Glucans with Different Degrees of Polymerization from Leuconostoc mesenteroides CICC6055: Analysis of Physicochemical Properties and Intestinal Prebiotic Function
Abstract
:1. Introduction
2. Results
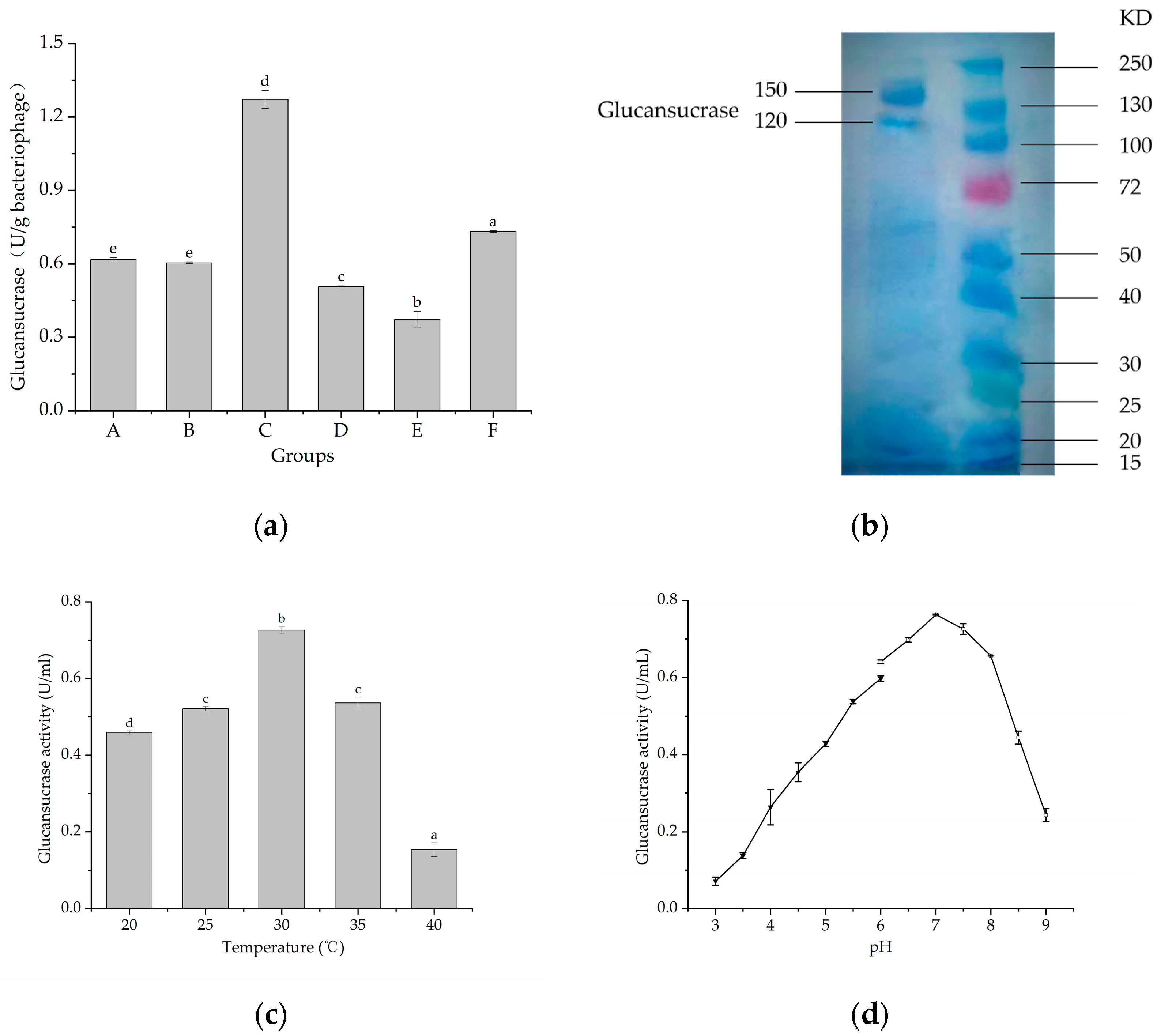
2.1. Properties of Glucansucrase from L. mesenteroides 6055
2.2. Analyses of Enzyme Synthesis
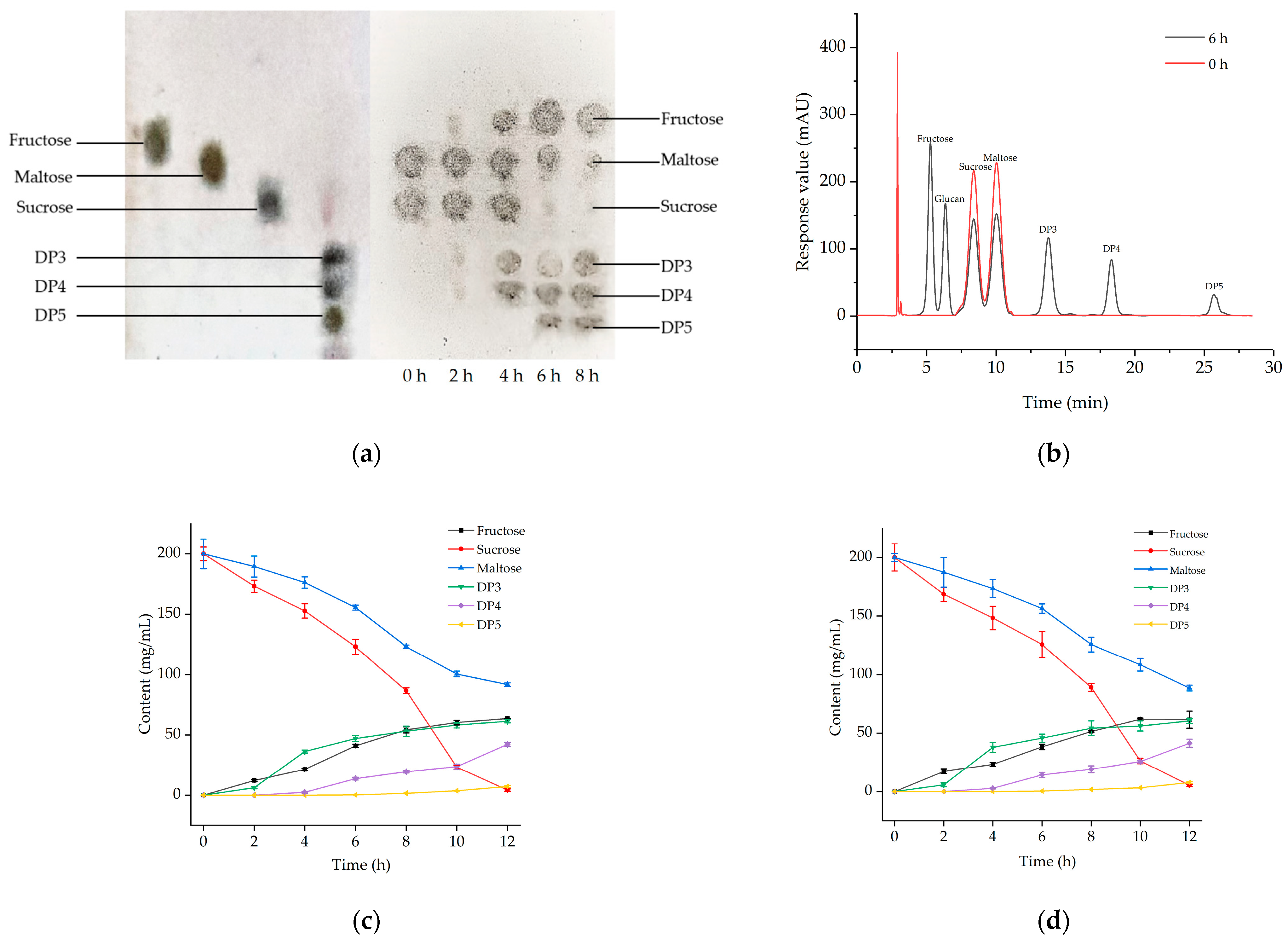
2.2.1. TLC Analysis of Oligosaccharide Products
2.2.2. HPLC Analysis of Oligosaccharide Products
2.2.3. Comparison of Microbial Fermentation and Enzyme Synthesis
2.3. Analysis of Physicochemical Properties of Glucan and Oligoglucan
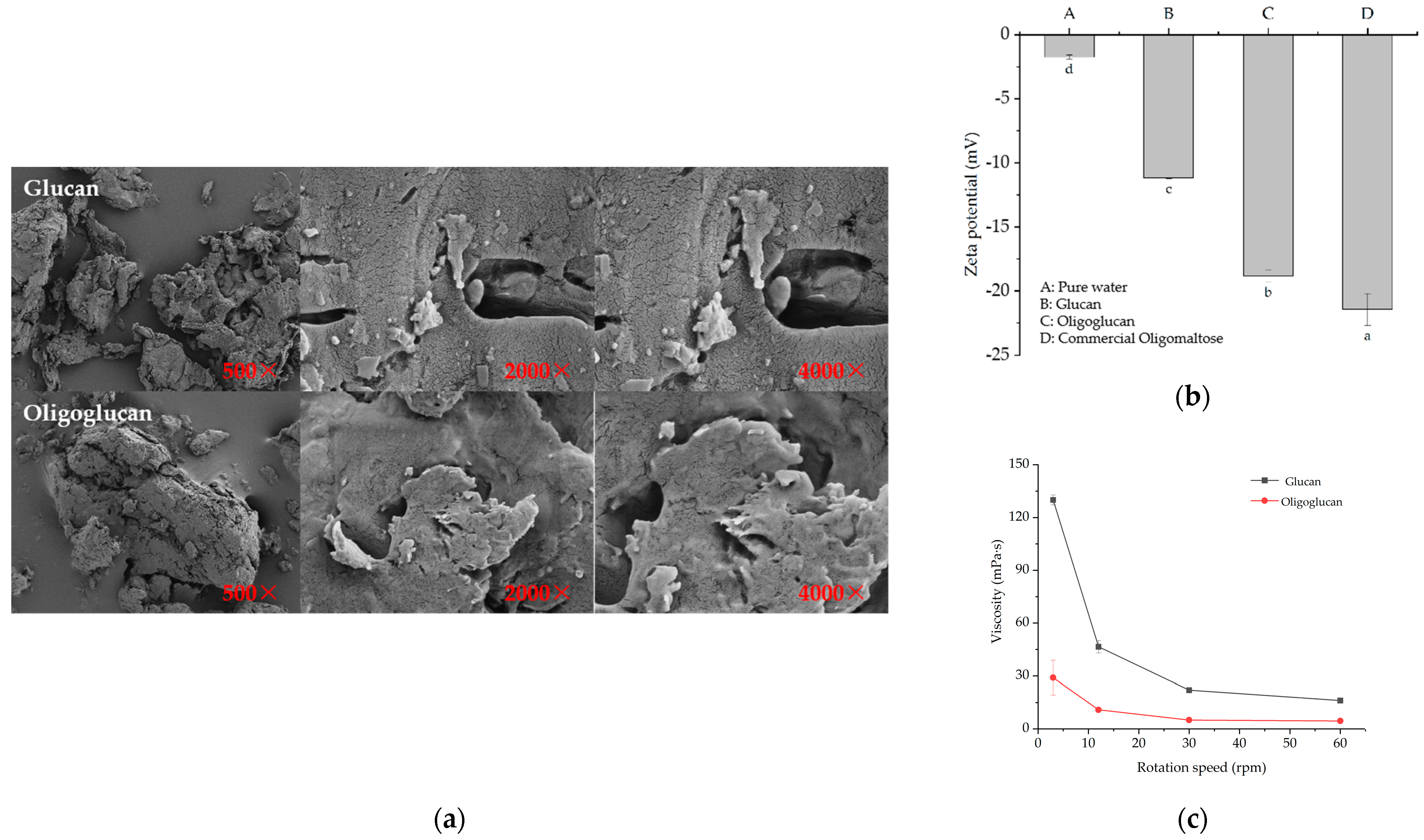
2.3.1. SEM, Zeta Potential, and Viscosity Analysis
2.3.2. Thermodynamic Analysis
2.3.3. Molecular Weight
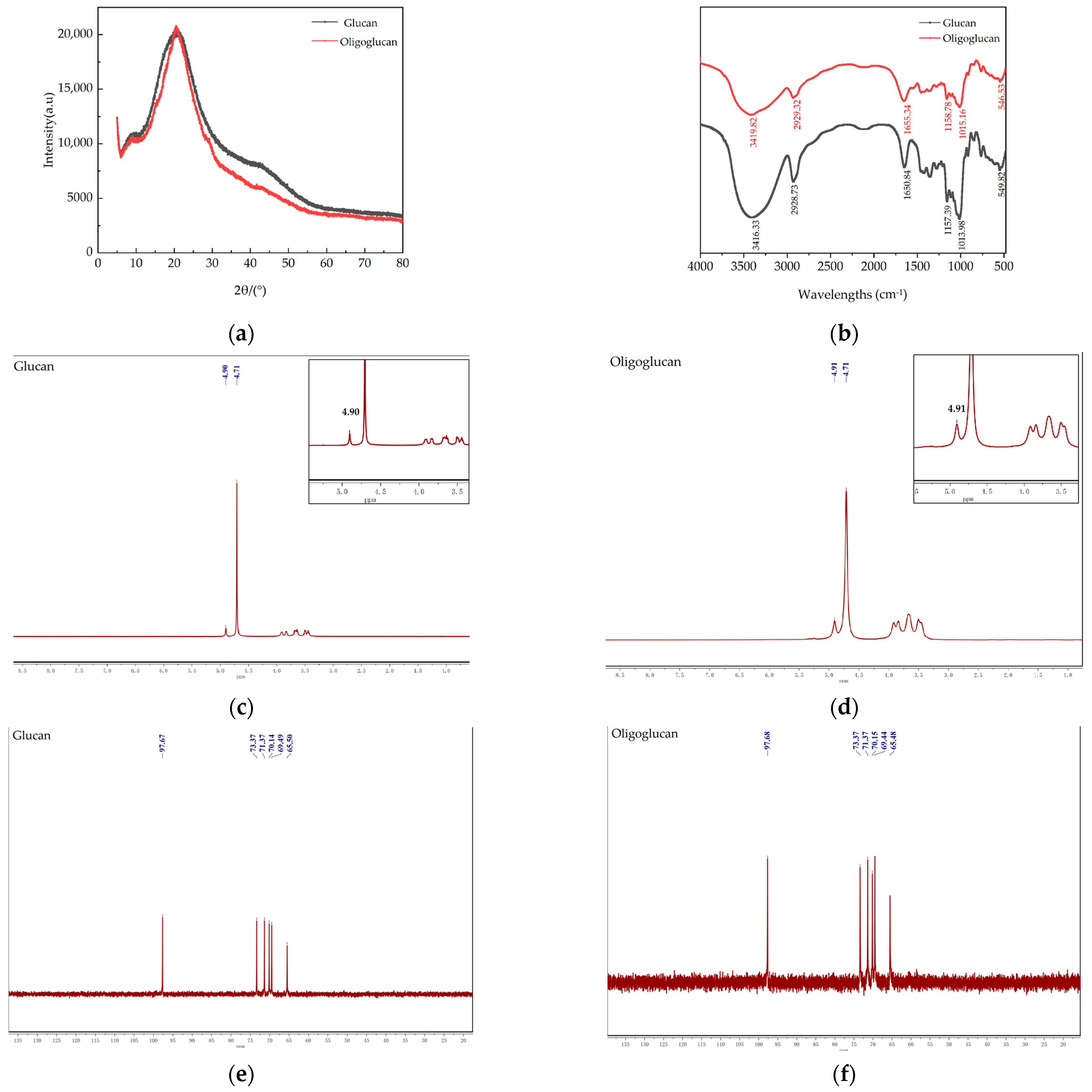
2.3.4. XRD Analysis
2.3.5. Fourier Transform-InfraRed (FT-IR) Analysis
2.3.6. Nuclear Magnetic Resonance (NMR) Analysis
2.4. In Vitro Simulation of Intestinal Probiotic Function
2.4.1. Changes of pH and Strain Density during Fermentation
2.4.2. Production of SCFAs during Fermentation
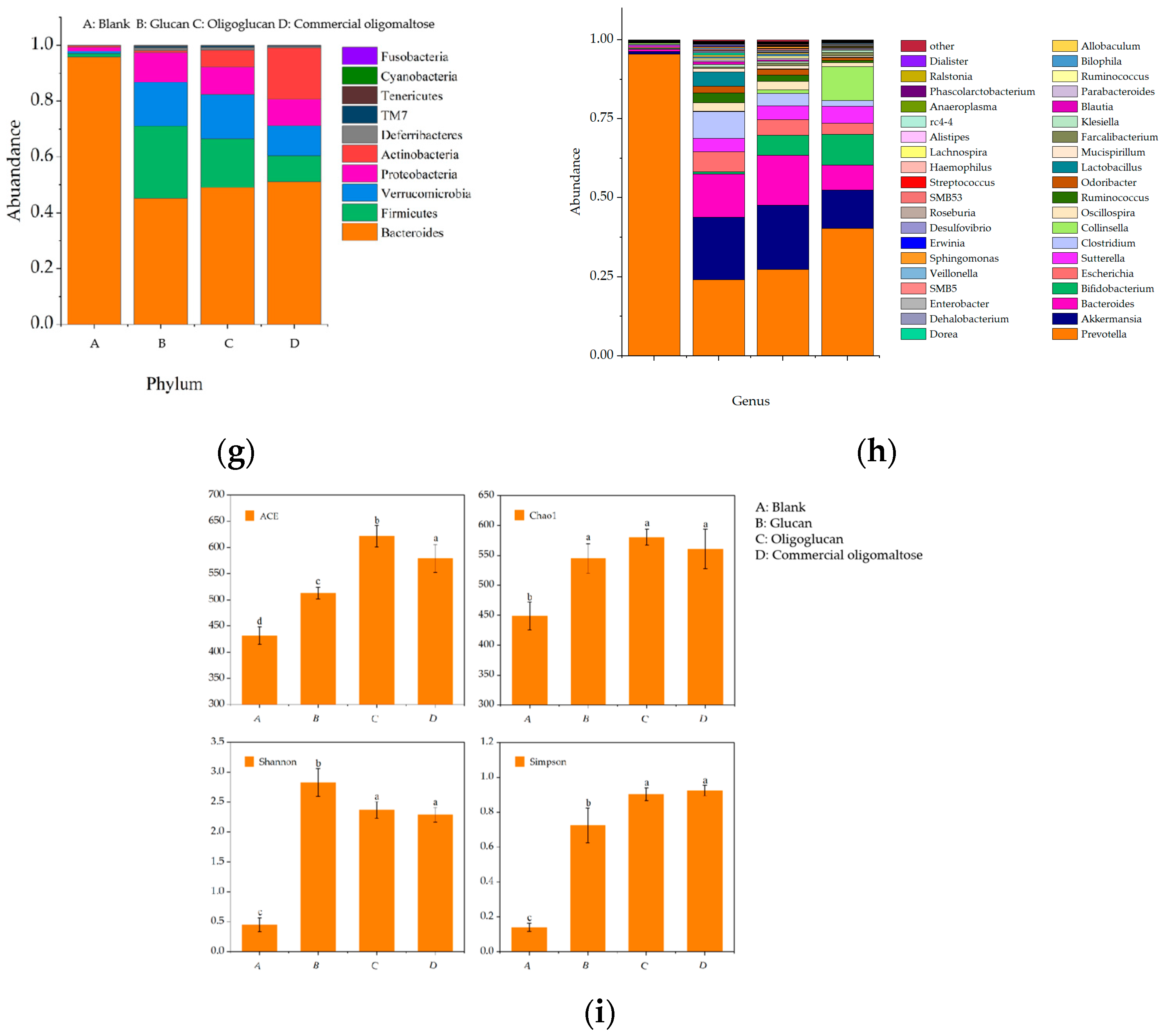
2.4.3. Changes in Intestinal Microbial Flora Growth
3. Discussion
3.1. Properties of Glucansucrase from L. mesenteroides 6055
3.2. Analyses of Enzyme Synthesis
3.3. Analysis of Physicochemical Properties of Glucan and Oligoglucan
3.4. In Vitro Simulation of Intestinal Probiotic Function
4. Materials and Methods
4.1. Preparation and Cultivation of Bacterial Strains
4.2. Extraction of Exopolysaccharides by L. mesenteroides 6055
4.2.1. Glucans from Strains Fermentation
4.2.2. Analyses of Properties of Glucansucrase
Extraction, Purification, and Enzyme Activity Assay
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Oligoglucans from Enzyme Synthesis
Thin-Layer Chromatography (TLC) Analysis
