Gemfibrozil Improves Microcirculatory Oxygenation of Colon and Liver without Affecting Mitochondrial Function in a Model of Abdominal Sepsis in Rats
Abstract
:1. Introduction
2. Results
2.1. Vital Parameters and Organ Damage Parameters
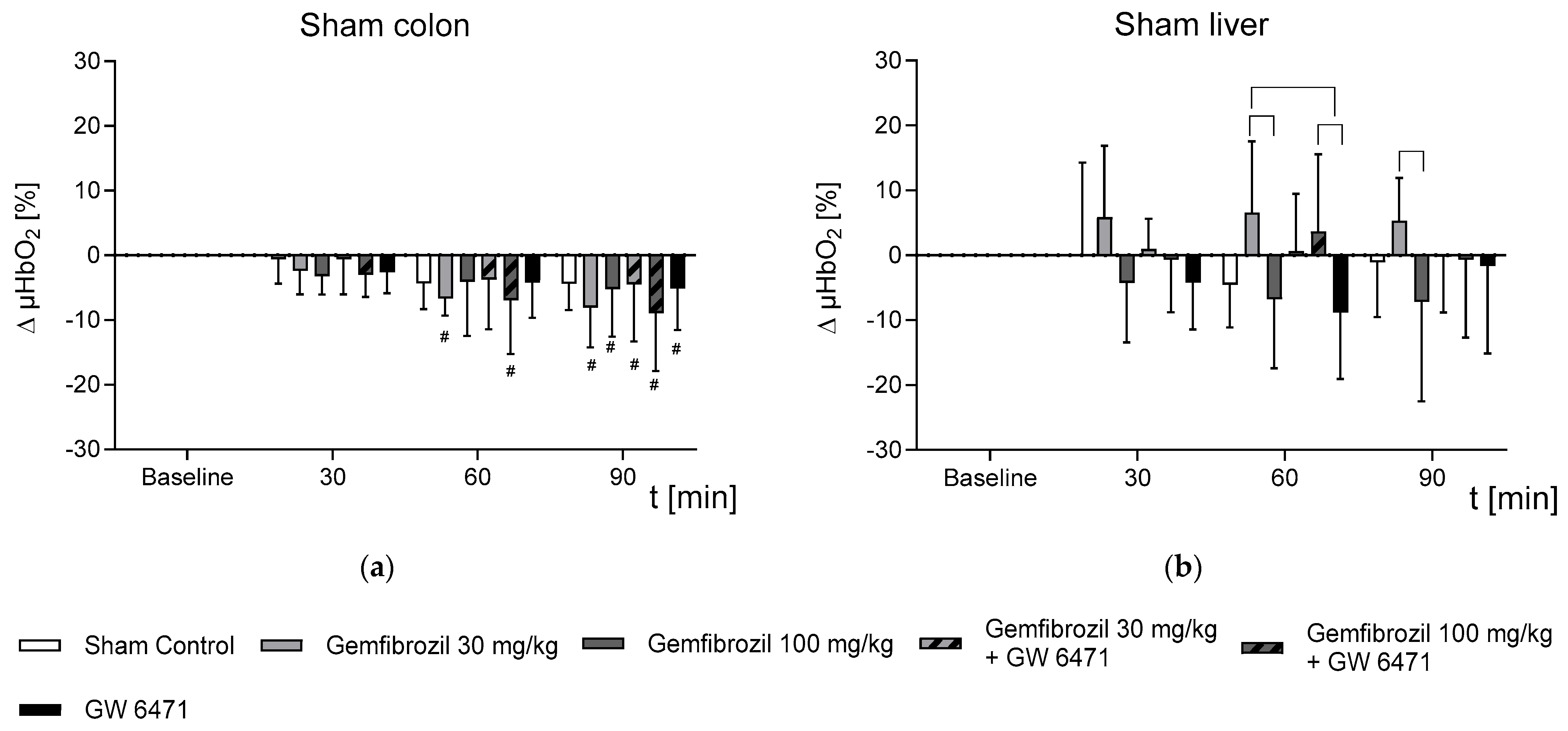
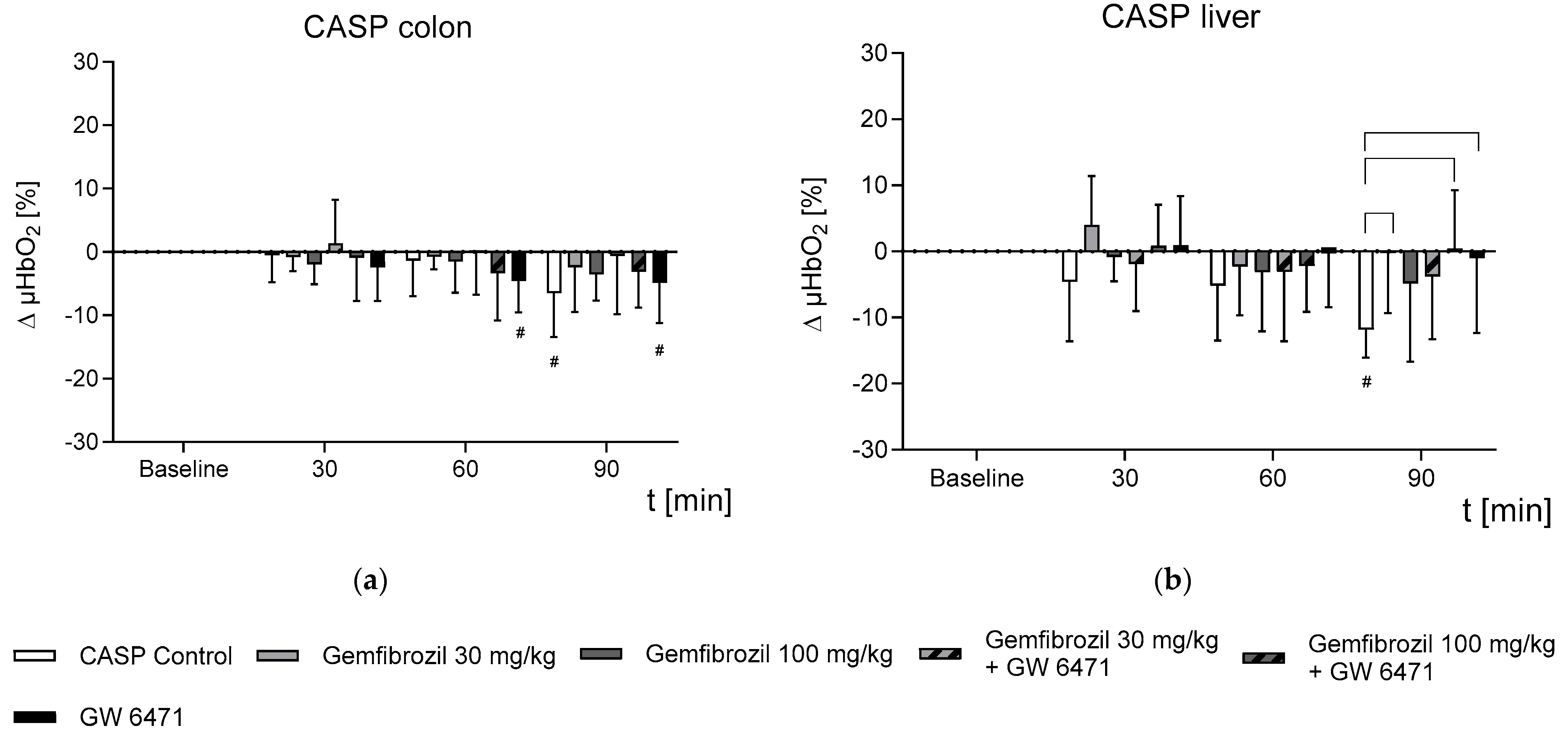
2.2. Effect of Gemfibrozil on Microvascular Oxygenation in the Colon and Liver
