Comprehensive Analysis of PANoptosis-Related Gene Signature of Ulcerative Colitis
Abstract
:1. Introduction
2. Results
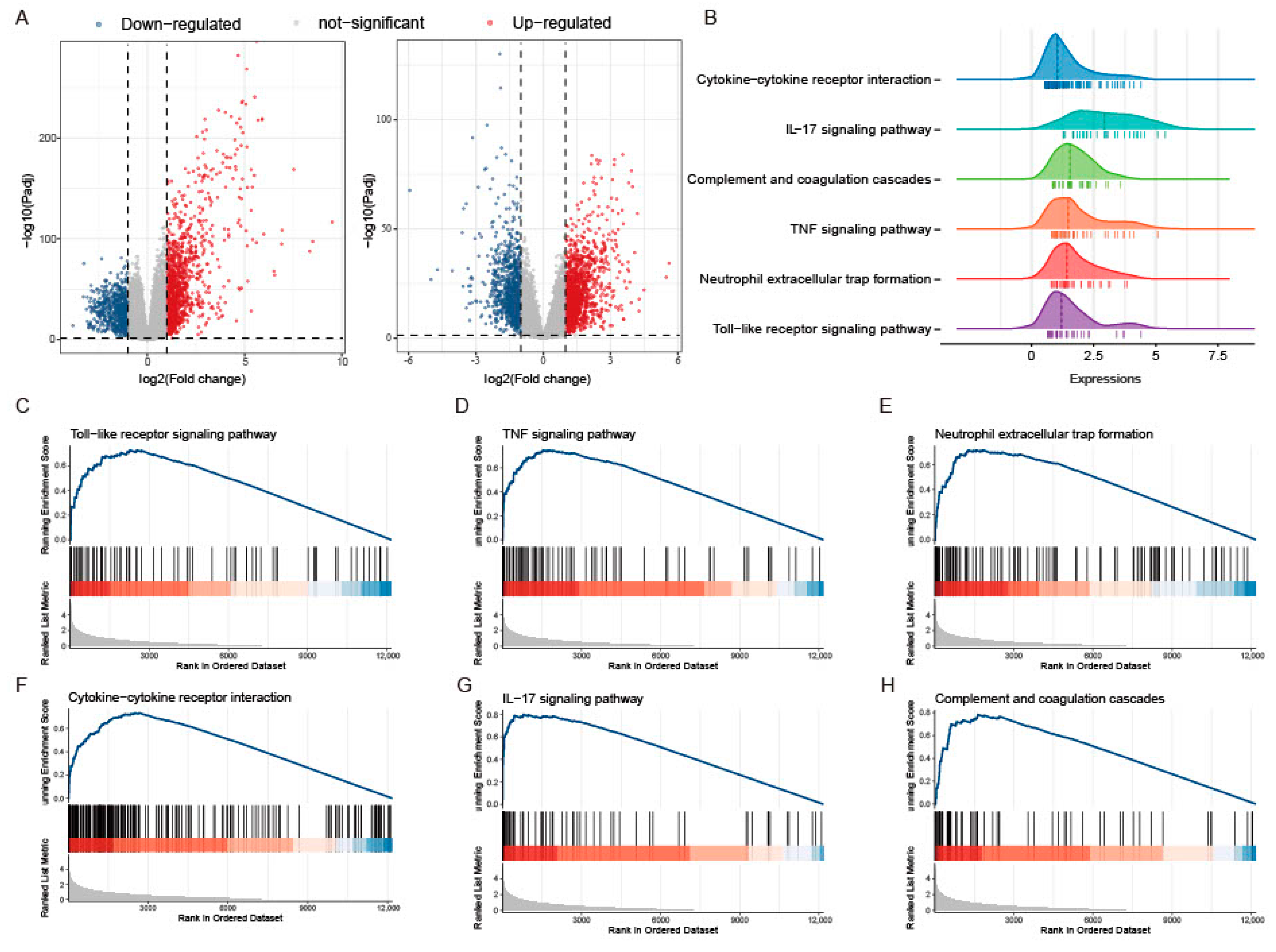
2.1. GSEA Revealed the Innate Immune-Related Biological Processes between Healthy Individuals and UC Patients
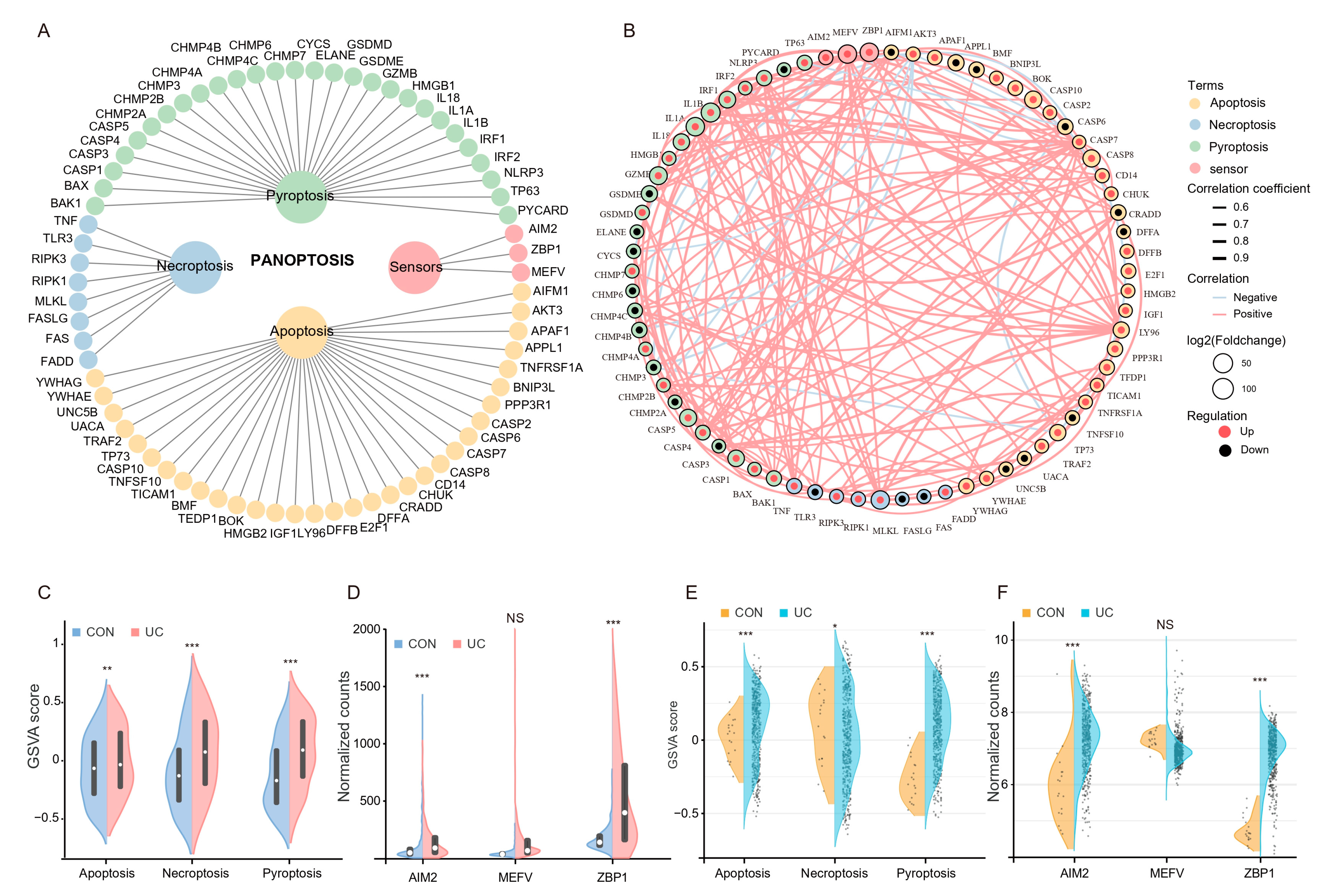
2.2. Colonic PANoptosis Signaling Is Significantly Activated in UC Patients
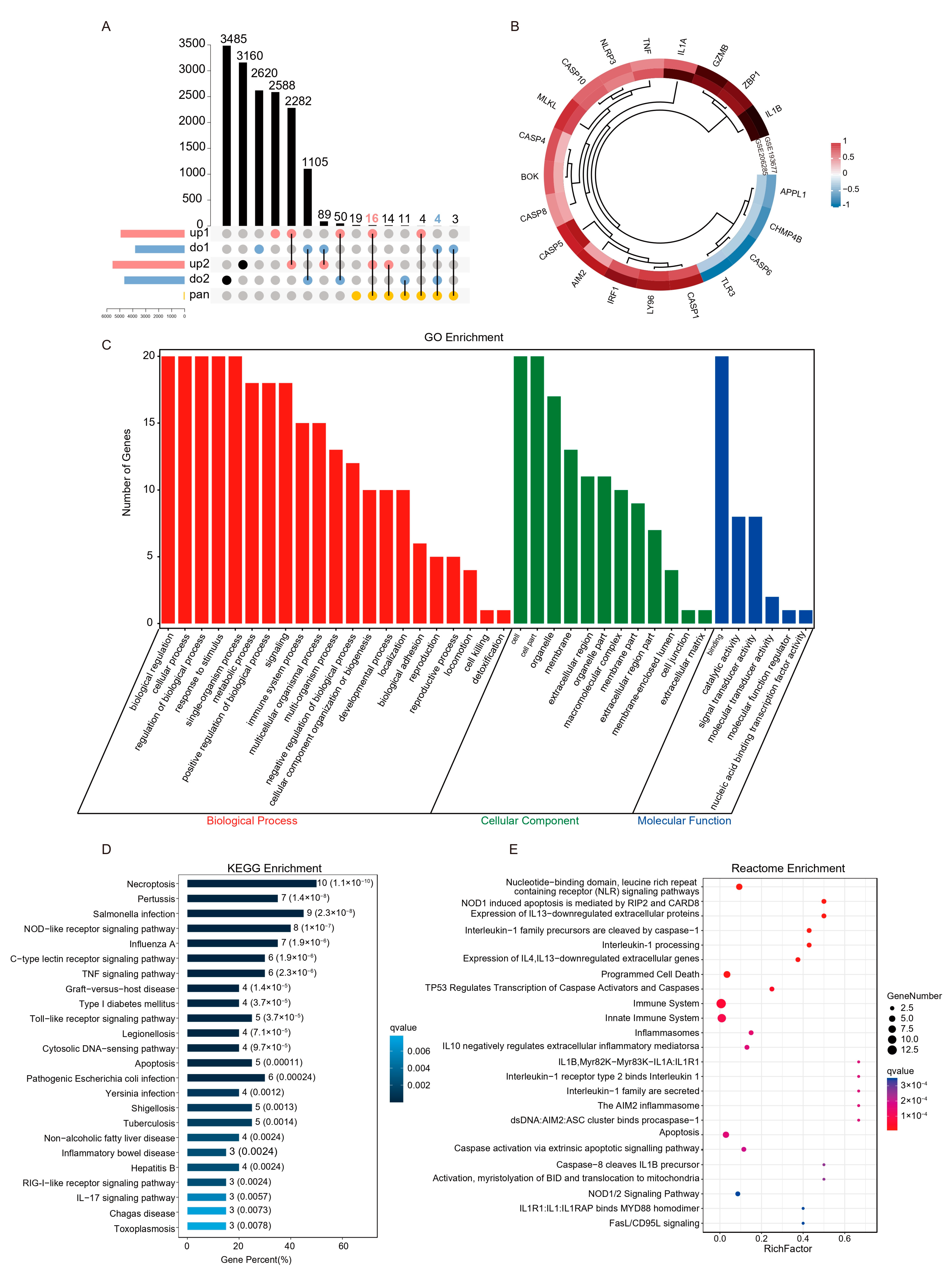
2.3. Screening and Functional Enrichment Analysis of Differential PANoptosis Genes
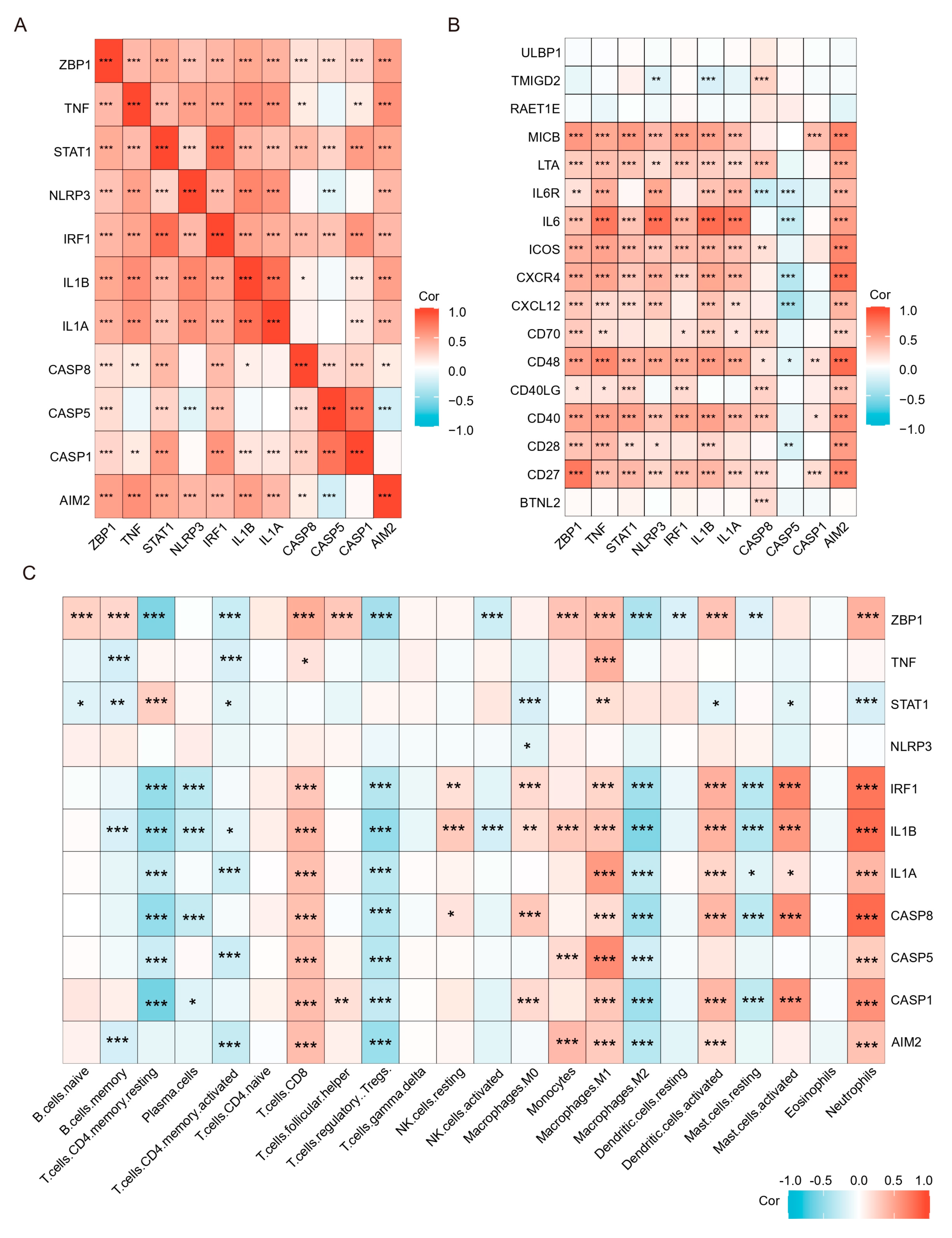
2.4. The PPI Network Analysis Indicated That the Hub Gene Mainly Comprises PANoptosis Sensors and Effectors
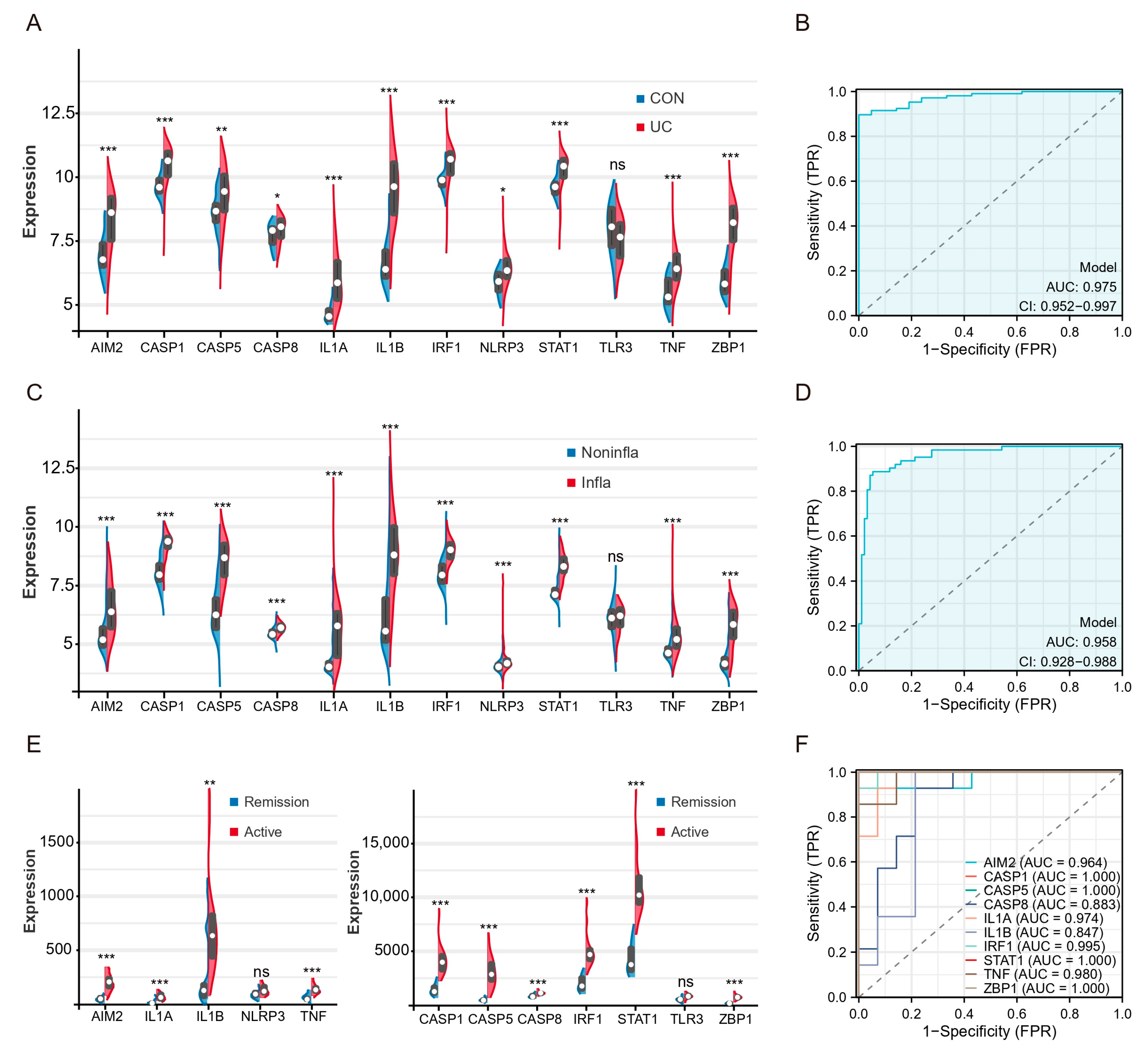
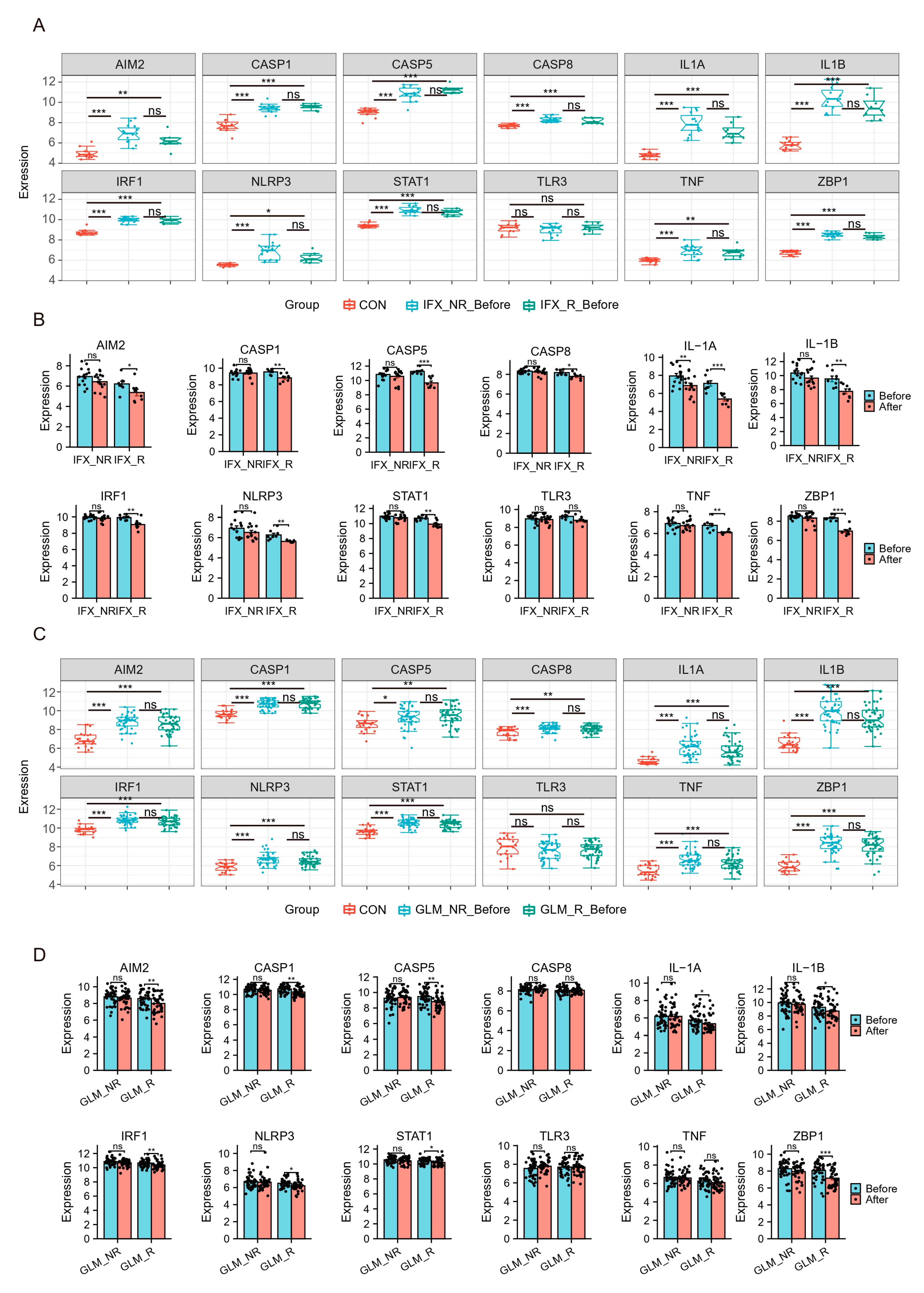
2.5. Patients with Active UC and Inflammatory Tissues of the Colon Exhibit High Expression of Hub Genes and TFs of PANoptosis
2.6. Hub Genes and TFs of PANoptosis Are Associated with UC Patients’ Response to Biologics