High-Performance Liquid Chromatography (HPLC)
4.3. Analysis of Physicochemical Properties of Glucan and Oligoglucan
4.3.1. Scanning Electron Microscopy (SEM)
4.3.2. Zeta Potential
4.3.3. Apparent Viscosity
4.3.4. Thermodynamic Properties
4.3.5. Molecular Weight Distribution
4.3.6. Crystalline Configuration
4.3.7. Fourier Transform-Infrared Spectroscopy (FT-IR)
4.3.8. Nuclear Magnetic Resonance (NMR)
4.4. Simulated Gastrointestinal Fermentation of Glucan and Oligoglucan
4.4.1. In Vitro Fermentation Procedure
4.4.2. Determination of pH, Strain Density, and Short-Chain Fatty Acids (SCFAs)
4.4.3. Identification of Intestinal Flora in Fermentation Broth
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Yassin, A.M.; Al-Madboly, L.A.; El-Hawiet, A. A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-kappaB inflammatory pathways in human colon cancer. Microb. Cell Fact. 2018, 17, 29. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Bhat, B.; Bajaj, B.K. Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from kalarei. Bioresour. Technol. 2018, 254, 264–267. [Google Scholar] [CrossRef]
- Dertli, E.; Toker, O.S.; Durak, M.Z.; Yilmaz, M.T.; Tatlisu, N.B.; Sagdic, O.; Cankurt, H. Development of a fermented ice-cream as influenced by in situ exopolysaccharide production: Rheological, molecular, microstructural and sensory characterization. Carbohydr. Polym. 2016, 136, 427–440. [Google Scholar] [CrossRef]
- Niu, M.; Guo, H.; Cai, J.; Duan, B.; Chen, Y.; Meng, X. Exopolysaccharide from Bifidobacterium breve alleviate dextran sulfate sodium-induced colitis in mice via inhibiting oxidative stress and regulating intestinal flora. Food Biosci. 2023, 56, 103020. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, B.; Wei, M.; He, Y.; Wang, Y.; Zhang, Q.; Wei, H.; Tao, X. Antitumor effect of exopolysaccharide from Lactiplantibacillus plantarum WLPL09 on melanoma mice via regulating immunity and gut microbiota. Int. J. Biol. Macromol. 