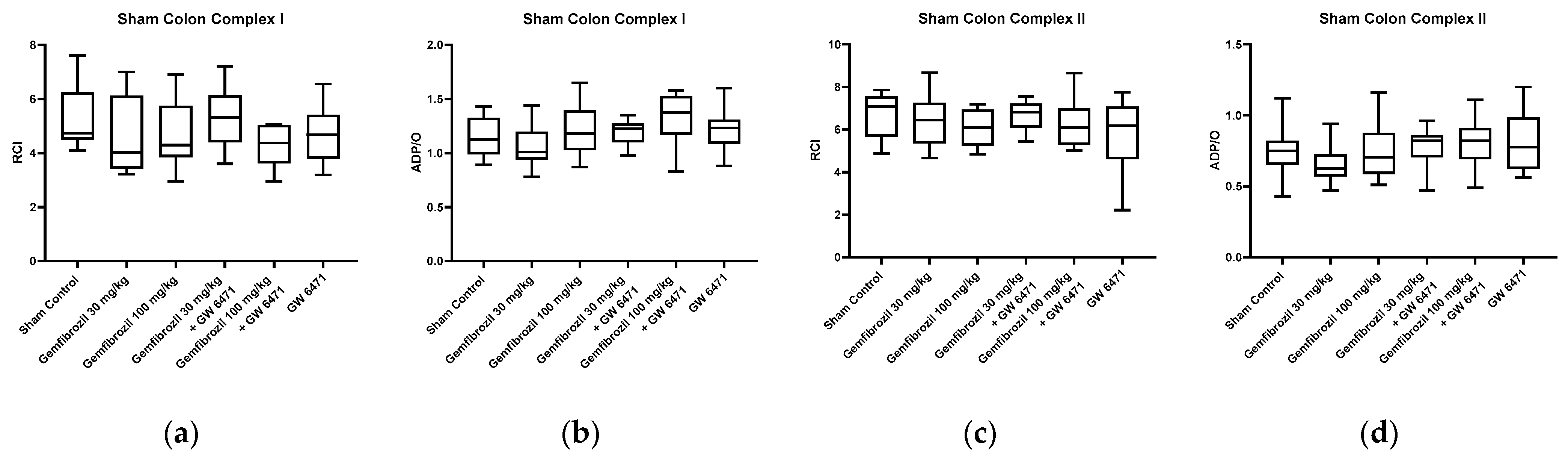
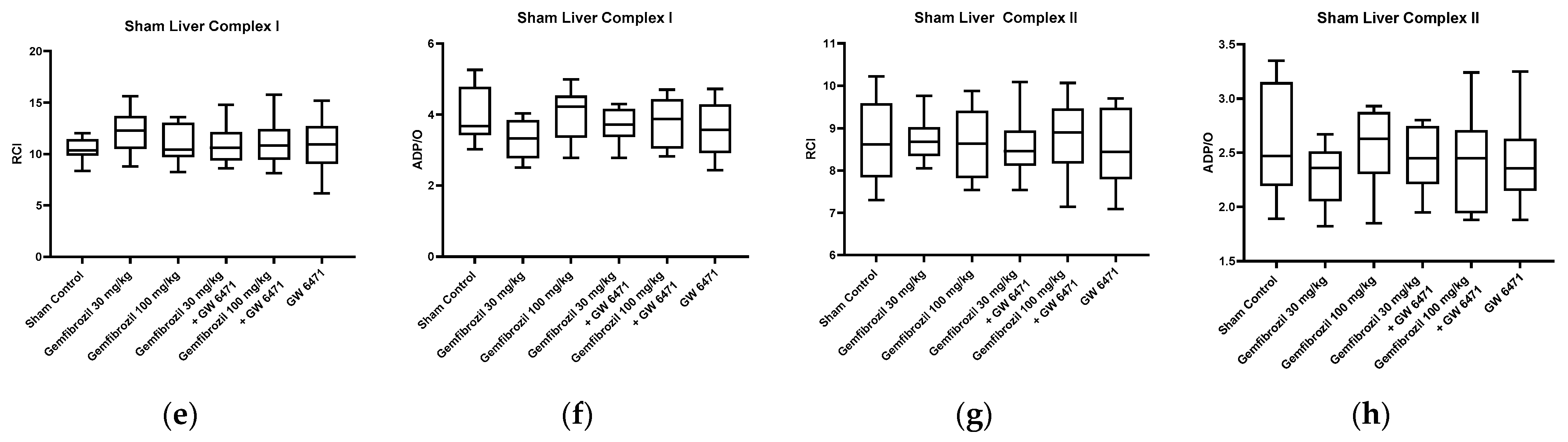
2.3. Mitochondrial Respiration and ATP Content
2.4. Lipid Peroxidation Levels as an Indicator of Oxidative Stress
3. Discussion
- In non-septic subjects, gemfibrozil treatment had an organ-dependent effect on microvascular oxygenation, displayed by stable µHbO2 measurements in the liver but a slight reduction in the colon.
