1. Introduction
Glioblastoma is a primary brain tumor and one of the most aggressive solid tumors in current oncological patients [
1]. The documented median survival time is 15 to 19 months. The current standard of care includes safe surgical resection followed by radiotherapy and Temozolomide (TMZ) per os and adjuvant chemotherapy with TMZ. Currently, the chemotherapy regimens approved for glioblastoma, except the TMZ, are limited to carmustine and bevacizumab, which are primarily inefficient [
2]. Therefore, we followed this unmet need, explored a new therapeutic target for this disease, and found that the androgen receptor (AR) is amplified at the DNA and RNA levels, and 56% of glioblastomas over-express AR protein [
3]. We also demonstrated that AR protein expression could be detected in glial tumors in real time by positron emission tomography/computed tomography scanning using [F18] DHT [
4].
AR functions as a steroid-hormone-activated transcription factor in the cytoplasm, bound to chaperone proteins in the heat-shock family. Upon binding of androgens like testosterone or dihydrotestosterone (DHT), the chaperones are released, allowing AR to homodimerize, translocate to the nucleus, and stimulate the transcription of AR-responsive genes, known for promoting cell proliferation and migration, primarily in prostate growth [
5]. Based on its natural role, AR’s involvement in prostate cancer has been prominent and known for many years [
6]. However, the involvement of AR in glioblastoma and the potential androgen receptor-regulated signaling pathways in glioblastoma were largely unknown.
The two most abounded AR antagonists are bicalutamide and enzalutamide. Bicalutamide is a second-generation AR antagonist designed to prevent androgens from binding to AR. Enzalutamide, a third-generation AR antagonist, inhibits AR nuclear translocation and AR binding to DNA [
7]. Our observations of AR overexpression in glioblastoma and the known efficacy of AR inhibitors in prostate cancer prompted the investigation of AR antagonists’ impact on glioblastoma cells. This exploration revealed a significant (
p < 0.05) concentration-dependent induction of cell death in three glioblastoma cell lines and two glioma-initiating cell lines upon treatment with AR antagonists. Furthermore, the administration of 20 mg/kg Xtandi (enzalutamide) via the oral route to nude mice hosting subcutaneous U87MG human glioblastoma xenografts yielded a remarkable 72% reduction in tumor volume (
p = 0.0027) [
3], consistent with Werner C.K. et al. [
8].
Motivated by these results, the current study aimed to ascertain the therapeutic potential of AR antagonists in intracranial models of human glioblastoma.
3. Discussion
The findings of this study align with and extend the growing body of literature that highlights the pivotal role of androgen receptor (AR) antagonists in the treatment of glioblastoma. Glioblastoma, characterized by its aggressive nature and limited treatment options, has long been a formidable challenge in the field of oncology [
1,
2]. A noteworthy development is the recognition of AR expression in a considerable percentage of glioblastoma cases, suggesting a promising avenue for potential therapeutic interventions [
3,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19].
Our study’s demonstration of the efficacy of enzalutamide and a newly formulated bicalutamide in extending the lifespan of mice bearing intracranial human glioblastoma is consistent with earlier in vitro and in vivo studies. Notably, both our group and others have conducted studies involving enzalutamide, which have shown a significant reduction in glioblastoma tumor volume in mouse subcutaneous xenograft models, echoing these findings [
8].
As all AR antagonists were initially developed for prostate cancer, there remains an unmet medical need for additional therapeutic modalities for brain tumors, such as glioblastoma, and a demand for improved formulations of AR antagonists specifically tailored for brain tumors to enhance bioavailability. Bicalutamide is well absorbed after oral administration due to its high lipophilicity (log P, 2.92); however, it exhibits very low water solubility (<40 mg/L) [
20]. Thus, bicalutamide poses challenges for effective oral administration due to its low bioavailability. Various strategies, including solid dispersions [
21], micellar solubilization [
22], nanoemulsions [
23], hydrotropic solubilization [
24], particle size reduction [
25], lipid-based delivery systems [
26], and complexation with cyclodextrins (CyDs) [
27], are employed to enhance the water solubility of lipophilic drugs. We addressed this need through the reformulation of bicalutamide with excipients known as “GRAS” and the development of novel drug formulations, “Bic-sol” introduced in this study. Our research shows that this formulation dramatically enhances the solubility of bicalutamide, leading to increased concentrations in brain tissue and improved bioavailability. In contrast to other solubility methods employed for enhancing the water solubility of lipophilic drugs, the approach utilized in this study—dissolving the active ingredient in acetone—offers distinct advantages. The choice to utilize a polar solvent like acetone was based on the anticipation that a clear solution would form when mixed with water and another polar solvent (isopropanol) containing surfactants. The key differentiator lies in achieving complete molecular-level dissolution of the active ingredient at this stage, as opposed to existing in the form of particles or droplets. The choice to utilize a polar solvent like acetone was based on the anticipation that a clear solution would form when mixed with water and another containing surfactants. The key differentiator lies in achieving complete molecular-level dissolution of the active ingredient at this stage, as opposed to existing in the form of particles or droplets. Comparatively, alternative methods, such as those relying on different solvents or co-solvents, may yield dispersed powders or encounter challenges in achieving the desired molecular-level solubility. Some methods might introduce potential issues related to the formation of particles—either too small and toxic or too large and hindered in penetrating biological barriers. The significance of our approach becomes apparent in the resulting water-soluble powder after the drying process, which demonstrates complete dissolution in water. This stands in contrast to methods that might produce powders with varying degrees of solubility or stability issues related to particle aggregation.