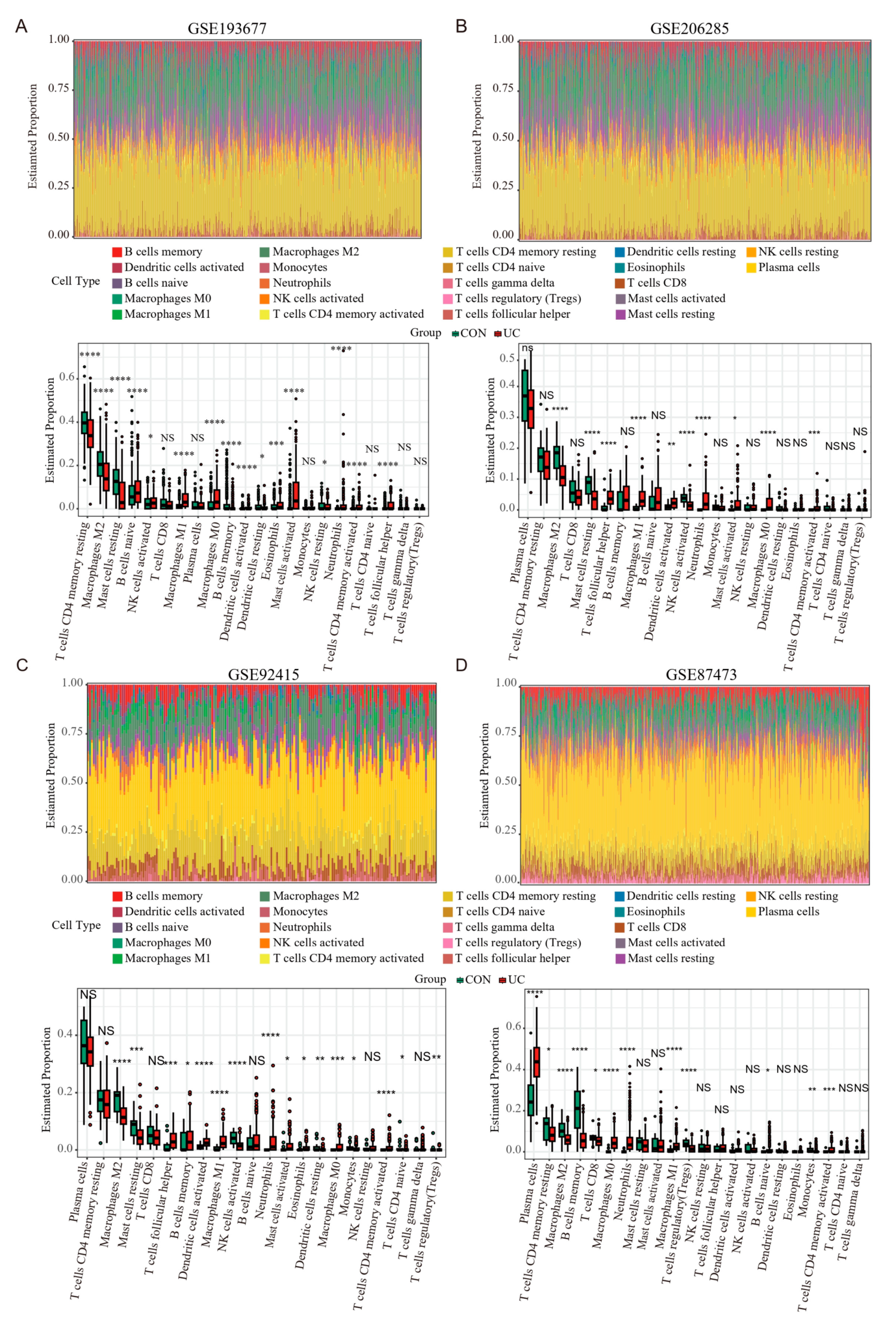
2.7. PANoptosis Is Associated with Increased Immune Cells and Proinflammatory Factors in the Colonic Mucosa of UC Patients
2.8. Prediction of Potential Drugs Targeting PANoptosis Signaling in UC Patients
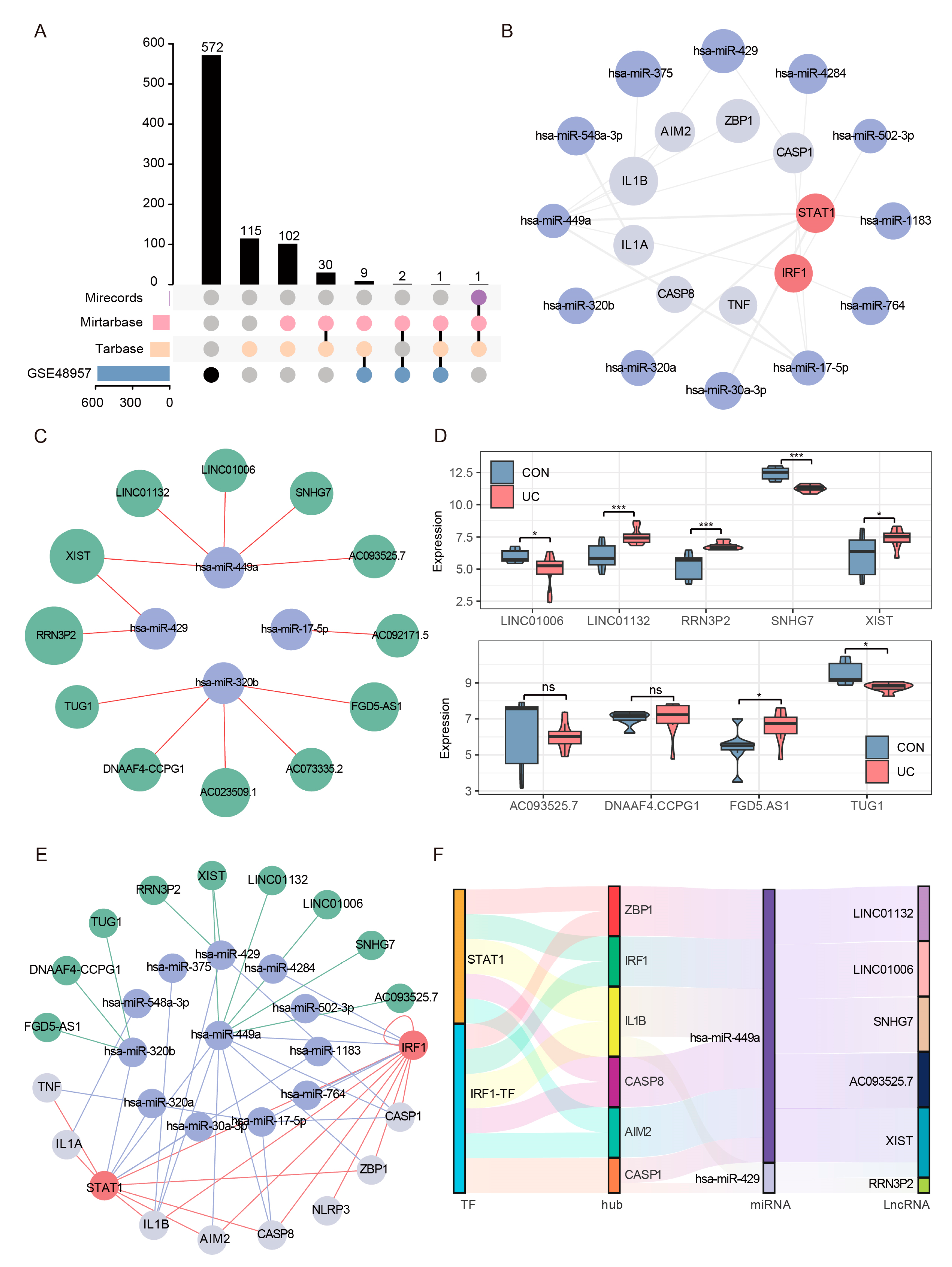
2.9. Construction of Reliable ceRNA Regulatory Network for PANoptosis Signaling
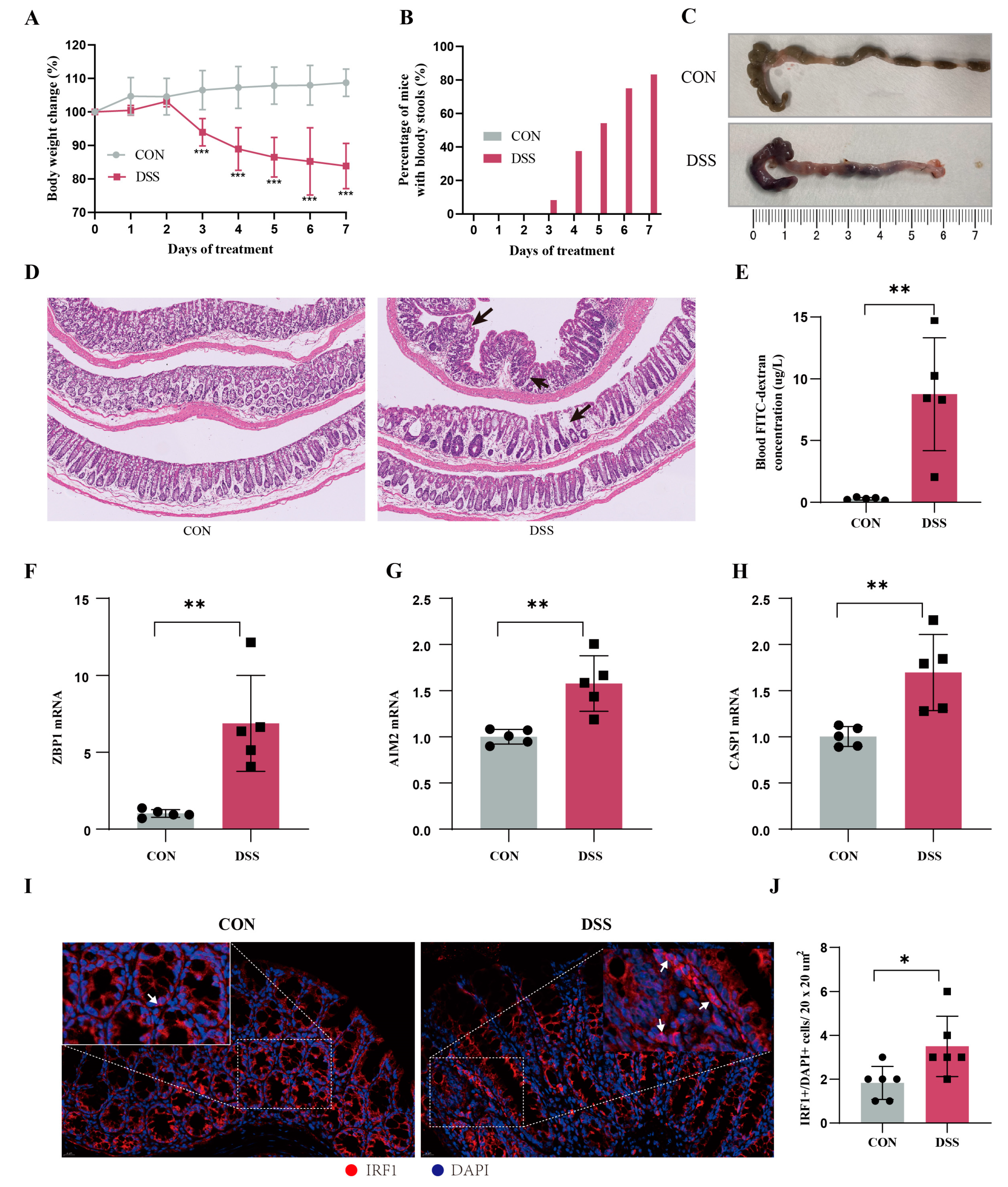
2.10. Animal Models Validate the Core Genes of PANoptosis Signaling
3. Discussion
4. Methods
4.1. Datasets and Sample Selection
4.2. Identification of Differentially Expressed Genes (DEGs)
4.3. Robust Rank Aggregation (RRA) Analysis
4.4. Biological Function and Pathway Enrichment Analysis
4.5. PANoptosis Gene Sets
4.6. Gene Set Variation Analysis (GSVA) Analysis
4.7. PPI Network Analysis
4.8. Evaluation of Tissue Infiltrating Immune Cells
4.9. Correction Analysis
4.10. Small Molecule Agents Screening and Molecular Docking Analysis