- Gemfibrozil treatment prevented colonic and hepatic microvascular oxygenation aberrances occurring under septic conditions.
- Gemfibrozil seems to affect the microcirculation PPARα-independently.
- Gemfibrozil treatment did not affect mitochondrial function and lipid peroxidation levels, which serve as an indicator of oxidative stress.
4. Materials and Methods
4.1. Animals
4.2. CASP Model
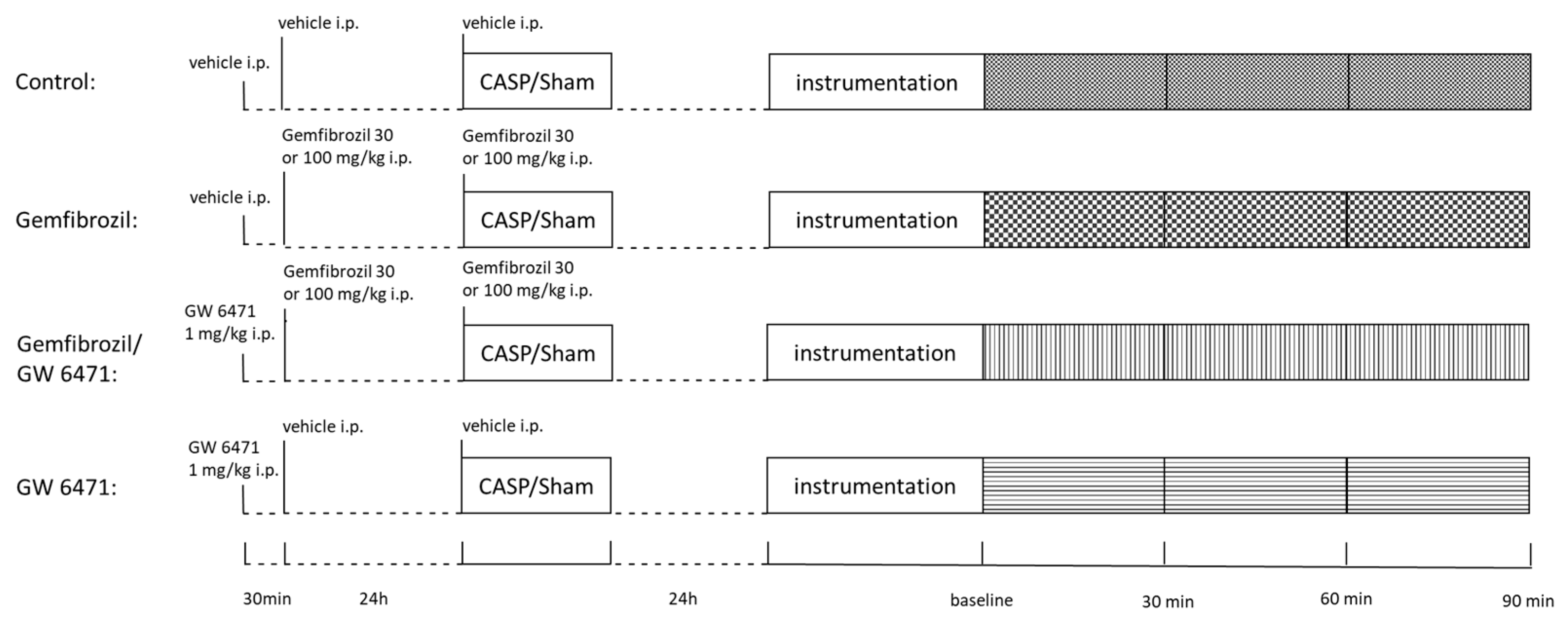
4.3. Treatment
4.4. Intervention
4.5. Microcirculation Evaluation
4.6. Mitochondrial Respiratory Rates
4.7. Malondialdehyde-Assay
4.8. ATP-Measurement
4.9. Plasma Analyses
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Reintam Blaser, A.; Poeze, M.; Malbrain, M.L.; Björck, M.; Oudemans-van Straaten, H.M.; Starkopf, J. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: A prospective multicentre study. Intensive Care Med. 2013, 39, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, C. Liver—Guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Ricottilli, F.; Ospina-Tascón, G.A. Septic shock: A microcirculation disease. Curr. Opin. Anaesthesiol. 2021, 34, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef]
- Cámara-Lemarroy, C.R.; Guzman, D.E.L.A.G.F.J.; Cordero-Perez, P.; Ibarra-Hernandez, J.M.; Muñoz-Espinosa, L.E.; Fernandez-Garza, N.E. Gemfibrozil attenuates the inflammatory response and protects rats from abdominal sepsis. Exp. Ther. Med. 2015, 9, 1018–1022. [Google Scholar] [CrossRef]
- Tancevski, I.; Nairz, M.; Duwensee, K.; Auer, K.; Schroll, A.; Heim, C.; Feistritzer, C.; Hoefer, J.; Gerner, R.R.; Moschen, A.R.; et al. Fibrates ameliorate the course of bacterial sepsis by promoting neutrophil recruitment via CXCR2. EMBO Mol. Med. 2014, 6, 810–820. [Google Scholar] [CrossRef]
- Meng, Y.-H.; Chen, K.-F. 1042: Effects of statin and fibrate on outcomes of sepsis. Crit. Care Med. 2015, 43, 262. [Google Scholar] [CrossRef]
- Issemann, I.; Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990, 347, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Orlando, P.; Pagano, E.; Aveta, T.; Buono, L.; Borrelli, F.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide normalizes intestinal motility in a model of post-inflammatory accelerated transit: Involvement of CB1 receptors and TRPV1 channels. Br. J. Pharmacol. 2014, 171, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.; Materozzi, M.; Galli, A. PPARs and Mitochondrial Metabolism: From NAFLD to HCC. PPAR Res. 2016, 2016, 7403230. [Google Scholar] [CrossRef] [PubMed]
- Nadanaciva, S.; Dykens, J.A.; Bernal, A.; Capaldi, R.A.; Will, Y. Mitochondrial impairment by PPAR agonists and statins identified via immunocaptured OXPHOS complex activities and respiration. Toxicol. Appl. Pharmacol. 2007, 223, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Price, E.T.; Welder, G.J.; Zineh, I. Modulatory effect of fenofibrate on endothelial production of neutrophil chemokines IL-8 and ENA-78. Cardiovasc. Drugs Ther. 2012, 26, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.; Jana, A.; Liu, X.; Ghosh, S.; Pahan, K. Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of I kappa B alpha in anti-inflammatory effect of gemfibrozil in microglia. J. Immunol. 2007, 179, 4142–4152. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Lee, M.-t.G.; Hsu, T.-C.; Porta, L.; Chang, S.-S.; Yo, C.-H.; Tsai, K.-C.; Lee, M. A Population-Based Cohort Study on the Drug-Specific Effect of Statins on Sepsis Outcome. Chest 2018, 153, 805–815. [Google Scholar] [CrossRef]