2024, 254, 127624. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Y.; Zheng, X.; Zhang, Y.; Deng, K.; Wu, T.; Cheng, H. Analysis of physicochemical properties of exopolysaccharide from Leuconostoc mesenteroides strain XR1 and its application in fermented milk. Lwt 2021, 146, 111449. [Google Scholar] [CrossRef]
- Matsuzaki, C.; Takagaki, C.; Tomabechi, Y.; Forsberg, L.S.; Heiss, C.; Azadi, P.; Matsumoto, K.; Katoh, T.; Hosomi, K.; Kunisawa, J.; et al. Structural characterization of the immunostimulatory exopolysaccharide produced by Leuconostoc mesenteroides strain NTM048. Carbohydr. Res. 2017, 448, 95–102. [Google Scholar] [CrossRef]
- Miao, M.; Jia, X.; Hamaker, B.; Cui, S.; Jiang, B.; Huang, C. Structure-prebiotic properties relationship for α-D-glucan from Leuconostoc citreum SK24.002. Food Hydrocoll. 2016, 57, 246–252. [Google Scholar] [CrossRef]
- İspirli, H.; Bowman, M.J.; Skory, C.D.; Dertli, E. Synthesis and characterization of cellobiose-derived oligosaccharides with Bifidogenic activity by glucansucrase E81. Food Biosci. 2021, 44, 101388. [Google Scholar] [CrossRef]
- Ko, J.A.; Nam, S.H.; Park, J.Y.; Wee, Y.; Kim, D.; Lee, W.S.; Ryu, Y.B.; Kim, Y.M. Synthesis and characterization of glucosyl stevioside using Leuconostoc dextransucrase. Food Chem. 2016, 211, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Kim, H.; Kang, C.G.; Park, C.; Kim, S.W.; Kim, D. Biochemical characterization of synthesized fisetin glucoside by dextransucrase from Leuconostoc mesenteroides NRRL B-1299CB4 with enhanced water solubility. Enzym. Microb. Technol. 2022, 161, 110111. [Google Scholar] [CrossRef] [PubMed]
