Developmental and Nutritional Dynamics of Malpighian Tubule Autofluorescence in the Asian Tiger Mosquito Aedes albopictus
Abstract
1. Introduction
2. Results
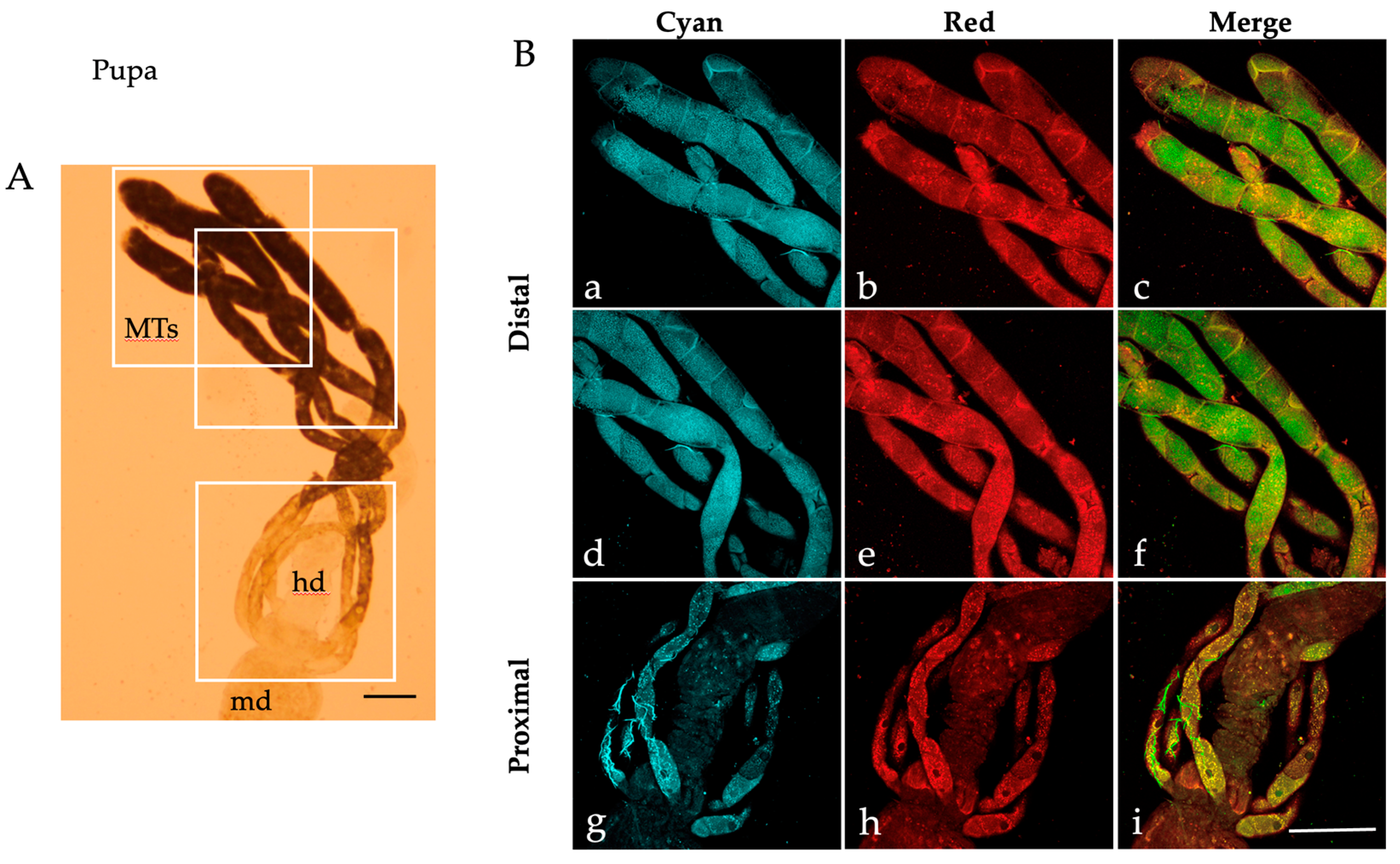
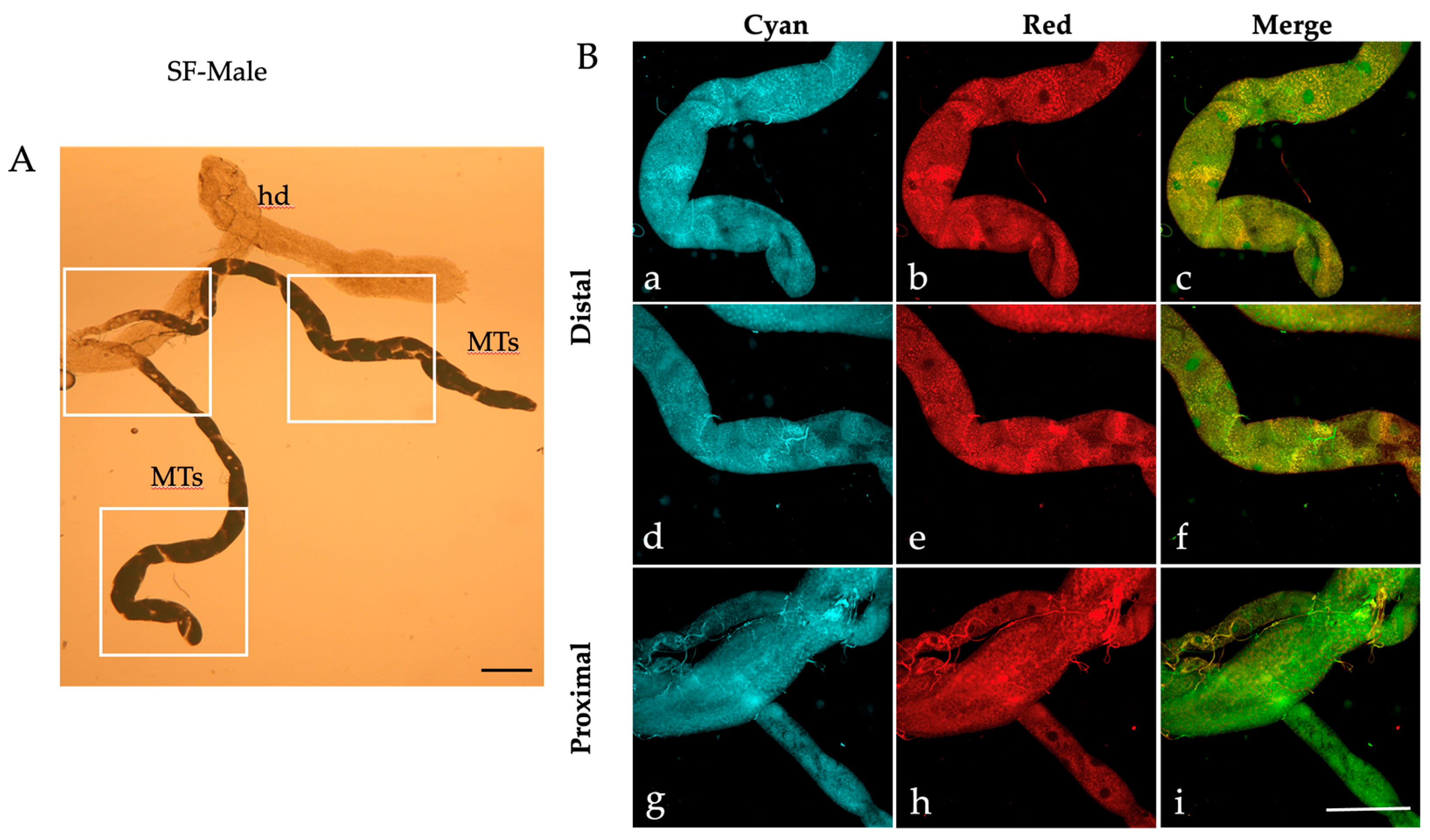
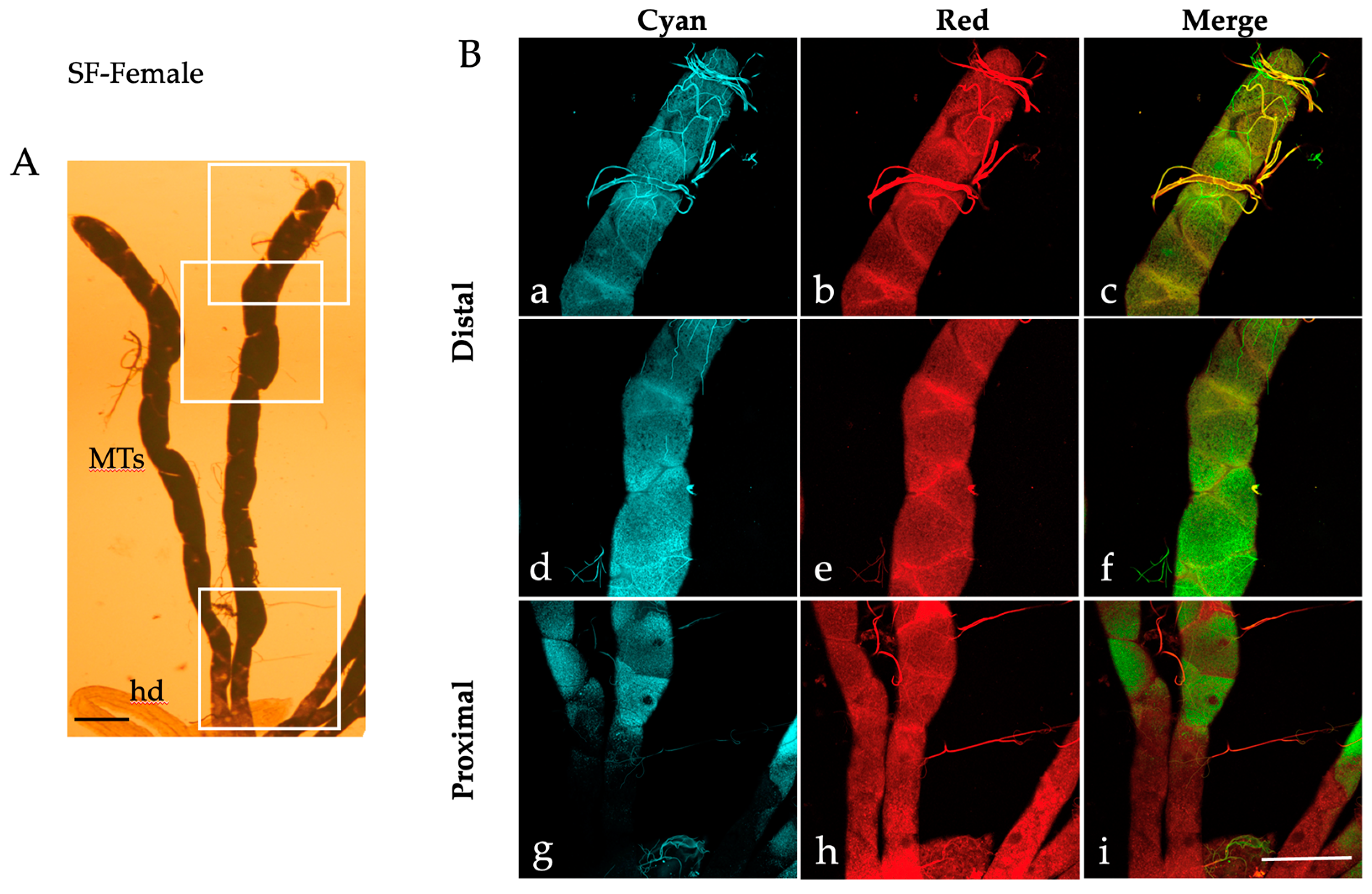
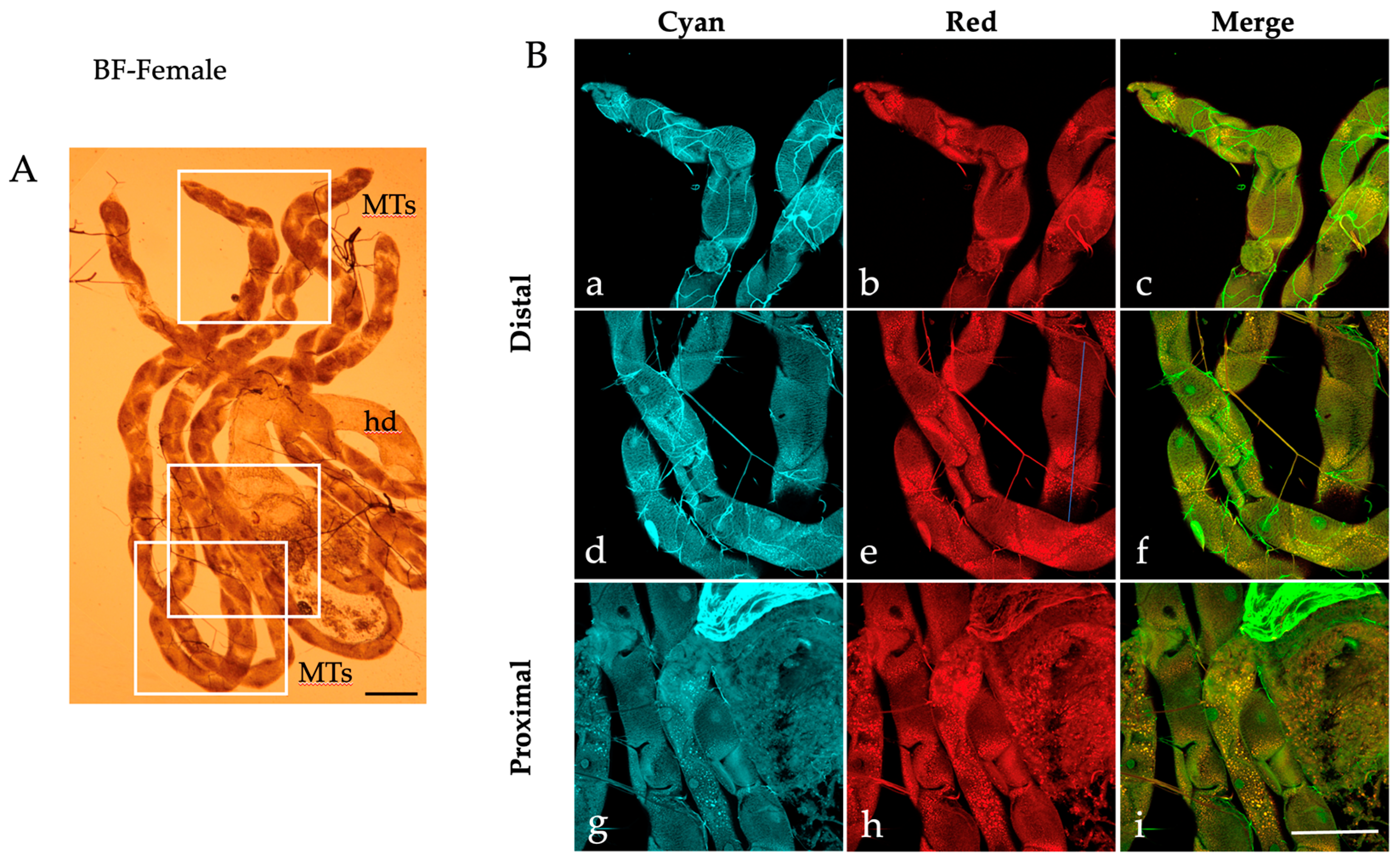
2.1. Autofluorescence Imaging of the Malpighian Tubules in the Different Developmental Stages of Ae. albopictus
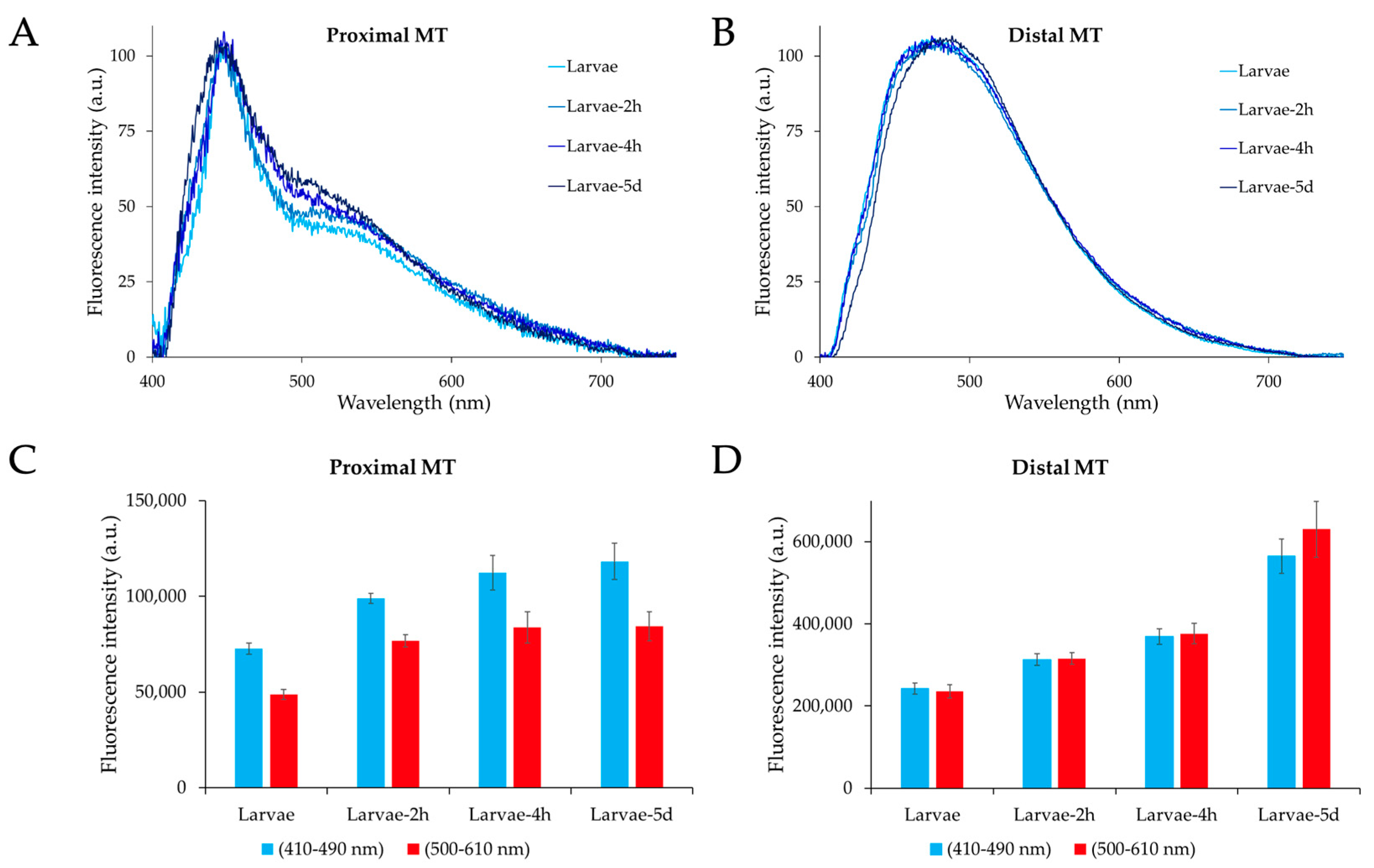
2.2. Microspectrofluorometry of Malpighian Tubule Autofluorescence in the Different Developmental Stages of Ae. albopictus
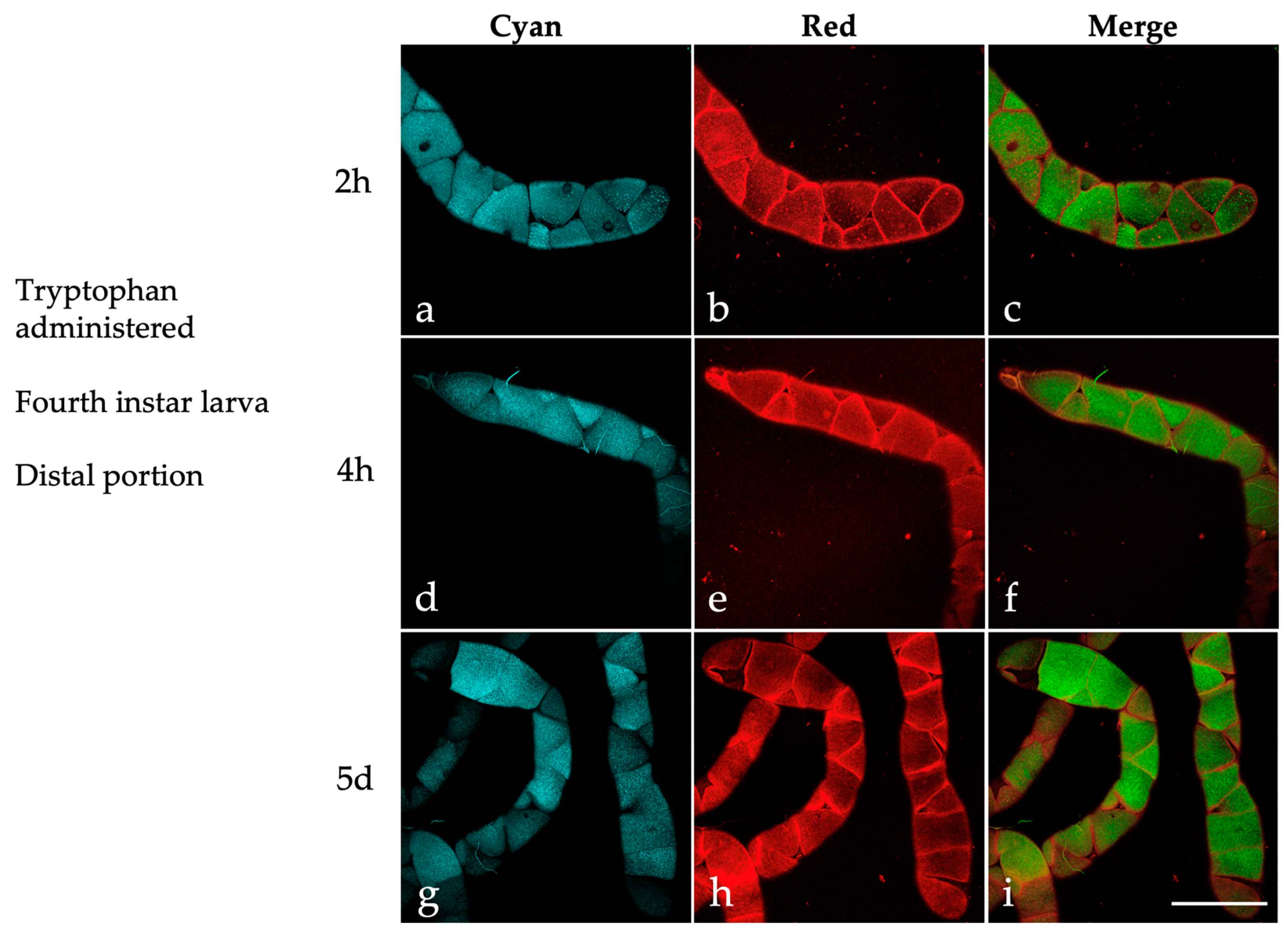
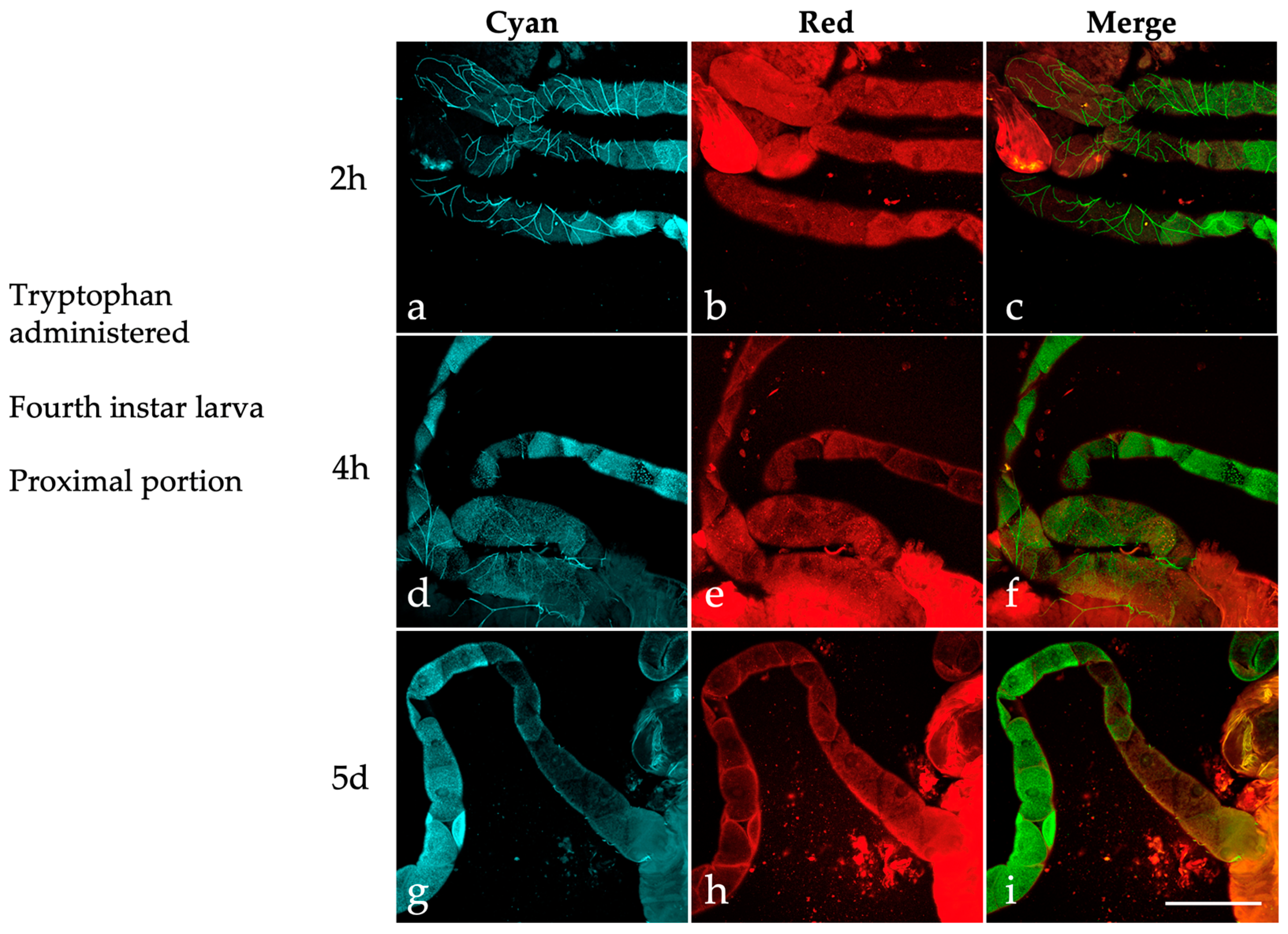
2.3. Effects of Dietary Tryptophan Administration on the AF of Larval Malpighian Tubules
3. Discussion
4. Materials and Methods
4.1. Insects and Malpighian Tubule Sample Preparation
4.2. Bright Field and AF Confocal Microscopy Imaging
4.3. Spectrofluorimetric Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farina, P.; Bedini, S.; Conti, B. Multiple functions of Malpighian tubules in insects: A review. Insects 2022, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Sawyer, J.K.; Peterson, N.G.; Dow, J.A.T.; Fox, D.T. Physiology, development, and disease modeling in the Drosophila excretory system. Genetics 2020, 214, 235–264. [Google Scholar] [CrossRef] [PubMed]
- Piermarini, P.M.; Gillen, C.M. Non-traditional models: The molecular physiology of sodium and water transport in mosquito Malpighian tubules. In Sodium and Water Homeostasis; Hyndman, K., Pannabecker, T., Eds.; Springer: New York, NY, USA, 2015; pp. 255–278. [Google Scholar]
- Xu, J.; Liu, Y.; Li, H.; Tarashansky, A.J.; Kalicki, C.H.; Hung, R.J.; Hu, Y.; Comjean, A.; Kolluru, S.S.; Wang, B.; et al. Transcriptional and functional motifs defining renal function revealed by single-nucleus RNA sequencing. Proc. Natl. Acad. Sci. USA 2022, 119, e2203179119. [Google Scholar] [CrossRef] [PubMed]
- Beyenbach, K.W.; Skaer, H.; Dow, J.A.T. The developmental, molecular, and transport biology of Malpighian tubules. Annu. Rev. Entomol. 2010, 55, 351–374. [Google Scholar] [CrossRef] [PubMed]
- Halberg, K.A.; Terhzaz, S.; Cabrero, P.; Davies, S.A.; Dow, J.A.T. Tracing the evolutionary origins of insect renal function. Nat. Commun. 2015, 6, 6800. [Google Scholar] [CrossRef] [PubMed]