4.11. CeRNA Network Construction
4.12. Animal Model of Colitis
4.13. Intestinal Permeability Assay to FITC-Dextran
4.14. H&E and IF Staining
4.15. RNA Extraction and RT-PCR
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IECs | Intestinal epithelial cells |
| UC | Ulcerative colitis |
| RRA | Robust Rank Aggregation |
| GSEA | Gene set enrichment analysis |
| GSVA | Geneset variation analysis |
| TFs | Transcription factors |
| CeRNA | Competitive endogenous RNA |
| PCD | Programmed cell death |
| FDR | False discovery rate |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| CMap | Connectivity map database |
| ZBP1 | Z-DNA-binding protein 1 |
| AIM2 | Absent in melanoma 2 |
| GSDMD | Gasdermin-D |
| IRF1 | Interferon Regulatory Factor 1 |
| CAC | Colorectal cancer |
References
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Geng, H.; Tan, X.-D. Cell death of intestinal epithelial cells in intestinal diseases. Sheng Li Xue Bao 2020, 72, 308–324. [Google Scholar] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet Lond. Engl. 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Guenin-Mace, L.; Konieczny, P.; Naik, S. Immune-Epithelial Cross Talk in Regeneration and Repair. Annu. Rev. Immunol. 2023, 41, 207–228. [Google Scholar] [CrossRef]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef]
- Christgen, S.; Tweedell, R.E.; Kanneganti, T.-D. Programming inflammatory cell death for therapy. Pharmacol. Ther. 2022, 232, 108010. [Google Scholar] [CrossRef]
- Bulek, K.; Zhao, J.; Liao, Y.; Rana, N.; Corridoni, D.; Antanaviciute, A.; Chen, X.; Wang, H.; Qian, W.; Miller-Little, W.A.; et al. Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis. J. Clin. Investig. 2020, 130, 4218–4234. [Google Scholar] [CrossRef]
- Schwarzer, R.; Jiao, H.; Wachsmuth, L.; Tresch, A.; Pasparakis, M. FADD and Caspase-8 Regulate Gut Homeostasis and Inflammation by Controlling MLKL- and GSDMD-Mediated Death of Intestinal Epithelial Cells. Immunity 2020, 52, 978–993.e6. [Google Scholar] [CrossRef]