- İspirli, H.; Yüzer, M.O.; Skory, C.; Colquhoun, I.J.; Sağdıç, O.; Dertli, E. Characterization of a glucansucrase from Lactobacillus reuteri E81 and production of malto-oligosaccharides. Biocatal. Biotransform. 2019, 37, 421–430. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Cao, C.; Zhu, X.; Wang, C.; Wu, R.; Wu, J. Extraction and biological activity of exopolysaccharide produced by Leuconostoc mesenteroides SN-8. Int. J. Biol. Macromol. 2020, 157, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Bisson, G.; Comuzzi, C.; Giordani, E.; Poletti, D.; Boaro, M.; Marino, M. An exopolysaccharide from Leuconostoc mesenteroides showing interesting bioactivities versus foodborne microbial targets. Carbohydr. Polym. 2023, 301, 120363. [Google Scholar] [CrossRef]
- Wang, B.; Song, Q.; Zhao, F.; Zhang, L.; Han, Y.; Zhou, Z. Isolation and characterization of dextran produced by Lactobacillus sakei L3 from Hubei sausage. Carbohydr. Polym. 2019, 223, 115111. [Google Scholar] [CrossRef] [PubMed]
- Bechtner, J.; Hassler, V.; Wefers, D.; Vogel, R.F.; Jakob, F. Insights into extracellular dextran formation by Liquorilactobacillus nagelii TMW 1.1827 using secretomes obtained in the presence or absence of sucrose. Enzyme Microb. Technol. 2021, 143, 109724. [Google Scholar] [CrossRef]
- Liu, L.; Xu, J.; Na, R.; Du, R.; Ping, W.; Ge, J.; Zhao, D. Purification, characterization and partial biological activities of exopolysaccharide produced by Saccharomyces cerevisiae Y3. Int. J. Biol. Macromol. 2022, 206, 777–787. [Google Scholar] [CrossRef]
- Du, R.; Pei, F.; Kang, J.; Zhang, W.; Wang, S.; Ping, W.; Ling, H.; Ge, J. Analysis of the structure and properties of dextran produced by Weissella confusa. Int. J. Biol. Macromol. 2022, 204, 677–684. [Google Scholar] [CrossRef]