- Gąsiorowski, L.; Andrikou, C.; Janssen, R.; Bump, P.; Budd, G.E.; Lowe, C.J.; Hejnol, A. Molecular evidence for a single origin of ultrafiltration-based excretory organs. Curr. Biol. 2021, 31, 3629–3638.e2. [Google Scholar] [CrossRef]
- Dow, J.A.T.; Davies, S.A. The Malpighian tubule: Rapid insights from post-genomic biology. J. Insect Physiol. 2006, 52, 365–378. [Google Scholar] [CrossRef]
- Dow, J.A.T.; Simons, M.; Romero, M.F. Drosophila melanogaster: A simple genetic model of kidney structure, function and disease. Nat. Rev. Nephrol. 2022, 18, 417–434. [Google Scholar] [CrossRef]
- Rodan, A.R. The Drosophila Malpighian tubule as a model for mammalian tubule function. Curr. Opin. Nephrol. Hypertens. 2019, 28, 455–464. [Google Scholar] [CrossRef]
- Koehler, S.; Huber, T.B. Insights into human kidney function from the study of Drosophila. Pediatr. Nephrol. 2023, 38, 3875–3887. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Turin, D.R.; Romero, M.F. Transporters and tubule crystals in the insect Malpighian tubule. Curr. Opin. Insect Sci. 2021, 47, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ju, Y.; Gao, L.; Miao, Y.; Qiao, H.; Wang, Y. The fruit fly kidney stone models and their application in drug development. Heliyon 2022, 8, e09232. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yoshida, H. Drosophila as a model organism. Adv. Exp. Med. Biol. 2018, 1076, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.; Kim, M.S.; Lang, S.; Bose, N.; Kahn, A.; Flechner, L.; Blaschko, S.D.; Zee, T.; Muteliefu, G.; Bond, N.; et al. A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease. PLoS ONE 2015, 10, e0124150. [Google Scholar] [CrossRef] [PubMed]
- Clemens, A.N. The Biology of Mosquitoes. Development, Nutrition and Reproduction; Chapman and Hall: London, UK, 1992; Volume 1, p. xxii+509. [Google Scholar]
- Melgarejo-Colmenares, K.; Cardo, M.V.; Vezzani, D. Blood feeding habits of mosquitoes: Hardly a bite in South America. Parasitol. Res. 2022, 121, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Becker, S.C. Mosquitoes as arbovirus vectors: From species identification to vector competence. In Mosquito-borne Diseases. Parasitology Research Monographs; Benelli, G., Mehlhorn, H., Eds.; Springer: Cham, Switzerland, 2018; Volume 10, pp. 163–212. [Google Scholar] [CrossRef]