- Günther, C.; Martini, E.; Wittkopf, N.; Amann, K.; Weigmann, B.; Neumann, H.; Waldner, M.J.; Hedrick, S.M.; Tenzer, S.; Neurath, M.F.; et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 2011, 477, 335–339. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Nagata, S.; Tanaka, M. Programmed cell death and the immune system. Nat. Rev. Immunol. 2017, 17, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Place, D.E.; Kanneganti, T.-D. The innate immune system and cell death in autoinflammatory and autoimmune disease. Curr. Opin. Immunol. 2020, 67, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Kanneganti, T.-D. PANoptosome signaling and therapeutic implications in infection: Central role for ZBP1 to activate the inflammasome and PANoptosis. Curr. Opin. Immunol. 2023, 83, 102348. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Wang, Y.; Kanneganti, T.-D. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput. Struct. Biotechnol. J. 2021, 19, 4641–4657. [Google Scholar] [CrossRef]
- Zhu, P.; Ke, Z.-R.; Chen, J.-X.; Li, S.-J.; Ma, T.-L.; Fan, X.-L. Advances in Mechanism and Regulation of PANoptosis: Prospects in Disease Treatment. Front. Immunol. 2023, 14, 1120034. [Google Scholar] [CrossRef]
- Innate Immune Inflammatory Cell Death: PANoptosis and PANoptosomes in Host Defense and Disease—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/36782083/ (accessed on 11 December 2023).
- NLRP12-PANoptosome activates PANoptosis and Pathology in Response to Heme and PAMPs—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/37267949/ (accessed on 11 December 2023).
- Therapeutic Potential of PANoptosis: Innate Sensors, Inflammasomes, and RIPKs in PANoptosomes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/37977994/ (accessed on 11 December 2023).