In summary, the chosen method prioritizes achieving molecular-level solubility, thus offering advantages over other techniques that may face challenges in controlling particle size, ensuring complete dissolution and addressing potential toxicity or stability concerns. Similar investigations [
28,
29,
30] have explored various solubilization techniques for AR antagonists, emphasizing the need for improved drug delivery systems to maximize their therapeutic potential. These studies, in conjunction with our findings, underscore the importance of enhancing drug solubility as a key strategy in neoplasm treatment development.
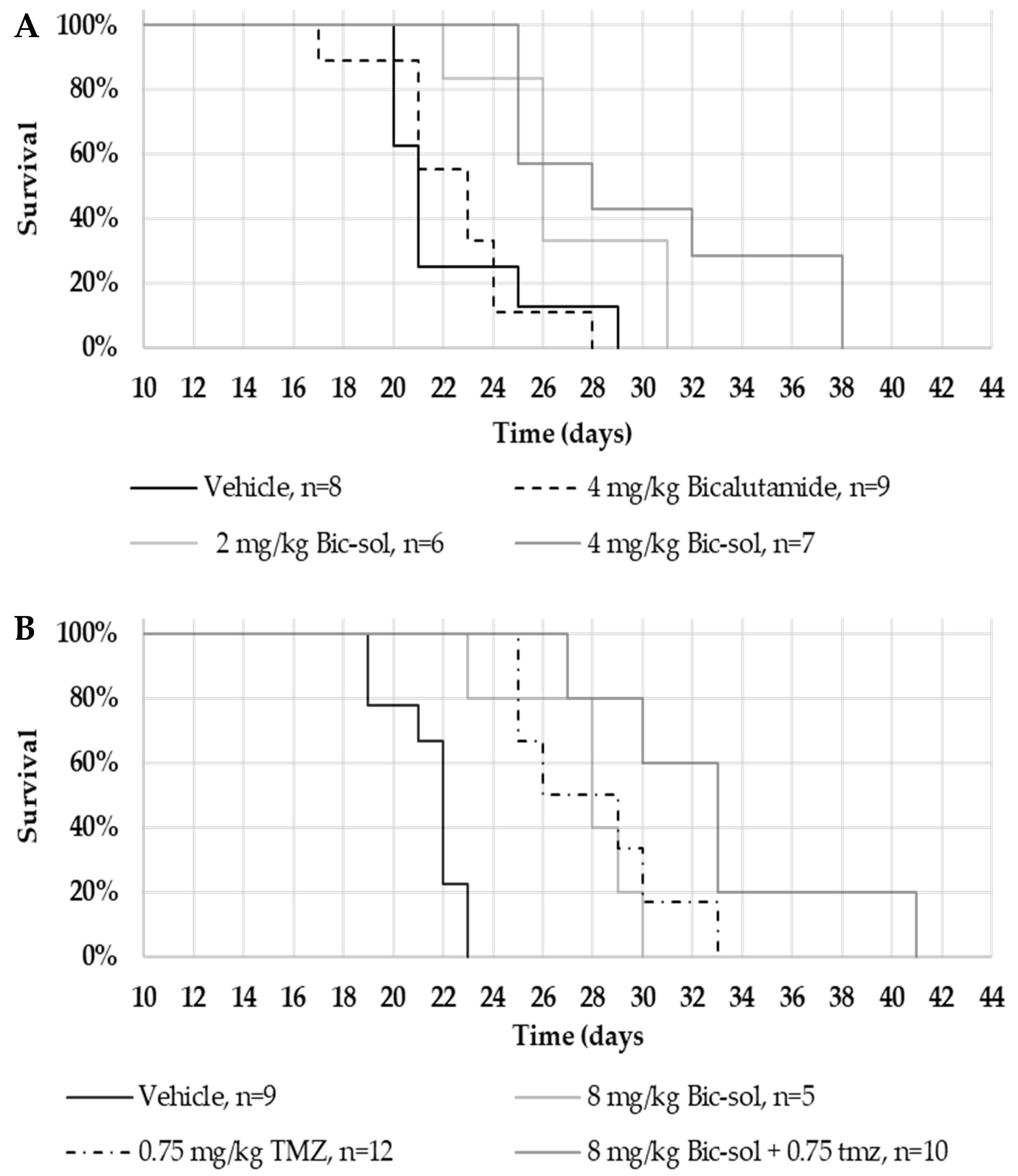
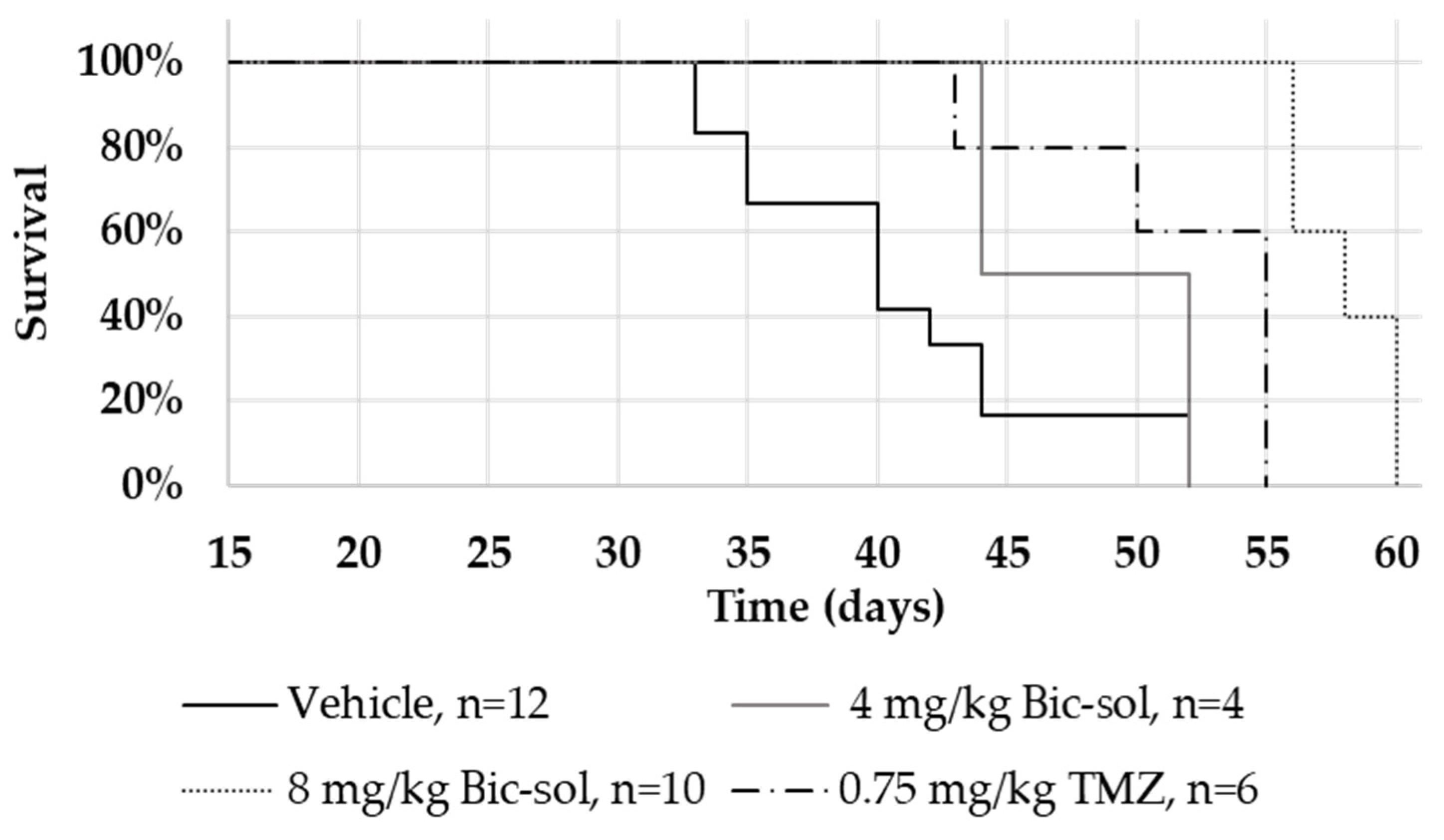
In the U87MG intracranial mouse model, Bic-sol monotherapy at 2 mg/kg and 4 mg/kg doses demonstrated significant efficacy, with distinct hazard rate differences compared to the control group. Combining Bic-sol with TMZ showed superior efficacy compared to monotherapy, highlighting its potential as a valuable addition to glioblastoma treatment. Importantly, Bic-sol monotherapy significantly extended mouse survival when compared to the control bicalutamide formulation, likely due to its improved brain bioavailability. These findings were consistent in the ZH-161 mouse model, with Bic-sol monotherapy proving notably more effective in extending the mouse lifespan compared to TMZ or the control group, emphasizing its therapeutic potential.