- Chen, B.; Sweeny, A.R.; Wu, V.Y.; Christofferson, R.C.; Ebel, G.; Fagre, A.C.; Gallichotte, E.; Kading, R.C.; Ryan, S.J.; Carlson, C.J. Exploring the mosquito-arbovirus network: A survey of vector competence experiments. Am. J. Trop. Med. Hyg. 2023, 108, 987–994. [Google Scholar] [CrossRef]
- Beyenbach, K.W.; Oviedo, A.; Aneshansley, D.J. Malpighian tubules of Aedes aegypti: Five tubules, one function. J. Insect Physiol. 1993, 39, 639–648. [Google Scholar] [CrossRef]
- Beyenbach, K.W.; Piermarini, P.M. Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti. Acta Physiol. 2011, 202, 387–407. [Google Scholar] [CrossRef]
- Mashinson, V.; Hopkins, C.R. Novel inhibitors of the renal inward rectifier potassium channel of the mosquito vector Aedes aegypti. Future Med. Chem. 2021, 13, 2015–2025. [Google Scholar] [CrossRef]
- Beyenbach, K.W.; Yu, Y.; Piermarini, P.M.; Denton, J. Targeting renal epithelial channels for the control of insect vectors. Tissue Barriers 2015, 3, e1081861. [Google Scholar] [CrossRef]
- Bottino-Rojas, V.; Ferreira-Almeida, I.; Nunes, R.D.; Feng, X.; Pham, T.B.; Kelsey, A.; Carballar-Lejarazú, R.; Gantz, V.; Oliveira, P.L.; James, A.A. Beyond the eye: Kynurenine pathway impairment causes midgut homeostasis dysfunction and survival and reproductive costs in blood-feeding mosquitoes. Insect Biochem. Mol. Biol. 2022, 142, 103720. [Google Scholar] [CrossRef] [PubMed]
- Piermarini, P.M.; Esquivel, C.J.; Denton, J.S. Malpighian tubules as novel targets for mosquito control. Int. J. Environ. Res. Public. Health 2017, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed]
- Klaessens, S.; Stroobant, V.; De Plaen, E.; Van den Eynde, B.J. Systemic tryptophan homeostasis. Front. Mol. Biosci. 2022, 9, 897929. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.D.; Güner, O.F.; Iradukunda, E.C.; Phillips, R.S.; Bowen, J.P. The kynurenine pathway and kynurenine 3-monooxygenase inhibitors. Molecules 2022, 27, 273. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Beerntsen, B.T.; Li, J. The tryptophan oxidation pathway in mosquitoes with emphasis on xanthurenic acid biosynthesis. J. Insect Physiol. 2007, 53, 254–263. [Google Scholar] [CrossRef]