- Du, R.; Xing, H.; Yang, Y.; Jiang, H.; Zhou, Z.; Han, Y. Optimization, purification and structural characterization of a dextran produced by L. mesenteroides isolated from Chinese sauerkraut. Carbohydr. Polym. 2017, 174, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhou, Z.; Han, Y. Exopolysaccharide from Leuconostoc pseudomesenteroides XG5 delay the onset of autoimmune diabetes by modulating gut microbiota and its metabolites SCFAs in NOD mice. J. Funct. Foods 2021, 79, 104427. [Google Scholar] [CrossRef]
- Hu, Y.; Ganzle, M.G. Effect of temperature on production of oligosaccharides and dextran by Weissella cibaria 10 M. Int. J. Food Microbiol. 2018, 280, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Tingirikari, J.M.; Kothari, D.; Shukla, R.; Goyal, A. Structural and biocompatibility properties of dextran from Weissella cibaria JAG8 as food additive. Int. J. Food Sci. Nutr. 2014, 65, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Miao, M.; Jiang, B.; Xu, T.; Cui, S.; Zhang, T. Leuconostoc citreum SK24.002 glucansucrase: Biochemical characterisation and de novo synthesis of α-glucan. Int. J. Biol. Macromol. 2016, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Ma, Y.; Jiang, B.; Cui, S.; Jin, Z.; Zhang, T. Characterisations of Lactobacillus reuteri SK24.003 glucansucrase: Implications for α-gluco-poly- and oligosaccharides biosynthesis. Food Chem. 2017, 222, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yeona, M.; Choia, N.; Chang, Y.; Jung, M.; Song, J.; Kim, J. Purification and characterization of a novel glucansucrase from Leuconostoc lactis EG001. Microbiol. Res. 2010, 165, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Stecka, K.; Grzybowski, R. The influence of pH and oxygen on the growth and probiotic activity of lactic acid bacteria. Food Biotechnol. 2000, 17, 275–281. [Google Scholar] [CrossRef]