It is important to acknowledge our prior publication, where we identified the expression of the AR variant (AR-V7) in ZH-161, potentially linked to androgen antagonist resistance in prostate cancer [
3]. The juxtaposition of this finding with our earlier revelation of only a moderate response of ZH-161 to androgen receptor antagonists in in vitro studies [
3] introduces an intriguing aspect. The efficacy of Bic-sol in the in vivo ZH-161 model prompts the consideration of potential explanations for this apparent discrepancy. Firstly, the resistance observed in prostate cancer may not seamlessly translate to glioblastomas, where distinct factors could influence the drug’s efficacy, potentially accounting for the observed sensitivity in the ZH-161 model. Additionally, despite the expression of AR-V7, the ZH-161 line co-expresses AR-FL, adding an extra layer of complexity to the observed responses. The concurrent presence of AR-FL might contribute to the efficacy gained by the drug. This observation could strengthen our results. If the drugs exhibit efficacy in a cell line with AR-V7, coupled with the fact that only 30% of glioblastoma patients have AR-V7, it raises the intriguing possibility that these drugs might exert even greater efficacy in patients lacking this specific transition. While we acknowledge that these assertions are drawn from our observations and represent assumptions, future investigations, supported by rigorous clinical studies, will be imperative to validate and build upon these preliminary findings, deepening our understanding of the intricate interplay between AR-V7, AR-FL, and drug efficacy in the context of glioblastoma.
Notably, this new formulation of Bic-sol offers the additional benefit of reducing the amount of bicalutamide [
31], potentially mitigating side effects associated with its use. This improvement in formulation underscores the potential for enhanced treatment outcomes with a more favorable safety profile. Furthermore, when compared to enzalutamide, it is noteworthy that Bic-sol demonstrated comparable efficacy at 10-times lower doses, emphasizing its efficiency in glioblastoma treatment. While we highlight the improved characteristics of Bic-sol, including enhanced solubility and increased bioavailability, comprehensive safety assessments are imperative, especially in combination with standard-of-care chemotherapy (e.g., TMZ). Future research should delve into the potential adverse effects and long-term safety implications associated with the use of AR antagonists.
The clinical translation of our research findings into a PET/CT-guided human clinical trial is a significant step forward, aligning with the vision of precision medicine. The utilization of the fluoro-5α dihydrotestosterone tracer for identifying high AR expression in glioblastoma tumors, as pioneered by Orevi et al. [
4], is a notable advancement. The work of Orevi et al. highlights the feasibility of certifying patients for AR antagonist therapy based on the presence of AR protein in their tumors, thereby opening doors for personalized treatment approaches. This aligns with our pursuit of personalized therapy based on the level of AR expression within glioblastoma tumors, bringing us closer to tailoring treatments to individual patients.
However, the journey from promising preclinical results to effective clinical therapy is complex [
32,
33]. The efficacy of AR antagonists, demonstrated in our mouse models, may not universally apply to all glioblastoma cases. Tumor heterogeneity and patient-specific factors such as MGMT promoter methylation status should be considered when assessing the potential generalization of our findings, including the emergence of resistance mechanisms, intricacies in combining multiple therapeutic agents, and the potential for side effects. Furthermore, ethical considerations, regulatory approvals, cost factors, accessibility, and patient preferences will significantly influence the translation of our research findings into a clinically viable treatment option [
32,
33,
34]. Gan X. et al.’s analysis underscores the importance of addressing these obstacles to ensure the ultimate clinical effectiveness of AR antagonist therapy in glioblastoma patients [
11]. It is crucial to emphasize that, in controlled clinical studies, 0.5% (10 out of 2051) of patients experienced seizures following exposure to enzalutamide. Notably, the incidence of seizures seems comparable among patients with metastatic prostate cancer, sharing similar seizure risk factors, whether exposed to enzalutamide or not. This suggests that enzalutamide may offer benefits for patients with a history of seizures or other predisposing factors [
31]. However, given the absence of exploration of this treatment in patients with brain tumors, it is imperative to closely monitor each patient throughout the duration of their treatment.
In conclusion, our study contributes to the expanding body of literature supporting AR antagonists and innovative drug formulations as potential game-changers in glioblastoma treatment. The promise they hold, coupled with personalized approaches based on AR expression, provides hope for improved therapeutic strategies in the battle against this challenging disease. However, it is clear that further interdisciplinary efforts are essential to navigate the intricate path from laboratory research to clinical success, ultimately benefiting glioblastoma patients and their families.
4. Materials and Methods
4.1. Cell Culture
U87MG was obtained from the American Type Culture Collection (Manassas, VA, USA). They were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 4 mmol/L L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, and with (as indicated) 10% of FBS. The glioma-initiating cell (GICs) lines, ZH-161, were kindly provided by Prof. Michael Weller from the Department of Neurology at the University Hospital Zurich, Switzerland, and maintained as described [
35,
36]. Briefly, cells were cultured in Neurobasal Medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with B-27 (20 μL/mL) and glutamax (10 μL/mL), fibroblast growth factor (FGF)-2, epidermal growth factor (EGF) (20 ng/mL each (Peprotech, Rocky Hill, PA, USA), and heparin (32 IE/mL; Sigma-Aldrich, St. Louis, MO, USA). All cells were maintained in a humidified incubator at 37 °C in 5% CO
2.