- Tearle, R. Tissue specific effects of ommochrome pathway mutations in Drosophila melanogaster. Genet Res. 1991, 57, 257–266. [Google Scholar] [CrossRef]
- Li, J.; Li, G. Transamination of 3-hydroxykynurenine to produce xanthurenic acid: A major branch pathway of tryptophan metabolism in the mosquito, Aedes aegypti, during larval development. Insect Biochem. Mol. Biol. 1997, 27, 859–867. [Google Scholar] [CrossRef]
- Linzen, B. The tryptophan-ommochrome pathway in insects. Adv. Insect Phys. 1974, 10, 117–246. [Google Scholar] [CrossRef]
- Nissani, M. Cell lineage analysis of kynurenine producing organs in Drosophila melanogaster. Genet Res. 1975, 26, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, W.G.; Jyoti, A.; Mishra, M.K.; Louissaint, N.; Romero, R.; Chugani, D.C.; Kannan, S.; Kannan, R.M. Concurrent quantification of tryptophan and its major metabolites. Anal. Biochem. 2013, 443, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, M.; Mawatari, K.I.; Morooka, A.; Yasuda, M.; Fukuuchi, T.; Yamaoka, N.; Kaneko, K.; Nakagomi, K.; Oku, N. Simultaneous determination of kynurenine and kynurenic acid by high-performance liquid chromatography photoirradiation system using a mobile phase containing 18-crown-6. Int. J. Tryptophan Res. 2019, 12, 1178646919834551. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Lam, Y.C.; Gaffney, J.P.; Weaver, J.C.; Krivoshik, S.R.; Hamchand, R.; Pieribone, V.; Gruber, D.F.; Crawford, J.M. Bright green biofluorescence in sharks derives from bromo-kynurenine metabolism. iScience 2019, 19, 1291–1336. [Google Scholar] [CrossRef]
- Rizki, T.M.; Rizki, R.M. An inducible enzyme system in the larval cells of Drosophila melanogaster. J. Cell Biol. 1963, 17, 87–92. [Google Scholar] [CrossRef]
- Rizki, T.M. Genetic control of cytodifferentiation. J. Cell Biol. 1963, 16, 513–520. [Google Scholar] [CrossRef]
- Rizki, M.T. Intracellular localization of kynurenine in the fatbody of Drosophila. J. Biophys. Biochem. Cytol. 1961, 9, 567–572. [Google Scholar] [CrossRef]