- Place, D.E.; Kanneganti, T.-D. Cell death-mediated cytokine release and its therapeutic implications. J. Exp. Med. 2019, 216, 1474–1486. [Google Scholar] [CrossRef]
- Gullett, J.M.; Tweedell, R.E.; Kanneganti, T.-D. It’s All in the PAN: Crosstalk, Plasticity, Redundancies, Switches, and Interconnectedness Encompassed by PANoptosis Underlying the Totality of Cell Death-Associated Biological Effects. Cells 2022, 11, 1495. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.-D. Inflammasome signaling in colorectal cancer. Transl. Res. J. Lab. Clin. Med. 2023, 252, 45–52. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Dai, Y.-H.; Wan, X.-X.; Hu, X.-M.; Zhao, W.-J.; Ban, X.-X.; Wan, H.; Huang, K.; Zhang, Q.; Xiong, K. ZBP1-Mediated Necroptosis: Mechanisms and Therapeutic Implications. Molecules 2022, 28, 52. [Google Scholar] [CrossRef]
- Zheng, M.; Karki, R.; Vogel, P.; Kanneganti, T.-D. Caspase-6 Is a Key Regulator of Innate Immunity, Inflammasome Activation, and Host Defense. Cell 2020, 181, 674–687.e13. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Gurung, P.; Kesavardhana, S.; Samir, P.; Burton, A.; Mummareddy, H.; Vogel, P.; Pelletier, S.; Burgula, S.; Kanneganti, T.-D. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J. Exp. Med. 2020, 217, jem.20191644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yin, C.; Boyd, D.F.; Quarato, G.; Ingram, J.P.; Shubina, M.; Ragan, K.B.; Ishizuka, T.; Crawford, J.C.; Tummers, B.; et al. Influenza Virus Z-RNAs Induce ZBP1-Mediated Necroptosis. Cell 2020, 180, 1115–1129.e13. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.K.S.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S.; et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Samir, P.; Malireddi, R.K.S.; Kanneganti, T.-D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, T.; Man, S.M.; Malireddi, R.K.S.; Karki, R.; Kesavardhana, S.; Place, D.E.; Neale, G.; Vogel, P.; Kanneganti, T.-D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016, 1, aag2045. [Google Scholar] [CrossRef] [PubMed]
- de Reuver, R.; Verdonck, S.; Dierick, E.; Nemegeer, J.; Hessmann, E.; Ahmad, S.; Jans, M.; Blancke, G.; Van Nieuwerburgh, F.; Botzki, A.; et al. ADAR1 prevents autoinflammation by suppressing spontaneous ZBP1 activation. Nature 2022, 607, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, N.W.; Ames, J.M.; Maurano, M.; Chu, L.H.; Somfleth, K.Y.; Gokhale, N.S.; Werner, M.; Snyder, J.M.; Lichauco, K.; Savan, R.; et al. ADAR1 mutation causes ZBP1-dependent immunopathology. Nature 2022, 607, 769–775. [Google Scholar] [CrossRef]
- Chen, I.-F.; Ou-Yang, F.; Hung, J.-Y.; Liu, J.-C.; Wang, H.; Wang, S.-C.; Hou, M.-F.; Hortobagyi, G.N.; Hung, M.-C. AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol. Cancer Ther. 2006, 5, 1–7. [Google Scholar] [CrossRef]
- Wang, B.; Bhattacharya, M.; Roy, S.; Tian, Y.; Yin, Q. Immunobiology and Structural Biology of AIM2 Inflammasome. Mol. Aspects Med. 2020, 76, 100869. [Google Scholar] [CrossRef]
- Du, L.; Wang, X.; Chen, S.; Guo, X. The AIM2 inflammasome: A novel biomarker and target in cardiovascular disease. Pharmacol. Res. 2022, 186, 106533. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Karki, R.; Wang, Y.; Nguyen, L.N.; Kalathur, R.C.; Kanneganti, T.-D. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature 2021, 597, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Kesavardhana, S.; Karki, R.; Kancharana, B.; Burton, A.R.; Kanneganti, T.-D. RIPK1 Distinctly Regulates Yersinia-Induced Inflammatory Cell Death, PANoptosis. ImmunoHorizons 2020, 4, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, Y.-B.; Gui, J.-F.; Lemon, S.M.; Yamane, D. Interferon regulatory factor 1 (IRF1) and anti-pathogen innate immune responses. PLoS Pathog. 2021, 17, e1009220. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Ishihara, M.; Lamphier, M.S.; Tanaka, N.; Oishi, I.; Aizawa, S.; Matsuyama, T.; Mak, T.W.; Taki, S.; Taniguchi, T. An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature 1995, 376, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tang, Y.-D.; Zheng, C. Revisiting IRF1-mediated antiviral innate immunity. Cytokine Growth Factor Rev. 2022, 64, 1–6. [Google Scholar] [CrossRef]
- Kuriakose, T.; Zheng, M.; Neale, G.; Kanneganti, T.-D. IRF1 Is a Transcriptional Regulator of ZBP1 Promoting NLRP3 Inflammasome Activation and Cell Death during Influenza Virus Infection. J. Immunol. 2018, 200, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Lee, E.; Banoth, B.; Malireddi, R.S.; Samir, P.; Tuladhar, S.; Mummareddy, H.; Burton, A.R.; Vogel, P.; et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. J. Clin. Investig. 2020, 5, e136720. [Google Scholar] [CrossRef]
- Tan, G.; Huang, C.; Chen, J.; Chen, B.; Shi, Y.; Zhi, F. An IRF1-dependent Pathway of TNFα-induced Shedding in Intestinal Epithelial Cells. J. Crohn’s Colitis 2022, 16, 133–142. [Google Scholar] [CrossRef]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Kong, Y.; Chen, N.; Peng, W.; Zi, R.; Jiang, M.; Zhu, J.; Wang, Y.; Yue, J.; Lv, J.; et al. Identification of Immune-Related Gene Signature and Prediction of CeRNA Network in Active Ulcerative Colitis. Front. Immunol. 2022, 13, 855645. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-C.; Zhu, W.; Li, S.-J.; Hu, W.; Zhang, J.; Zhuo, Y.; Zhang, H.; Wang, J.; Zhang, Y.; Huang, S.X.; et al. FOXC1-mediated LINC00301 facilitates tumor progression and triggers an immune-suppressing microenvironment in non-small cell lung cancer by regulating the HIF1α pathway. Genome Med. 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.; Hastie, A.; Diez-Domingo, J.; Tinoco, J.C.; Yu, C.J.; Andrews, C.; Beytout, J.; Caso, C.; Cheng, H.S.; Cheong, H.J.; et al. The Adjuvanted Recombinant Zoster Vaccine Confers Long-Term Protection Against Herpes Zoster: Interim Results of an Extension Study of the Pivotal Phase 3 Clinical Trials ZOE-50 and ZOE-70. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhao, H.; Zheng, L.; Zhou, C.; Han, Y.; Wu, A.; Jia, Z.; Xia, T.; Zhi, Q. Non-coding RNAs and colitis-associated cancer: Mechanisms and clinical applications. Clin. Transl. Med. 2023, 13, e1253. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.-H.; Lin, S.-C.; Wang, W.-C.; Yang, Y.-C.; Lee, J.-C.; Lin, P.-W.; Chu, M.-L.; Lan, K.-Y.; Zuchini, R.; Liu, H.-S.; et al. Autophagy Upregulates miR-449a Expression to Suppress Progression of Colorectal Cancer. Front. Oncol. 2021, 11, 738144. [Google Scholar] [CrossRef]