4.2. In Vivo Inhibition of Glioblastoma Growth
The potential of androgen receptor antagonists to inhibit human glioblastoma growth was studied in an intracranial in vivo model.
Ethical statement: This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Hebrew University Medical School (Permit Number: MD-16-14864-5 and MD-18-15591-5). To minimize the suffering of the animals, injections of tumor cells were performed under light anesthesia (ketamine + xylazine, 100 and 5 mg/kg body weight, respectively). The animals were monitored twice weekly for body weight and neurological signs. Upon termination of the experiments, the animals were euthanized by exposure to excess CO2.
In this study, 6- to 8-week-old athymic male nude (nu/nu) mice were intracranially implanted with U87MG glioblastoma cells (4 × 105 cells) or spheres of ZH-161 glioblastoma-initiating cells (GICs) (1 × 105 cells) into their right cerebral hemisphere (1 mm posterior and 2.3 mm lateral to the bregma, to a depth of 3 mm). After 7 days, once tumors had been established, tumor-bearing mice were randomized into the treatment groups (n = 5–10 per group), as indicated. Each group was treated 5 times weekly by oral gavage with either vehicle (vehicle of Xtandi was composed of 220 mg/kg caprylocaproyl polyoxylglycerides in saline and vehicle of Bic-sol contained empty powder soluble in DW); 20, 50, or 100 mg/kg of a commercially available enzalutamide formulation (Xtandi, Astellas Pharma Inc., Tokyo, Japan); bicalutamide (Sigma-Aldrich) dissolved in 5% DMSO and 95% corn oil; the newly formulated bicalutamide (Bic-sol) prepared as described below; or with TMZ (0.75 mg/kg 3% DMSO in saline) (Tocris Bioscience, Bristol, UK). The endpoint of the experiment was defined as the number of days that elapsed from tumor implantation to the day of overt symptoms (significant weight loss, lethargy, hunched posture, or other neurological signs).
4.3. Preparation of an Improved AR Antagonist Formulation
The process for producing the test formulations comprising AR antagonists is as follows: 0.225 g of Lecithin S75 (LIPOID® S75, Lipoid, Newark, NJ, USA), 0.225 g of ammonium glycyrrhizinate (Sigma), 9.5 g of isopropanol (Alfaaesar), and 5.5 g of TDW (Triple Distilled Water) were combined in a glass vial and mixed by magnetic stirrer until a clear solution was obtained. Then, 0.05 g of the active drug (Bicalutamide, Apex Biotech LLC (Boston, MA, USA), or enzalutamide, A2S (Yavne, Israel)) was dissolved in 1.5 g acetone. The two solutions were combined and mixed with a magnetic stirrer until a clear solution was obtained. The solution was spray dried (Mini Spray Dryer B-290) (buchi Sarl, Villebon sur Yvette, France) at an air inlet temperature of 120 °C and a liquid feed rate of 10 mL·min−1. The powder obtained at the end of the spray-dry process was dispersed, 1% w/w, in distilled water by vortex for 5 min.
4.4. Characterization of the New Formulation
4.4.1. Cryo-TEM Analysis
The formulation products were analyzed using transmission electron cryomicroscopy (Cryo-TEM). The formulations comprising active ingredients were compared to those without any active material. The amount of the active ingredient (bicalutamide) in the powder before dispersion was 10% w/w. To prepare samples for analysis, 1% or 5% (as indicated) of the total weight of the powder was added to a vial with water (TDW) and vortexed for 5 min. Then, 2–4 µL drop of the test sample was applied to a TEM grid (300 mesh Cu Lacey substrate, Ted Pella, Ltd., Redding, CA, USA) following a short pre-treatment of the grid by glow discharge. The excess liquid was blotted off, and the specimen was vitrified by rapid plunging into liquid ethane pre-cooled by liquid nitrogen using a vitrification robot system (Vitrobot mark IV, FEI) (Thermo Fisher Scientific Inc., Waltham, MA, USA).
4.4.2. Particle Size Distribution Measurements by Dynamic Light Scattering (DLS)
Particle sizes after re-dispersion of the obtained powders (with and without bicalu-tamide) in water were measured at room temperature by DLS using a Nano-ZS Zetasiz-er (Malvern Instrument Ltd., Worcestershire, UK). The instrument is equipped with 633 nm laser, and the light scattering is detected at 173 degrees by backscattering technology (NIBS, Non-Invasive Backscatter). The re-dispersed powders in water (1% w/w of powder in water) were performed three times, and each measurement was performed in triplicate.