- Yu, A.S.; Liang, B.; Yang, S.-J.T.; Kim, B.J.; Huang, C.-W.; Sim, J.J. Statin use and survival among ESKD patients hospitalized with sepsis. Clin. Kidney J. 2021, 14, 1710–1712. [Google Scholar] [CrossRef]
- Esposito, E.; Rinaldi, B.; Mazzon, E.; Donniacuo, M.; Impellizzeri, D.; Paterniti, I.; Capuano, A.; Bramanti, P.; Cuzzocrea, S. Anti-inflammatory effect of simvastatin in an experimental model of spinal cord trauma: Involvement of PPAR-α. J. Neuroinflamm. 2012, 9, 81. [Google Scholar] [CrossRef]
- Rinaldi, B.; Donniacuo, M.; Esposito, E.; Capuano, A.; Sodano, L.; Mazzon, E.; Di Palma, D.; Paterniti, I.; Cuzzocrea, S.; Rossi, F. PPARα mediates the anti-inflammatory effect of simvastatin in an experimental model of zymosan-induced multiple organ failure. Br. J. Pharmacol. 2011, 163, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Basso, P.J.; Sales-Campos, H.; Nardini, V.; Duarte-Silva, M.; Alves, V.B.F.; Bonfá, G.; Rodrigues, C.C.; Ghirotto, B.; Chica, J.E.L.; Nomizo, A.; et al. Peroxisome Proliferator-Activated Receptor Alpha Mediates the Beneficial Effects of Atorvastatin in Experimental Colitis. Front. Immunol. 2021, 12, 618365. [Google Scholar] [CrossRef] [PubMed]
- Lustig, M.K.; Bac, V.H.; Pavlovic, D.; Maier, S.; Gründling, M.; Grisk, O.; Wendt, M.; Heidecke, C.D.; Lehmann, C. Colon ascendens stent peritonitis--a model of sepsis adopted to the rat: Physiological, microcirculatory and laboratory changes. Shock 2007, 28, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Stübs, C.C.; Picker, O.; Schulz, J.; Obermiller, K.; Barthel, F.; Hahn, A.M.; Bauer, I.; Beck, C. Acute, short-term hypercapnia improves microvascular oxygenation of the colon in an animal model of sepsis. Microvasc. Res. 2013, 90, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ozansoy, G.; Akin, B.; Aktan, F.; Karasu, C. Short-term gemfibrozil treatment reverses lipid profile and peroxidation but does not alter blood glucose and tissue antioxidant enzymes in chronically diabetic rats. Mol. Cell Biochem. 2001, 216, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, G.; Liu, X.; Namura, S. Effects of gemfibrozil on outcome after permanent middle cerebral artery occlusion in mice. Brain Res. 2009, 1279, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.E.; Stanley, T.B.; Montana, V.G.; Lambert, M.H.; Shearer, B.G.; Cobb, J.E.; McKee, D.D.; Galardi, C.M.; Plunket, K.D.; Nolte, R.T.; et al. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARα. Nature 2002, 415, 813–817. [Google Scholar] [CrossRef] [PubMed]
- El-Sisi, A.; Hegazy, S.; El-Khateeb, E. Effects of Three Different Fibrates on Intrahepatic Cholestasis Experimentally Induced in Rats. PPAR Res. 2013, 2013, 781348. [Google Scholar] [CrossRef]
- More, V.R.; Campos, C.R.; Evans, R.A.; Oliver, K.D.; Chan, G.N.; Miller, D.S.; Cannon, R.E. PPAR-α, a lipid-sensing transcription factor, regulates blood–brain barrier efflux transporter expression. J. Cereb. Blood Flow. Metab. 2017, 37, 1199–1212. [Google Scholar] [CrossRef]
- Roy, A.; Pahan, K. Gemfibrozil, stretching arms beyond lipid lowering. Immunopharmacol. Immunotoxicol. 2009, 31, 339–351. [Google Scholar] [CrossRef]