- Yagi, S.; Ogawa, H. Effect of tryptophan metabolites on fluorescent granules in the Malpighian tubules of eye color mutants of Drosophila melanogaster. Zoolog Sci. 1996, 13, 97–104. [Google Scholar] [CrossRef]
- Garay, E.; Schuth, N.; Barbanente, A.; Tejeda-Guzmán, C.; Vitone, D.; Osorio, B.; Clark, A.H.; Nachtegaal, M.; Haumann, M.; Dau, H.; et al. Tryptophan regulates Drosophila zinc stores. Proc. Natl. Acad. Sci. USA 2022, 119, e2117807119. [Google Scholar] [CrossRef]
- Croce, A.C.; Scolari, F. Characterization of spontaneous melanization by fluorescence spectroscopy: A basis for analytical application to biological substrates. Biology 2023, 12, 433. [Google Scholar] [CrossRef]
- Croce, A.C.; Scolari, F. The bright side of the tiger: Autofluorescence patterns in Aedes albopictus (Diptera, Culicidae) male and female mosquitoes. Molecules 2022, 27, 713. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F.; Girella, A.; Croce, A.C. Imaging and spectral analysis of autofluorescence patterns in larval head structures of mosquito vectors. Eur. J. Histochem. 2022, 66, 3462. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Gorb, S.N. Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J. Microsc. 2012, 245, 1–16. [Google Scholar] [CrossRef]
- Saltin, B.D.; Matsumura, Y.; Reid, A.; Windmill, J.F.; Gorb, S.N.; Jackson, J.C. Material stiffness variation in mosquito antennae. J. R. Soc. Interface 2019, 16, 20190049. [Google Scholar] [CrossRef] [PubMed]
- Pentzold, S.; Marion-Poll, F.; Grabe, V.; Burse, A. Autofluorescence-based identification and functional validation of antennal gustatory sensilla in a specialist leaf beetle. Front. Physiol. 2019, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.L.A.; Dias, F.; Nunes, R.D.; Pereira, L.O.; Santos, T.S.R.; Chiarini, L.B.; Ramos, T.D.; Silva-Mendes, B.J.; Perales, J.; Valente, R.H.; et al. The antioxidant role of xanthurenic acid in the Aedes aegypti midgut during digestion of a blood meal. PLoS ONE 2012, 7, e38349. [Google Scholar] [CrossRef] [PubMed]
- Maya-Maldonado, K.; Cardoso-Jaime, V.; González-Olvera, G.; Osorio, B.; Recio-Tótoro, B.; Manrique-Saide, P.; Rodríguez-Sánchez, I.P.; Lanz-Mendoza, H.; Missirlis, F.; Hernández-Hernández, F.d.l.C. Mosquito metallomics reveal copper and iron as critical factors for Plasmodium infection. PLoS Negl. Trop. Dis. 2021, 15, e0009509. [Google Scholar] [CrossRef]
