. [Google Scholar] [CrossRef]
- Tevis, S.E.; Hunt, K.K.; Miranda, R.N.; Lange, C.; Butler, C.E.; Clemens, M.W. Differences in Human Leukocyte Antigen Expression Between Breast Implant–Associated Anaplastic Large Cell Lymphoma Patients and the General Population. Aesthetic Surg. J. 2019, 39, 1065–1070. [Google Scholar] [CrossRef]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014, 12, 87. [Google Scholar] [CrossRef]
- Wally, N.; Schneider, M.; Thannesberger, J.; Kastner, M.T.; Bakonyi, T.; Indik, S.; Rattei, T.; Bedarf, J.; Hildebrand, F.; Law, J.; et al. Plasmid DNA contaminant in molecular reagents. Sci. Rep. 2019, 9, 1652. [Google Scholar] [CrossRef]
- Epps, M.T.; Langsdon, S.; Pels, T.K.; Noyes, V.; Levine, D.; Thurston, T.E.; Spratt, H.G.; Brzezienski, M.A. Pocket Irrigation and Technique During Reconstructive Surgery. Ann. Plast. Surg. 2019, 82, S427–S432. [Google Scholar] [CrossRef] [PubMed]
- Jewell, M.L.; Bionda, N.; Moran, A.V.; Bevels, E.J.; Jewell, H.L.; Hariri, S.; Leung, B.K. In Vitro Evaluation of Common Antimicrobial Solutions Used for Breast Implant Soaking and Breast Pocket Irrigation—Part 2: Efficacy Against Biofilm-Associated Bacteria. Aesthetic Surg. J. 2021, 41, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Ngaage, L.M.M.C.M.B.; Elegbede, A.; Brao, K.B.; Chopra, K.; Gowda, A.U.; Nam, A.J.; Ernst, R.K.; Shirtliff, M.E.; Harro, J.; Rasko, Y.M. The Efficacy of Breast Implant Irrigant Solutions: A Comparative Analysis Using an In Vitro Model. Plast. Reconstr. Surg. 2020, 146, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, E.J.; Felder-Scott, C.; Deva, A.K.; E Greenberg, D.; Adams, W.P. Optimizing Breast Pocket Irrigation: The Breast Implant–Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) Era. Aesthetic Surg. J. 2019, 40, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E. The Limitations of Three-Dimensional Simulations in Breast Augmentation. Aesthetic Surg. J. 2015, 35, Np62-4. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.; Yim, N.; Tummala, S.; Parsa, A.A.; Parsa, F.D. Povidone-Iodine versus antibiotic irrigation in breast implant surgery: Revival of the ideal solution. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O.; Wall, M.J.; Itani, K.M.; Otterson, M.F.; Webb, A.L.; Carrick, M.M.; Miller, H.J.; Awad, S.S.; Crosby, C.T.; Mosier, M.C.; et al. Chlorhexidine–Alcohol versus Povidone–Iodine for Surgical-Site Antisepsis. N. Engl. J. Med. 2010, 362, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Makihara, S.; Kariya, S.; Okano, M.; Naito, T.; Tsumura, M.; Nishizaki, K. Orbital complications of infected mucocele in the paranasal sinuses. Auris Nasus Larynx 2020, 47, 990–995. [Google Scholar] [CrossRef]
- Jongsma, T.E.; Puylaert, J.B. Infection of a malignant appendiceal mucocele masquerading as an appendiceal abscess: Role of preoperative sonography and CT. J. Clin. Ultrasound 2009, 37, 236–238. [Google Scholar] [CrossRef]
- Rangarathnam, C.; Linscheid, R.L. Infected mucous cyst of the finger. J. Hand Surg. 1984, 9, 245–247. [Google Scholar] [CrossRef]
- Eichenfield, D.Z.; Sprague, J.; Eichenfield, L.F. Management of Acne Vulgaris. JAMA 2021, 326, 2055–2067. [Google Scholar] [CrossRef]
- Clemens, M.W.; Medeiros, L.J.; Butler, C.E.; Hunt, K.K.; Fanale, M.A.; Horwitz, S.; Weisenburger, D.D.; Liu, J.; Morgan, E.A.; Kanagal-Shamanna, R.; et al. Complete Surgical Excision Is Essential for the Management of Patients with Breast Implant–Associated Anaplastic Large-Cell Lymphoma. J. Clin. Oncol. 2016, 34, 160–168. [Google Scholar] [CrossRef]
- Patzelt, M.; Zarubova, L.; Klener, P.; Barta, J.; Benkova, K.; Brandejsova, A.; Trneny, M.; Gürlich, R.; Sukop, A. Anaplastic Large-Cell Lymphoma Associated with Breast Implants: A Case Report of a Transgender Female. Aesthetic Plast. Surg. 2017, 42, 451–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).