- Seki, T.; Yokoshiki, H.; Sunagawa, M.; Nakamura, M.; Sperelakis, N. Angiotensin II stimulation of Ca2+-channel current in vascular smooth muscle cells is inhibited by lavendustin-A and LY-294002. Pflügers Arch. 1999, 437, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kawanabe, Y.; Hashimoto, N.; Masaki, T. Effects of phosphoinositide 3-kinase on endothelin-1-induced activation of voltage-independent Ca2+ channels and vasoconstriction. Biochem. Pharmacol. 2004, 68, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Morello, F.; Perino, A.; Hirsch, E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc. Res. 2008, 82, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Zahradka, L.S.P. Angiotensin II Activates Phosphatidylinositol 3-Kinase in Vascular Smooth Muscle Cells. Circ. Res. 1997, 81, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Gong, N. Role of the PI3K/Akt signaling pathway in liver ischemia reperfusion injury: A narrative review. Ann. Palliat. Med. 2021, 11, 806–817. [Google Scholar] [CrossRef]
- Guo, J.Y.; Yang, T.; Sun, X.G.; Zhou, N.Y.; Li, F.S.; Long, D.; Lin, T.; Li, P.Y.; Feng, L. Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway. J. Biomed. Sci. 2011, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Donadello, K.; Sakr, Y.; Ospina-Tascon, G.; Salgado, D.; Scolletta, S.; Vincent, J.-L. Microcirculatory Alterations in Patients With Severe Sepsis: Impact of Time of Assessment and Relationship With Outcome. Crit. Care Med. 2013, 41, 791–799. [Google Scholar] [CrossRef]
- Kondo, K.; Sugioka, T.; Tsukada, K.; Aizawa, M.; Takizawa, M.; Shimizu, K.; Morimoto, M.; Suematsu, M.; Goda, N. Fenofibrate, a Peroxisome Proliferator-Activated Receptor α Agonist, Improves Hepatic Microcirculatory Patency and Oxygen Availability in a High-Fat-Diet-Induced Fatty Liver in Mice. In Proceedings of the Oxygen Transport to Tissue XXXI, Boston, MA, USA, 24 October 2009; pp. 77–82. [Google Scholar]
- Goodwill, A.G.; Frisbee, S.J.; Stapleton, P.A.; James, M.E.; Frisbee, J.C. Impact of chronic anticholesterol therapy on development of microvascular rarefaction in the metabolic syndrome. Microcirculation 2009, 16, 667–684. [Google Scholar] [CrossRef]
- Harmer, J.A.; Keech, A.C.; Veillard, A.S.; Skilton, M.R.; Marwick, T.H.; Watts, G.F.; Meredith, I.T.; Celermajer, D.S. Fenofibrate effects on arterial endothelial function in adults with type 2 diabetes mellitus: A FIELD substudy. Atherosclerosis 2015, 242, 295–302. [Google Scholar] [CrossRef]
- Haak, T.; Haak, E.; Kusterer, K.; Weber, A.; Kohleisen, M.; Usadel, K.H. Fenofibrate improves microcirculation in patients with hyperlipidemia. Eur. J. Med. Res. 1998, 3, 50–54. [Google Scholar]
- Standage, S.W.; Caldwell, C.C.; Zingarelli, B.; Wong, H.R. Reduced Peroxisome Proliferator-Activated Receptor α Expression Is Associated With Decreased Survival and Increased Tissue Bacterial Load in Sepsis. Shock. 2012, 37, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Van Wyngene, L.; Vanderhaeghen, T.; Timmermans, S.; Vandewalle, J.; Van Looveren, K.; Souffriau, J.; Wallaeys, C.; Eggermont, M.; Ernst, S.; Van Hamme, E.; et al. Hepatic PPARα function and lipid metabolic pathways are dysregulated in polymicrobial sepsis. EMBO Mol. Med. 2020, 12, e11319. [Google Scholar] [CrossRef] [PubMed]