- Esquivel, C.J.; Cassone, B.J.; Piermarini, P.M. A de novo transcriptome of the Malpighian tubules in non-blood-fed and blood-fed Asian tiger mosquitoes Aedes albopictus: Insights into diuresis, detoxification, and blood meal processing. PeerJ 2016, 2016, e1784. [Google Scholar] [CrossRef]
- Neff, D.; Frazier, S.F.; Quimby, L.; Wang, R.T.; Zill, S. Identification of resilin in the leg of cockroach, Periplaneta americana: Confirmation by a simple method using pH dependence of UV fluorescence. Arthropod Struct. Dev. 2000, 29, 75–83. [Google Scholar] [CrossRef]
- Cohen, E. Chitin synthesis and inhibition: A revisit. Pest. Manag. Sci. 2001, 57, 946–950. [Google Scholar] [CrossRef]
- Schaub, G.A.; Schnitker, A. Influence of Blastocrithidia triatomae (Trypanosomatidae) on the reduviid bug Triatoma infestans: Alterations in the Malpighian tubules. Parasitol. Res. 1988, 75, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Vega, L.; Mikó, I. Know your insect: Malpighian tubules in Trichoplusia ni (Lepidoptera: Noctuidae). Res. Ideas Outcomes 2017, 3, e11827. [Google Scholar] [CrossRef][Green Version]
- Mello, M.L.S.; Vidal, B.D. Microspectrofluorimetry of the naturally fluorescent substances of the Malpighian tubules of Triatoma infestans and Panstrongylus megistus. Acta Histochem. Cytoc. 1985, 18, 365–373. [Google Scholar] [CrossRef]
- Cabrero, P.; Terhzaz, S.; Dornan, A.J.; Ghimire, S.; Holmes, H.L.; Turin, D.R.; Romero, M.F.; Davies, S.A.; Dow, J.A.T. Specialized stellate cells offer a privileged route for rapid water flux in Drosophila renal tubule. Proc. Natl. Acad. Sci. USA 2020, 117, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.K.; Tapadia, M.G. Ecdysone signaling is required for proper organization and fluid secretion of stellate cells in the Malpighian tubules of Drosophila melanogaster. Int. J. Dev. Biol. 2010, 54, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Piermarini, P.M.; Dunemann, S.M.; Rouhier, M.F.; Calkins, T.L.; Raphemot, R.; Denton, J.S.; Hine, R.M.; Beyenbach, K.W. Localization and role of inward rectifier K+ channels in Malpighian tubules of the yellow fever mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2015, 67, 59–73. [Google Scholar] [CrossRef]