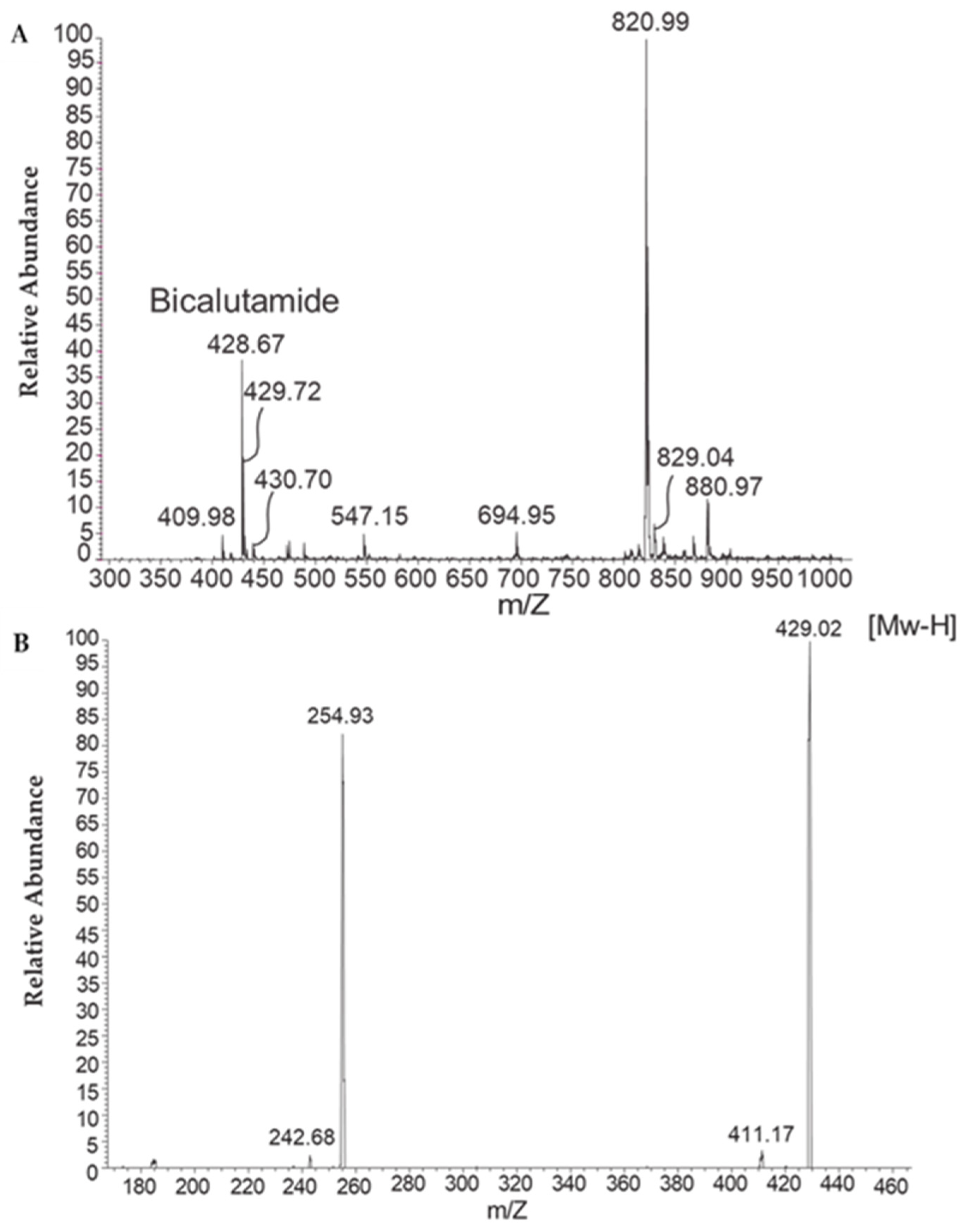
4.4.3. HPLC Liquid Chromatography and Tandem Mass Spectrometry (HPLC-MS/MS)
To examine whether the bicalutamide active ingredient is present in the aqueous phase of the reconstituted formulation, samples were subjected to microfiltration, and high-performance liquid chromatography (HPLC) analysis was performed on the aqueous filtrate. To this end, formulations prepared as described above, with or without bicalutamide, were reconstituted in water (1% w/w) and filtered through a 0.22-micron Millipore filter. Then, 100 µL of the filtrate was injected into the HPLC system.
The presence of bicalutamide in the aqueous filtrate was further confirmed by liquid chromatography and tandem mass spectrometry (HPLC-MS/MS), essentially as described by Kim et al. [
9]. The mass spectrometer was tuned in negative ionization mode with deprotonated ions (M-H).