- Kuebart, A.; Gross, K.; Ripkens, J.J.; Tenge, T.; Raupach, A.; Schulz, J.; Truse, R.; Hof, S.; Marcus, C.; Vollmer, C.; et al. Pravastatin Improves Colonic and Hepatic Microcirculatory Oxygenation during Sepsis without Affecting Mitochondrial Function and ROS Production in Rats. Int. J. Mol. Sci. 2023, 24, 5455. [Google Scholar] [CrossRef] [PubMed]
- McGown, C.C.; Brookes, Z.L.S. Beneficial effects of statins on the microcirculation during sepsis: The role of nitric oxide. Br. J. Anaesth. 2007, 98, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, L.; Wang, M.-M.; Cao, L.-P.; Sun, Z.-R.; Yang, Z.-Z.; Zhang, W.; Zhang, P.; Nie, S.-N. Pravastatin attenuates sepsis-induced acute lung injury through decreasing pulmonary microvascular permeability via inhibition of Cav-1/eNOS pathway. Int. Immunopharmacol. 2021, 100, 108077. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.E.; Kaplon, R.E.; Lucking, S.M.S.; Russell-Nowlan, M.J.; Eckel, R.H.; Seals, D.R. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension 2012, 60, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Goya, K.; Sumitani, S.; Xu, X.; Kitamura, T.; Yamamoto, H.; Kurebayashi, S.; Saito, H.; Kouhara, H.; Kasayama, S.; Kawase, I. Peroxisome Proliferator-Activated Receptor α Agonists Increase Nitric Oxide Synthase Expression in Vascular Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Herminghaus, A.; Laser, E.; Schulz, J.; Truse, R.; Vollmer, C.; Bauer, I.; Picker, O. Pravastatin and Gemfibrozil Modulate Differently Hepatic and Colonic Mitochondrial Respiration in Tissue Homogenates from Healthy Rats. Cells 2019, 8, 983. [Google Scholar] [CrossRef]
- Kar, D.; Bandyopadhyay, A. Targeting Peroxisome Proliferator Activated Receptor α (PPAR α) for the Prevention of Mitochondrial Impairment and Hypertrophy in Cardiomyocytes. Cell Physiol. Biochem. 2018, 49, 245–259. [Google Scholar] [CrossRef]
- Schulz, J.; Vollmer, C.; Truse, R.; Bauer, I.; Beck, C.; Picker, O.; Herminghaus, A. Effect of Pravastatin Pretreatment and Hypercapnia on Intestinal Microvascular Oxygenation and Blood Flow During Sepsis. Shock 2020, 53, 88–94. [Google Scholar] [CrossRef]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.S.M.; Farouk, A.A.O.; Mohamad, T.A.S.T.; Sulaiman, M.R.; Zakaria, H.; Hassan, N.I.; Perimal, E.K. Zerumbone Ameliorates Neuropathic Pain Symptoms via Cannabinoid and PPAR Receptors Using In Vivo and In Silico Models. Molecules 2021, 26, 3849. [Google Scholar] [CrossRef] [PubMed]
- Hof, S.; Truse, R.; Weber, L.; Herminghaus, A.; Schulz, J.; Weber, A.P.M.; Maleckova, E.; Bauer, I.; Picker, O.; Vollmer, C. Local Mucosal CO2 but Not O2 Insufflation Improves Gastric and Oral Microcirculatory Oxygenation in a Canine Model of Mild Hemorrhagic Shock. Front. Med. (Lausanne) 2022, 9, 867298. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, W.G.; Buursma, A.; Meeuwsen-van der Roest, W.P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar] [CrossRef]
- Beauvoit, B.; Evans, S.M.; Jenkins, T.W.; Miller, E.E.; Chance, B. Correlation between the light scattering and the mitochondrial content of normal tissues and transplantable rodent tumors. Anal. Biochem. 1995, 226, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Krug, A. Mikrozirkulation und Sauerstoffversorgung des Gewebes: Methode des so genannten O2C (oxygen to see). Phlebologie 2006, 35, 300–312. [Google Scholar] [CrossRef]