- Armstrong, D.; Wilhelm, J.; Smid, F.; Elleder, M. Chromatography and spectrofluorometry of brain fluorophores in neuronal ceroid lipofuscinosis (NCL). Mech. Ageing Dev. 1992, 64, 293–302. [Google Scholar] [CrossRef]
- Kim, C.E.; Park, K.B.; Ko, H.J.; Keshavarz, M.; Bae, Y.M.; Kim, B.; Patnaik, B.B.; Jang, H.A.; Lee, Y.S.; Han, Y.S.; et al. Aedes albopictus autophagy-related gene 8 (AaAtg8) is required to confer anti-bacterial gut immunity. Int. J. Mol. Sci. 2020, 21, 2944. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, J.; Zhang, D.; Zhong, C.; Wang, S.; Li, X.; Feng, L.; Shi, S.; Wang, B.; Liu, Q. Systematic identification of autophagy-related proteins in Aedes albopictus. PLoS ONE 2021, 16, e0245694. [Google Scholar] [CrossRef]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef]
- Weiss, B.L.; Wang, J.; Aksoy, S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011, 9, e1000619. [Google Scholar] [CrossRef]
- Kajla, M.; Gupta, K.; Gupta, L.; Kumar, S. A Fine-tuned management between physiology and immunity maintains the gut microbiota in insects. Biochem. Physiol. 2015, 4, 2. [Google Scholar] [CrossRef]
- Kumar, S.; Molina-Cruz, A.; Gupta, L.; Rodrigues, J.; Barillas-Mury, C. A Peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 2010, 327, 1644–1648. [Google Scholar] [CrossRef]
- Kajla, M.; Choudhury, T.P.; Kakani, P.; Gupta, K.; Dhawan, R.; Gupta, L.; Kumar, S. Silencing of Anopheles stephensi heme peroxidase HPX15 activates diverse immune pathways to regulate the growth of midgut bacteria. Front. Microbiol. 2016, 7, 217893. [Google Scholar] [CrossRef]
- Seabourn, P.S.; Weber, D.E.; Spafford, H.; Medeiros, M.C.I. Aedes albopictus microbiome derives from environmental sources and partitions across distinct host tissues. Microbiologyopen 2023, 12, e1364. [Google Scholar] [CrossRef]
- Dontsov, A.E.; Sakina, N.L.; Yakovleva, M.A.; Bastrakov, A.I.; Bastrakova, I.G.; Zagorinsky, A.A.; Ushakova, N.A.; Feldman, T.B.; Ostrovsky, M.A. Ommochromes from the compound eyes of insects: Physicochemical properties and antioxidant activity. Biochemistry 2020, 85, 668–678. [Google Scholar] [CrossRef]