- Zeng, M.; Li, N.; Astmann, T.; Oh, J.H.; van Pijkeren, J.P.; Pan, X. Facile and efficient chemical synthesis of gluco-oligosaccharides (GlcOS) with diverse glycosidic linkages as potential prebiotics to promote the growth of probiotic bacteria. Food Res. Int. 2023, 165, 112436. [Google Scholar] [CrossRef]
- Shukla, R.; Shukla, S.; Bivolarski, V.; Iliev, I. Structural Characterization of Insoluble Dextran Produced by Leuconostoc mesenteroides NRRL B-1149 in the Presence of Maltose. Food Technol. Biotechnol. 2011, 49, 291–296. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Q.; Guo, Y.; Han, Y.; Xiao, H.; Zhou, Z. Isolation and characterization of dextran produced by Leuconostoc citreum NM105 from manchurian sauerkraut. Carbohydr. Polym. 2015, 133, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Qi, J.R.; Feng, J.L.; Cao, J.; Wang, J.M.; Yang, X.Q. Pea soluble polysaccharides obtained from two enzyme-assisted extraction methods and their application as acidified milk drinks stabilizers. Food Res. Int. 2018, 109, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Jeddou, K.B.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhou, J.; Xu, Q.; Dong, M.; Fan, X.; Rui, X.; Zhang, Q.; Chen, X.; Jiang, M.; Wu, J.; et al. In vitro digestion and fermentation of released exopolysaccharides (r-EPS) from Lactobacillus delbrueckii ssp. bulgaricus SRFM-1. Carbohydr. Polym. 2020, 230, 115593. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Wang, B. Synthesis, characterization and antioxidant activity of selenium modified polysaccharides from Hohenbuehelia serotina. Int. J. Biol. Macromol. 