- Tomar, N.; Zhang, X.; Kandel, S.M.; Sadri, S.; Yang, C.; Liang, M.; Audi, S.H.; Cowley, A.W., Jr.; Dash, R.K. Substrate-dependent differential regulation of mitochondrial bioenergetics in the heart and kidney cortex and outer medulla. Biochim. Biophys. Acta Bioenerg. 2022, 1863, 148518. [Google Scholar] [CrossRef]
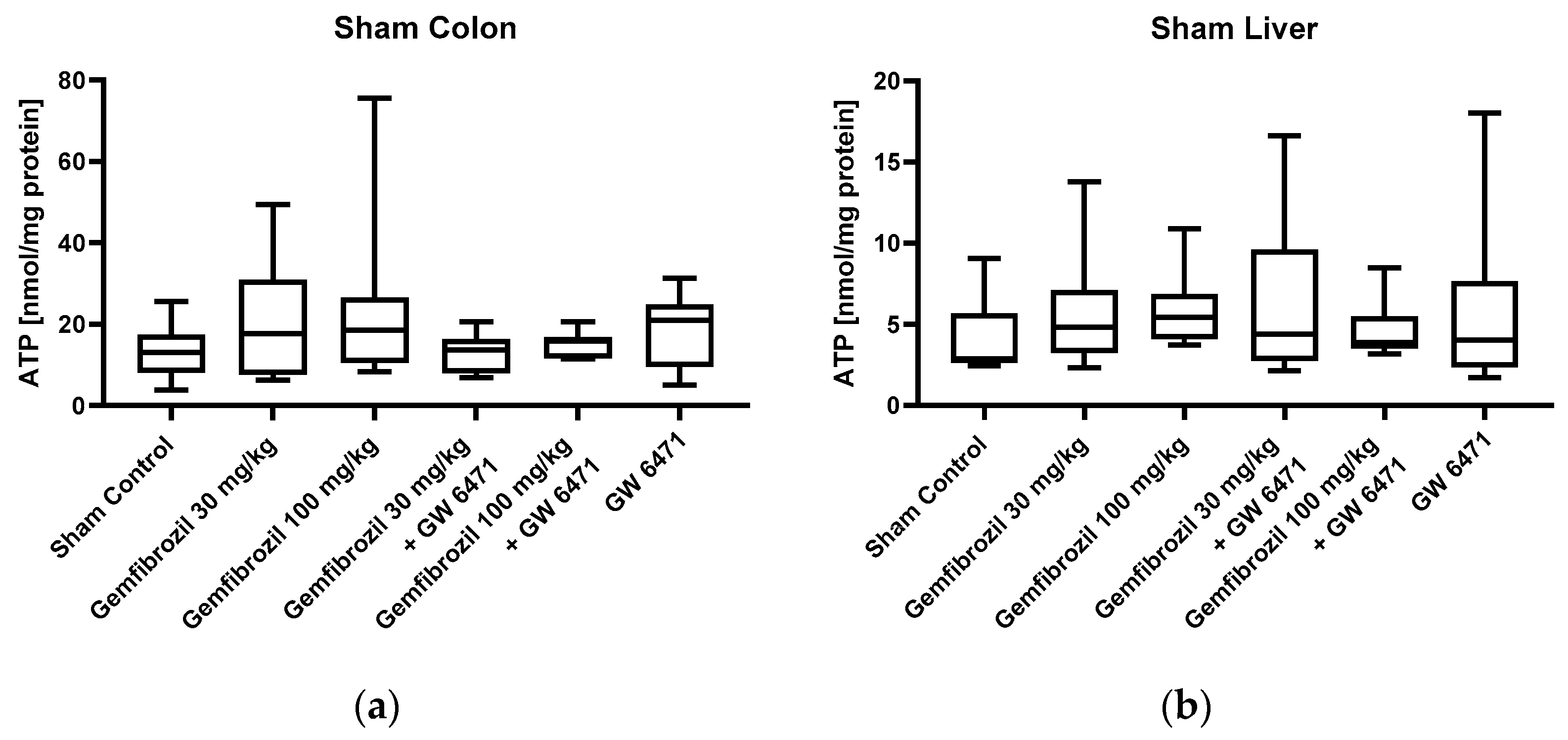
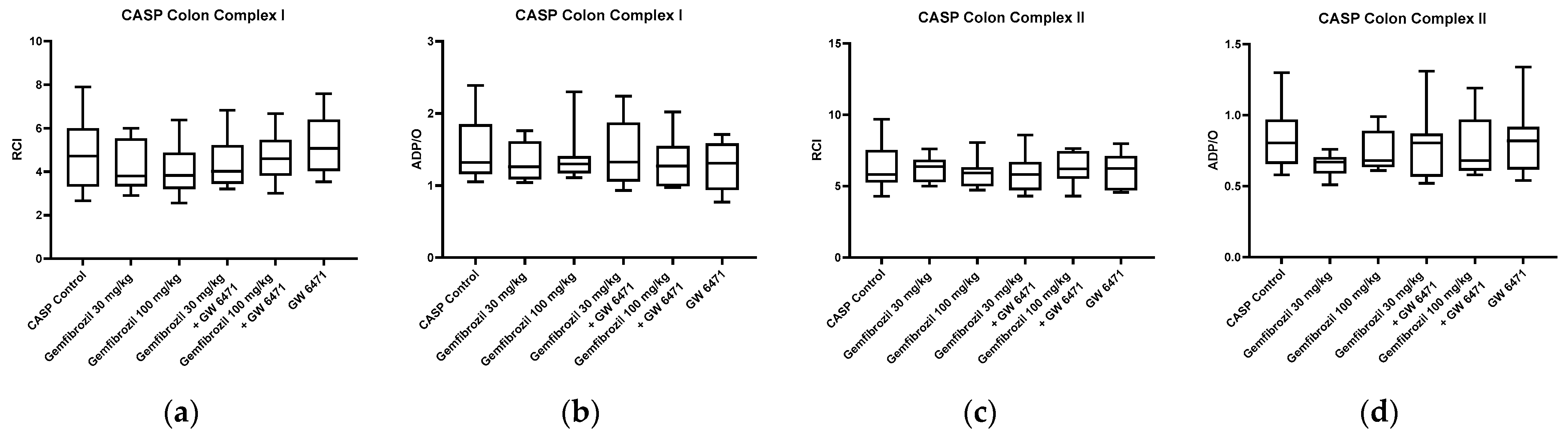
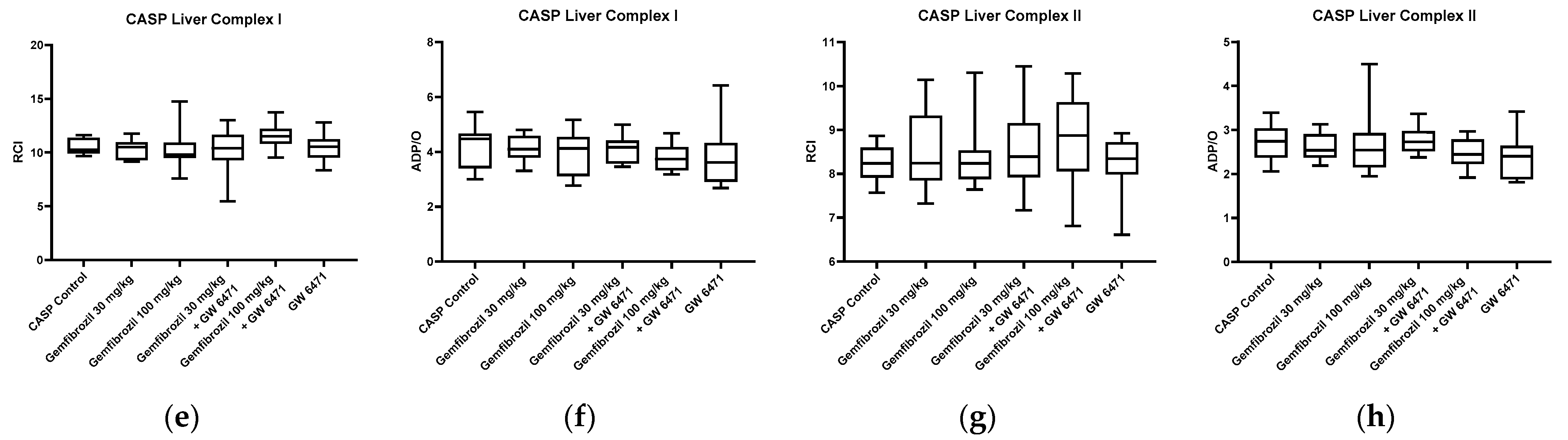


| Sham Control | Gemfibrozil 30 mg/kg BW | Gemfibrozil 100 mg/kg BW | Gemfibrozil 30 mg/kg BW + GW 6471 | Gemfibrozil 100 mg/kg BW + GW 6471 | GW 6471 | |
|---|---|---|---|---|---|---|
| Mean arterial pressure (MAP) [mmHg] | ||||||
| Baseline | 110 ± 22 | 106 ± 36 | 130 ± 19 | 124 ± 26 | 136 ± 10 | 127 ± 32 |
| 30 min | 104 ± 24 | 97 ± 30 | 114 ± 31 | 108 ± 22 | 104 ± 34 * | 113 ± 36 |
| 60 min | 96 ± 27 | 86 ± 21 * | 104 ± 37 * | 93 ± 22 * | 99 ± 26 * | 102 ± 35 * |
| 90 min | 95 ± 32 | 84 ± 23 * | 108 ± 37 * | 91 ± 32 * | 94 ± 34 * | 105 ± 31 * |
| Heart rate (HR) [beats/min] | ||||||
| Baseline | 436 ± 58 | 485 ± 74 | 459 ± 35 | 481 ± 43 | 489 ± 58 | 467 ± 49 |
| 30 min | 406 ± 58 | 474 ± 72 | 447 ± 40 | 450 ± 55 * | 441 ± 45 * | 444 ± 49 * |
| 60 min | 396 ± 51 * | 445 ± 71 * | 419 ± 40 * | 430 ± 35 * | 416 ± 39 * | 396 ± 62 * |
| 90 min | 383 ± 63 * | 429 ± 66 *§ | 430 ± 50 | 410 ± 53 *§ | 415 ± 43 * | 380 ± 54 *§ |
| Lactate [mmol/L] | ||||||
| Baseline | 1.7 ± 0.6 | 1.5 ± 0.4 | 1.2 ± 0.1 | 1.3 ± 0.6 | 1.3 ± 0.5 | 1.3 ± 0.70 |
| 30 min | 1.2 ± 0.4 * | 1.1 ± 0.3 | 1.2 ± 0.2 | 1.0 ± 0.1 | 1.4 ± 0.3 | 1.2 ± 0.67 |
| 60 min | 1.1 ± 0.2 * | 1.1 ± 0.4 | 1.2 ± 0.2 | 0.9 ± 0.4 | 1.3 ± 0.2 | 1.1 ± 0.33 |
| 90 min | 1.1 ± 0.3 * | 0.9 ± 0.2 * | 0.9 ± 0.1 | 0.8 ± 0.3 | 1.3 ± 0.3 | 0.9 ± 0.28 * |
| CASP Control | Gemfibrozil 30 mg/kg BW | Gemfibrozil 100 mg/kg BW | Gemfibrozil 30 mg/kg BW + GW 6471 | Gemfibrozil 100 mg/kg BW + GW 6471 | GW 6471 | |
|---|---|---|---|---|---|---|
| Mean Arterial Pressure (MAP) [mmHg] | ||||||
| Baseline | 105 ± 23 | 108 ± 18 | 108 ± 20 | 123 ± 26 | 109 ± 27 | 123 ± 26 |
| 30 min | 93 ± 26 | 97 ± 16 | 107 ± 22 | 102 ± 21 * | 102 ± 39 | 111 ± 36 |
| 60 min | 85 ± 31 * | 93 ± 21 | 98 ± 28 | 100 ± 24 * | 103 ± 40 | 103 ± 29 * |
| 90 min | 90 ± 38 | 92 ± 21 * | 99 ± 34 | 101 ± 25 * | 97 ± 32 | 101 ± 27 * |
| Heart rate (HR) [beats/min] | ||||||
| Baseline | 477 ± 46 | 496 ± 40 | 484 ± 51 | 470 ± 75 | 478 ± 37 | 480 ± 64 |
| 30 min | 432 ± 60 * | 454 ± 57 * | 461 ± 46 | 436 ± 65 | 441 ± 35 * | 448 ± 62 |
| 60 min | 420 ± 81 * | 430 ± 54 * | 443 ± 46 * | 425 ± 66 * | 431 ± 69 * | 437 ± 56 * |
| 90 min | 417± 78 * | 423 ± 62 * | 427 ± 81 * | 404 ± 56 * | 440 ± 45 * | 415 ± 48 * |
| Lactate [mmol/L] | ||||||
| Baseline | 1.5 ± 0.5 | 1.9 ± 0.5 | 1.5 ± 0.6 | 1.4 ± 0.8 | 1.4 ± 0.6 | 1.4 ± 0.3 |
| 30 min | 1.4 ± 0.9 | 1.6 ± 0.6 | 1.3 ± 0.5 | 1.2 ± 0.5 | 1.3 ± 0.6 | 1.3 ± 0.3 |
| 60 min | 1.4 ± 0.6 | 1.3 ± 0.4 * | 1.2 ± 0.4 | 0.9 ± 0.3 * | 1.2 ± 0.5 | 1.5 ± 0.5 |
| 90 min | 1.2 ± 0.5 | 1.0 ± 0.2 *§ | 1.0 ± 0.3 * | 0.8 ± 0.2 * | 1.2 ± 0.5 | 1.4 ± 0.3 |
| Sham Control | Gemfibrozil 30 mg/kg BW | Gemfibrozil 100 mg/kg BW | Gemfibrozil 30 mg/kg BW + GW 6471 | Gemfibrozil 100 mg/kg BW + GW 6471 | GW 6471 | |
|---|---|---|---|---|---|---|