- Campos-Blázquez, J.P.; Schuth, N.; Garay, E.; Clark, A.H.; Vogelsang, U.; Nachtegaal, M.; Contreras, R.G.; Quintanar, L.; Missirlis, F. Chloroquine disrupts zinc storage granules in primary Malpighian tubule cells of Drosophila melanogaster. Metallomics 2022, 14, 75. [Google Scholar] [CrossRef]
- Merritt, R.W.; Dadd, R.H.; Walker, E.D. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 1992, 37, 349–376. [Google Scholar] [CrossRef]
- Pudar, D.; Puggioli, A.; Balestrino, F.; Sy, V.; Carrieri, M.; Bellini, R.; Petrić, D. Effect of cage size on Aedes albopictus wing length, survival and egg production. Heliyon 2021, 7, e07381. [Google Scholar] [CrossRef]
- Gomulski, L.M.; Mariconti, M.; Di Cosimo, A.; Scolari, F.; Manni, M.; Savini, G.; Malacrida, A.R.; Gasperi, G. The Nix locus on the male-specific homologue of chromosome 1 in Aedes albopictus is a strong candidate for a male-determining factor. Parasit. Vectors 2018, 11, 85–95. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croce, A.C.; Garbelli, A.; Moyano, A.; Soldano, S.; Tejeda-Guzmán, C.; Missirlis, F.; Scolari, F. Developmental and Nutritional Dynamics of Malpighian Tubule Autofluorescence in the Asian Tiger Mosquito Aedes albopictus. Int. J. Mol. Sci. 2024, 25, 245. https://doi.org/10.3390/ijms25010245
Croce AC, Garbelli A, Moyano A, Soldano S, Tejeda-Guzmán C, Missirlis F, Scolari F. Developmental and Nutritional Dynamics of Malpighian Tubule Autofluorescence in the Asian Tiger Mosquito Aedes albopictus. International Journal of Molecular Sciences. 2024; 25(1):245. https://doi.org/10.3390/ijms25010245
Chicago/Turabian StyleCroce, Anna Cleta, Anna Garbelli, Andrea Moyano, Sara Soldano, Carlos Tejeda-Guzmán, Fanis Missirlis, and Francesca Scolari. 2024. "Developmental and Nutritional Dynamics of Malpighian Tubule Autofluorescence in the Asian Tiger Mosquito Aedes albopictus" International Journal of Molecular Sciences 25, no. 1: 245. https://doi.org/10.3390/ijms25010245
APA StyleCroce, A. C., Garbelli, A., Moyano, A., Soldano, S., Tejeda-Guzmán, C., Missirlis, F., & Scolari, F. (2024). Developmental and Nutritional Dynamics of Malpighian Tubule Autofluorescence in the Asian Tiger Mosquito Aedes albopictus. International Journal of Molecular Sciences, 25(1), 245. https://doi.org/10.3390/ijms25010245