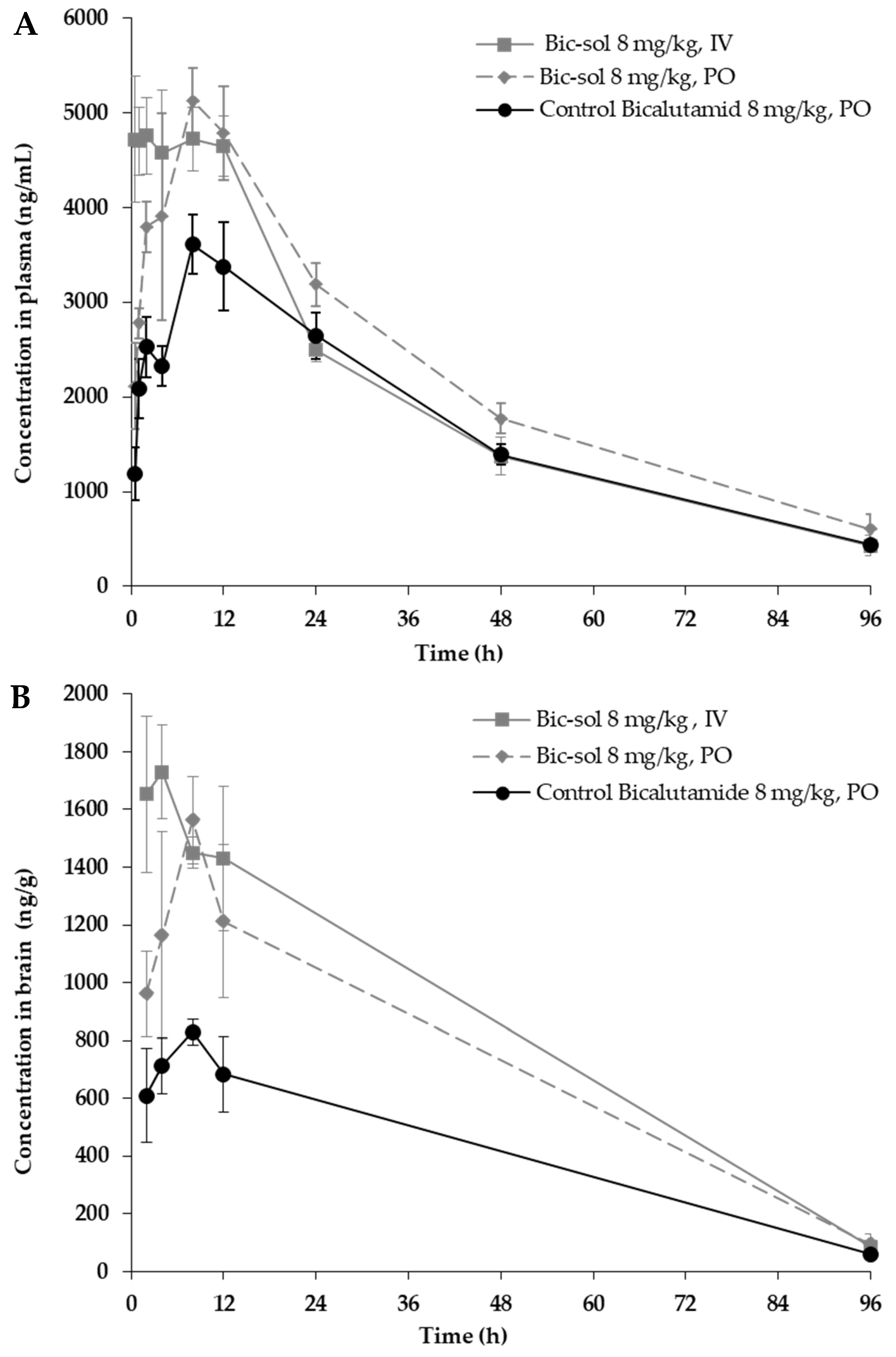
4.4.4. Pharmacokinetic Analyses of Bic-sol
The plasma pharmacokinetics (PK) with brain penetration of bicalutamide following a single intravenous [
7] or oral (PO) administration of the new bicalutamide formulation was tested in male CD1 mice and compared to that of the control bicalutamide formulation.
For the preparation of the new bicalutamide formulation for IV/PO dosing in mice, a bicalutamide formulation was prepared, as described in Example 1, combined with water to obtain a concentration of 20 mg/mL of the bicalutamide formulation in DDW, and this was vortexed for two minutes. The appearance of the resulting test formulation (herein designated “new bicalutamide formulation” or “bicalutamide test formulation”) was of a colorless transparent solution.
To prepare the control bicalutamide formulation for PO dosing for mice, bicalutamide was dissolved to obtain a concentration of 2 mg/mL bicalutamide in 5% DMSO and 95% corn oil as follows. Bicalutamide was first dissolved in DMSO, combined with corn oil, and vortexed for two minutes. The appearance of the resulting control formulation (herein designated “control bicalutamide formulation” or “bicalutamide control”) was of a clear yellow solution.
The concentration of the bicalutamide active ingredient in both the resulting test formulation and control formulation was 2 mg/mL.