| Creatinine (mg/dL) | 0.35 ± 0.13 | 0.37 ± 0.1 | 0.46 ± 0.3 | 0.29 ± 0.1 | 0.37 ± 0.2 | 0.33 ± 0.1 |
| Urea (mg/dL) | 47.4 ± 11.7 | 50.6 ± 12.3 | 51.8 ± 14.0 | 44.7 ± 8.5 | 43.6 ± 13.1 | 47.8 ± 7.9 |
| AST (U/L) | 123.8 ± 74.2 | 200.5 ± 140.3 | 143.5 ± 60.7 | 117.8 ± 46.2 | 150.6 ± 109.1 | 103.3 ± 34.7 |
| ALT (U/L) | 55.6 ± 20.9 | 82.8 ± 56 | 52 ± 17.8 | 58.6 ± 23.6 | 80.8 ± 68.1 | 48.5 ± 14.1 |
| CASP-Control | Gemfibrozil 30 mg/kg BW | Gemfibrozil 100 mg/kg BW | Gemfibrozil 30 mg/kg BW + GW 6471 | Gemfibrozil 100 mg/kg BW + GW 6471 | GW 6471 | |
|---|---|---|---|---|---|---|
| Creatinine (mg/dL) | 0.33 ± 0.1 | 0.32 ± 0.1 | 0.29 ± 0.1 | 0.33 ± 0.1 | 0.36 ± 0.1 | 0.35 ± 0.1 |
| Urea (mg/dL) | 45.5 ± 12.5 | 46.8 ± 11.4 | 43.9 ± 12.9 | 44.4 ± 11.2 | 46.1 ± 8.3 | 47.8 ± 10.8 |
| AST (U/L) | 149.7 ± 96.6 | 144.3 ± 36.6 | 175.5 ± 133.6 | 131.4 ± 40.1 | 204.3 ± 156 | 236.2 ± 307.9 |
| ALT (U/L) | 68.1 ± 51.1 | 56.4 ± 23.1 | 77.8 ± 71.5 | 57.6 ± 26.4 | 103.5 ± 91.6 | 141.6 ± 240.9 |
| Group 1 | Sham + DMSO 50% (gemfibrozil carrier) and DMSO 5% (GW 6471 carrier) |
| Group 2 | Sham + Gemfibrozil 100 mg/kg BW + DMSO 5% |
| Group 3 | Sham + Gemfibrozil 30 mg/kg BW + DMSO 5% |
| Group 4 | Sham + Gemfibrozil 100 mg/kg BW + GW 6471 1 mg/kg BW |
| Group 5 | Sham + Gemfibrozil 30 mg/kg BW + GW 6471 1 mg/kg BW |
| Group 6 | Sham + GW 6471 1 mg/kg BW + DMSO 50% |
| Group 7 | CASP + DMSO 5% + DMSO 50% |
| Group 8 | CASP + Gemfibrozil 100 mg/kg BW + DMSO 5% |
| Group 9 | CASP + Gemfibrozil 30 mg/kg BW + DMSO 5% |
| Group 10 | CASP + Gemfibrozil 100 mg/kg BW + GW 6471 1 mg/kg BW |
| Group 11 | CASP + Gemfibrozil 30 mg/kg BW + GW 6471 1 mg/kg BW |
| Group 12 | CASP + GW 6471 1 mg/kg BW + DMSO 50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuebart, A.; Gross, K.; Maicher, C.; Sonnenschein, M.; Raupach, A.; Schulz, J.; Truse, R.; Hof, S.; Marcus, C.; Vollmer, C.; et al. Gemfibrozil Improves Microcirculatory Oxygenation of Colon and Liver without Affecting Mitochondrial Function in a Model of Abdominal Sepsis in Rats. Int. J. Mol. Sci. 2024, 25, 262. https://doi.org/10.3390/ijms25010262
Kuebart A, Gross K, Maicher C, Sonnenschein M, Raupach A, Schulz J, Truse R, Hof S, Marcus C, Vollmer C, et al. Gemfibrozil Improves Microcirculatory Oxygenation of Colon and Liver without Affecting Mitochondrial Function in a Model of Abdominal Sepsis in Rats. International Journal of Molecular Sciences. 2024; 25(1):262. https://doi.org/10.3390/ijms25010262
Chicago/Turabian StyleKuebart, Anne, Katharina Gross, Charlotte Maicher, Max Sonnenschein, Annika Raupach, Jan Schulz, Richard Truse, Stefan Hof, Carsten Marcus, Christian Vollmer, and et al. 2024. "Gemfibrozil Improves Microcirculatory Oxygenation of Colon and Liver without Affecting Mitochondrial Function in a Model of Abdominal Sepsis in Rats" International Journal of Molecular Sciences 25, no. 1: 262. https://doi.org/10.3390/ijms25010262
APA StyleKuebart, A., Gross, K., Maicher, C., Sonnenschein, M., Raupach, A., Schulz, J., Truse, R., Hof, S., Marcus, C., Vollmer, C., Bauer, I., Picker, O., Relja, B., & Herminghaus, A. (2024). Gemfibrozil Improves Microcirculatory Oxygenation of Colon and Liver without Affecting Mitochondrial Function in a Model of Abdominal Sepsis in Rats. International Journal of Molecular Sciences, 25(1), 262. https://doi.org/10.3390/ijms25010262