- Feng, Y.; Dong, Y.-W.; Song, Y.-N.; Xiao, J.-H.; Guo, X.-Y.; Jiang, W.-L.; Lu, L.-G. MicroRNA-449a is a potential predictor of colitis-associated colorectal cancer progression. Oncol. Rep. 2018, 40, 1684–1694. [Google Scholar] [CrossRef]
- Ma, M.; Pei, Y.; Wang, X.; Feng, J.; Zhang, Y.; Gao, M.-Q. LncRNA XIST mediates bovine mammary epithelial cell inflammatory response via NF-κB/NLRP3 inflammasome pathway. Cell Prolif. 2019, 52, e12525. [Google Scholar] [CrossRef]
- Argmann, C.; Hou, R.; Ungaro, R.C.; Irizar, H.; Al-Taie, Z.; Huang, R.; Kosoy, R.; Venkat, S.; Song, W.M.; Di’Narzo, A.F.; et al. Biopsy and blood-based molecular biomarker of inflammation in IBD. Gut 2023, 72, 1271–1287. [Google Scholar] [CrossRef]
- Pavlidis, P.; Tsakmaki, A.; Pantazi, E.; Li, K.; Cozzetto, D.; Bell, J.D.; Yang, F.; Lo, J.W.; Alberts, E.; Sa, A.C.C.; et al. Interleukin-22 regulates neutrophil recruitment in ulcerative colitis and is associated with resistance to ustekinumab therapy. Nat. Commun. 2022, 13, 5820. [Google Scholar] [CrossRef]
- Li, K.; Strauss, R.; Ouahed, J.; Chan, D.; Telesco, S.E.; Shouval, D.S.; Canavan, J.B.; Brodmerkel, C.; Snapper, S.B.; Friedman, J.R. Molecular Comparison of Adult and Pediatric Ulcerative Colitis Indicates Broad Similarity of Molecular Pathways in Disease Tissue. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 45–52. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.-F.; Reinisch, W.; et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 85–95; quiz e14–15. [Google Scholar] [CrossRef]
- Van der Goten, J.; Vanhove, W.; Lemaire, K.; Van Lommel, L.; Machiels, K.; Wollants, W.-J.; De Preter, V.; De Hertogh, G.; Ferrante, M.; Van Assche, G.; et al. Integrated miRNA and mRNA expression profiling in inflamed colon of patients with ulcerative colitis. PLoS ONE 2014, 9, e116117. [Google Scholar] [CrossRef]
- Padua, D.; Mahurkar-Joshi, S.; Law, I.K.M.; Polytarchou, C.; Vu, J.P.; Pisegna, J.R.; Shih, D.; Iliopoulos, D.; Pothoulakis, C. A long noncoding RNA signature for ulcerative colitis identifies IFNG-AS1 as an enhancer of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G446–G457. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kolde, R.; Laur, S.; Adler, P.; Vilo, J. Robust rank aggregation for gene list integration and meta-analysis. Bioinforma Oxf. Engl. 2012, 28, 573–580. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov. Camb. Mass. 2021, 2, 100141. [Google Scholar] [CrossRef]
- Pan, H.; Pan, J.; Li, P.; Gao, J. Characterization of PANoptosis patterns predicts survival and immunotherapy response in gastric cancer. Clin. Immunol. 2022, 238, 109019. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chin, C.-H.; Wu, H.-H.; Chen, S.-H.; Ho, C.-W.; Ko, M.-T. Hubba: Hub objects analyzer–A framework of interactome hubs identification for network biology. Nucleic Acids Res. 2008, 36, W438–W443. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Janky, R.; Verfaillie, A.; Imrichová, H.; Van de Sande, B.; Standaert, L.; Christiaens, V.; Hulselmans, G.; Herten, K.; Sanchez, M.N.; Potier, D.; et al. iRegulon: From a gene list to a gene regulatory network using large motif and track collections. PLoS Comput. Biol. 2014, 10, e1003731. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef]




| GEO ID | RNA Type | Platform | Tissues | Attribute |
|---|---|---|---|---|
| GSE193677 | mRNA | GPL16791 | Colonic mucosal | Training set |
| GSE206285 | mRNA | GPL13158 | Colonic mucosal | Training set |
| GSE87466 | mRNA | GPL13158 | Colonic mucosal | Validation set |
| GSE66407 | mRNA | GPL19833 | Colonic mucosal | Validation set |
| GSE128682 | mRNA | GPL21697 | Colonic mucosal | Validation set |
| GSE73661 | mRNA | GPL6244 | Colonic mucosal | Validation set |
| GSE92415 | mRNA | GPL13158 | Colonic mucosal | Validation set |
| GSE48957 | miRNA | GPL14613 | Colonic mucosal | Validation set |
| GSE77013 | lncRNA | GPL16956 | Colonic mucosal | Validation set |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-M.; Yang, J.; Xia, W.-Y.; Wang, Y.-M.; Zhu, Y.-B.; Huang, Q.; Feng, T.; Xie, L.-S.; Li, S.-H.; Liu, S.-Q.; et al. Comprehensive Analysis of PANoptosis-Related Gene Signature of Ulcerative Colitis. Int. J. Mol. Sci. 2024, 25, 348. https://doi.org/10.3390/ijms25010348
Wang J-M, Yang J, Xia W-Y, Wang Y-M, Zhu Y-B, Huang Q, Feng T, Xie L-S, Li S-H, Liu S-Q, et al. Comprehensive Analysis of PANoptosis-Related Gene Signature of Ulcerative Colitis. International Journal of Molecular Sciences. 2024; 25(1):348. https://doi.org/10.3390/ijms25010348
Chicago/Turabian StyleWang, Jun-Meng, Jiao Yang, Wan-Yu Xia, Yue-Mei Wang, Yuan-Bing Zhu, Qin Huang, Tong Feng, Lu-Shuang Xie, Si-Hui Li, Shu-Qing Liu, and et al. 2024. "Comprehensive Analysis of PANoptosis-Related Gene Signature of Ulcerative Colitis" International Journal of Molecular Sciences 25, no. 1: 348. https://doi.org/10.3390/ijms25010348
APA StyleWang, J.-M., Yang, J., Xia, W.-Y., Wang, Y.-M., Zhu, Y.-B., Huang, Q., Feng, T., Xie, L.-S., Li, S.-H., Liu, S.-Q., Yu, S.-G., & Wu, Q.-F. (2024). Comprehensive Analysis of PANoptosis-Related Gene Signature of Ulcerative Colitis. International Journal of Molecular Sciences, 25(1), 348. https://doi.org/10.3390/ijms25010348