2018, 120, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Hamazaki, T.; Jokaji, H.; Minami, S.; Kobayashi, M. Effect of HMG-CoA reductase inhibitors on plasma polyunsaturated fatty acid concentrations in patients with hyperlipidemia. Int. J. Clin. Lab. Res. 1998, 28, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Schilderink, R.; Verseijden, C.; Seppen, J.; Muncan, V.; van den Brink, G.R.; Lambers, T.T.; van Tol, E.A.; de Jonge, W.J. The SCFA butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial HDAC. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G1138–G1146. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Fang, Q.; Zhou, W.; Ping, Q.; Xiao, Y.; Wang, Z. Performance and mechanism of sodium citrate pretreatment to promote waste activated sludge disintegration and short-chain fatty acid production during anaerobic fermentation. J. Environ. Chem. Eng. 2023, 11, 109161. [Google Scholar] [CrossRef]
- Du, H.; Zhao, A.; Wang, Q.; Yang, X.; Ren, D. Supplementation of Inulin with Various Degree of Polymerization Ameliorates Liver Injury and Gut Microbiota Dysbiosis in High Fat-Fed Obese Mice. J. Agric. Food Chem. 2020, 68, 779–787. [Google Scholar] [CrossRef]
- Ghotaslou, R.; Nabizadeh, E.; Memar, M.Y.; Law, W.M.H.; Ozma, M.A.; Abdi, M.; Yekani, M.; Kadkhoda, H.; Hosseinpour, R.; Bafadam, S.; et al. The metabolic, protective, and immune functions of Akkermansia muciniphila. Microbiol. Res. 2023, 266, 127245. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yan, Y.; Peng, Y.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2019, 125, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jiang, S.; Zeng, M. In vitro simulated digestion and fermentation characteristics of polysaccharide from oyster (Crassostrea gigas), and its effects on the gut microbiota. Food Res. Int. 2021, 149, 110646. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Li, L.; Zhang, Z.; Zhang, J.; Zhang, J.; Wang, J. Characterization of polysaccharide fractions from Allii macrostemonis bulbus and assessment of their antioxidant. Lwt 2022, 165. [Google Scholar] [CrossRef]