A total of 45 male CD1 mice, approximately 24~25 g of body weight, were purchased from JH Laboratory Animal Co., Ltd (Seoul, Republic of Korea). The animals had free access to food and water. The IV dosing was conducted via the tail vein, and the PO dosing was conducted via oral gavage. The animals were anesthetized by carbon dioxide inhalation, and blood samples (~110 µL) were collected via facial vein puncture or cardiac puncture into K2EDTA tubes. Blood samples were put on ice and centrifuged at 4 °C and 2000× g for 5 min to obtain plasma samples within 15 min.
After blood collection, the animals were euthanized by carbon dioxide inhalation; a mid-line incision was made in the scalp, and the skin retracted. The skull overlying the brain was removed, and whole brain samples were collected, rinsed with cold saline, dried on filtrate paper, weighted, and snap-frozen by placing them into dry ice. Tissue samples were homogenized with homogenizing solution (PBS) before analysis.
Three treatment groups (N = 15) were tested: new bicalutamide formulation 80 mg/Kg, IV or PO administration, and control bicalutamide formulation (8 mg/kg), PO administration (the dose of the active ingredient administered in all treatment groups was 8 mg/kg). Sampling was performed at 0.5, 1, 2, 4, 8, 12, 24, 48, and 96 h after dosing (nine time points, semi-serial bleeding for plasma, N = 3/time point). Terminal collection of brain samples was performed at 2, 4, 8, 12, and 96 h.
Blood samples were processed into plasma and analyzed for bicalutamide using a qualified liquid chromatography–tandem mass spectrometry (LC-MS/MS) method. Brain samples were analyzed for bicalutamide using a qualified liquid chromatography–tandem mass spectrometry (LC-MS/MS) method.
LC-MS/MS analysis was performed with the following parameters: instrument LC-MS/MS-19 (Triple Quad 5500) (SCIEX, Toronto, ON, Canada); Matrix Male CD1 mouse plasma and brain homogenate; analyte(s) bicalutamide; internal stand glipizide; MS conditions negative ion, ESI; SRM detection; compound name Parent (
m/
z)/Daught (
m/
z) bicalutamide; Q1/Q3 masses: 429.20/255.10 Da; glipizide Q1/Q3 masses: 444.30/319.10 Da; HPLC conditions Mobile phase: Mobile Phase A: H
2O—0.025% FA—1 mM NH
4OAC; Mobile Phase B: MeOH—0.025% FA—1 mM NH
4OAC.
| Time | Parameter |
| 0.20 | 5 |
| 0.60 | 95 |
| 1.30 | 95 |
| 1.31 | 5 |
| 1.80 | Stop |
Column: ACQUITY UPLC BEH C18 2.1 × 50 mm, 1.7 µm; column temperature: 60 °C; retention time: bicalutamide: 1.21 min; glipizide: 1.20 min.
Sample preparation: For non-diluted plasma samples: 1. An aliquot of 30 µL sample was added with 200 µL IS (Glipizide, 40 ng/mL) in ACN. 2. The mixture was vortexed for 1 min and centrifuged at 5800 rpm for 10 min. 3. A 100 µL supernatant was transferred to a new plate. 4. A solvent of 0.5 µL was injected into LC-MS/MS for analysis.
For brain samples:
The sample was homogenized with 3 volumes (v/w) of PBS. The diluted factor was 4. The following operation was the same as the undiluted ones.
The calibration curve was 10–10,000 ng/mL for bicalutamide in male CD1 mouse plasma and brain homogenate.
The PK parameters were determined based on group means by non-compartmental analysis using Pharsight Phoenix WinNonlin® 8.2 software. “BQL” rule: concentration data under LLOQ (LLOQ = 10.00 ng/mL for bicalutamide in mouse plasma and brain homogenate) were replaced with “BQL” and excluded from graphing and PK parameter estimation. Terminal t½ calculation: time points were automatically selected by the “best fit” model for terminal half-life estimation as the first option. The manual selection was applied when “best fit” could not define the terminal phase well. When the number of detected time points after Tmax was less than 3, the terminal half-life and AUCINF were not calculated.
4.5. Statistical Analysis
Survival analysis, specifically employing Kaplan–Meier survival curves, was conducted to assess and compare survival rates among different treatment groups. Log-rank tests were utilized to identify statistically significant differences in survival distributions, and hazard ratios were calculated to quantify the risk of an event occurring in one group compared to another. All analyses were performed using the Evan Miller online tool (
https://www.evanmiller.org/ab-testing/survival-curves.html, last accessed on 26 November 2023).
Pharmacokinetics data analysis, including parameters, such as CL (clearance), Vss (volume of distribution at steady state), Tmax (time to maximum concentration), Cmax (maximum concentration), T1/2 (half-life), AUC (area under the curve), F% (bioavailability), etc., was conducted using the non-compartmental model in WinNonlin V 8.2 statistical software (Pharsight Corporation, Sunnyval, CA, USA).