- Liang, D.; Li, N.; Dai, X.; Zhang, H.; Hu, H. Effects of different types of potato resistant starches on intestinal microbiota and short-chain fatty acids under in vitro fermentation. Int. J. Food Sci. Technol. 2021, 56, 2432–2442. [Google Scholar] [CrossRef]


| 0–2000 | 2000–10,000 | >10,000 | Mn (Da) | Mw (Da) | PD | |
|---|---|---|---|---|---|---|
| Glucan | 0 | 0 | 100% | 844,190 | 1,117,981 | 1.32432 |
| Oligoglucan | 93.3% | 6.483% | 0.187% | 725 | 3342 | 4.60966 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Jiao, Z.; Wang, T.; Zhang, B.; Zhao, H. Glucans with Different Degrees of Polymerization from Leuconostoc mesenteroides CICC6055: Analysis of Physicochemical Properties and Intestinal Prebiotic Function. Int. J. Mol. Sci. 2024, 25, 258. https://doi.org/10.3390/ijms25010258
Gu J, Jiao Z, Wang T, Zhang B, Zhao H. Glucans with Different Degrees of Polymerization from Leuconostoc mesenteroides CICC6055: Analysis of Physicochemical Properties and Intestinal Prebiotic Function. International Journal of Molecular Sciences. 2024; 25(1):258. https://doi.org/10.3390/ijms25010258
Chicago/Turabian StyleGu, Jiabao, Ziyan Jiao, Tao Wang, Bolin Zhang, and Hongfei Zhao. 2024. "Glucans with Different Degrees of Polymerization from Leuconostoc mesenteroides CICC6055: Analysis of Physicochemical Properties and Intestinal Prebiotic Function" International Journal of Molecular Sciences 25, no. 1: 258. https://doi.org/10.3390/ijms25010258
APA StyleGu, J., Jiao, Z., Wang, T., Zhang, B., & Zhao, H. (2024). Glucans with Different Degrees of Polymerization from Leuconostoc mesenteroides CICC6055: Analysis of Physicochemical Properties and Intestinal Prebiotic Function. International Journal of Molecular Sciences, 25(1), 258. https://doi.org/10.3390/ijms25010258







