Distinct Metabolic Profiles of Ocular Hypertensives in Response to Hypoxia
Abstract
1. Introduction
2. Results
- Age, BMI, and Sex: Ns
- IOP OD: Control vs. NTG (ns); Control vs. OHT (p < 0.0001); NTG vs. OHT (p < 0.0001)
- IOP OS: Control vs. NTG (ns); Control vs. OHT (p < 0.0001); NTG vs. OHT (p < 0.0001)
- MD OD: Control vs. NTG (p < 0.01); Control vs. OHT (ns); NTG vs. OHT (p < 0.05)
- MD OS: Control vs. NTG (p < 0.0001); Control vs. OHT (ns); NTG vs. OHT (p < 0.0001)
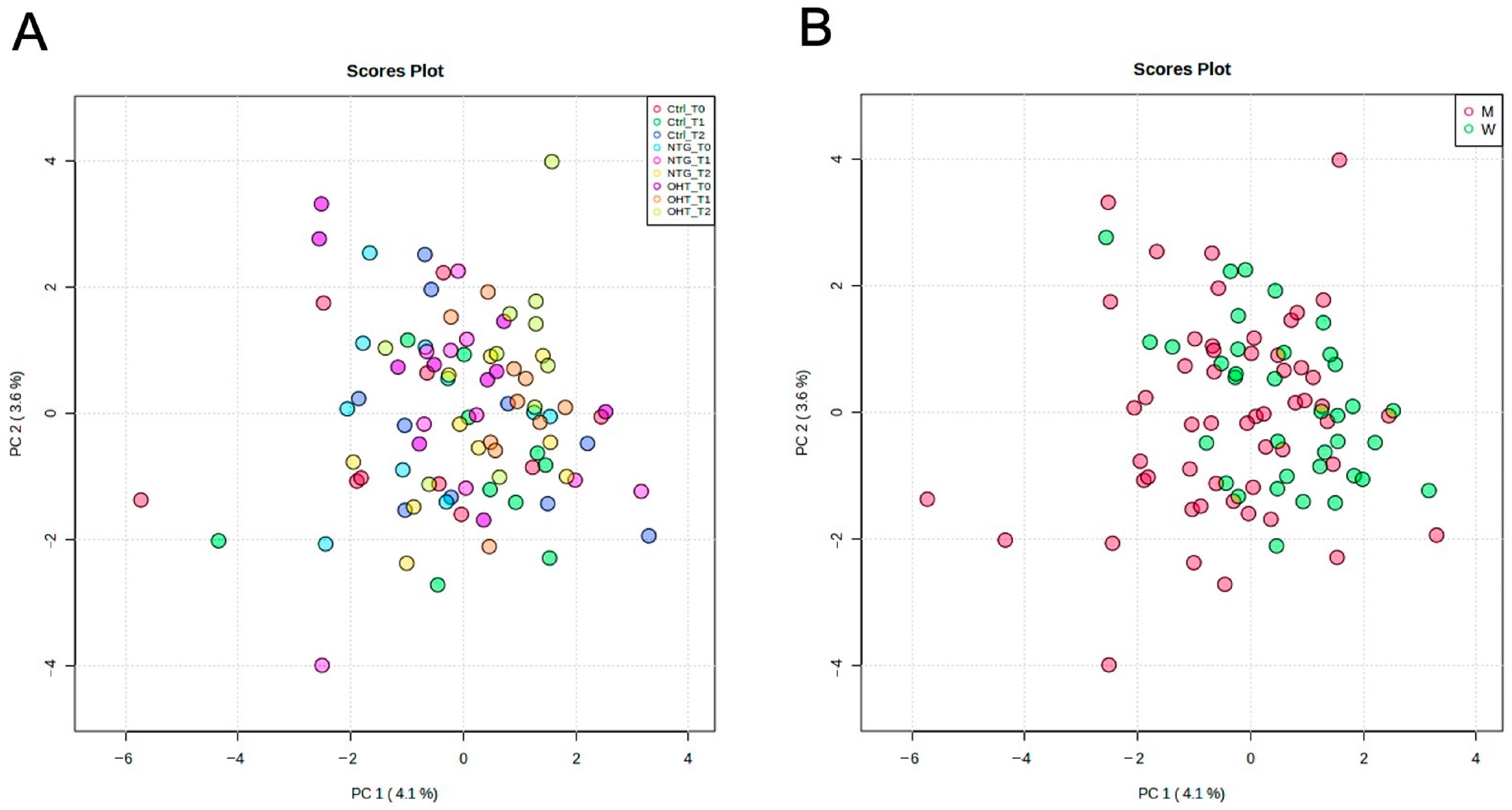
2.1. Metabolite Detection
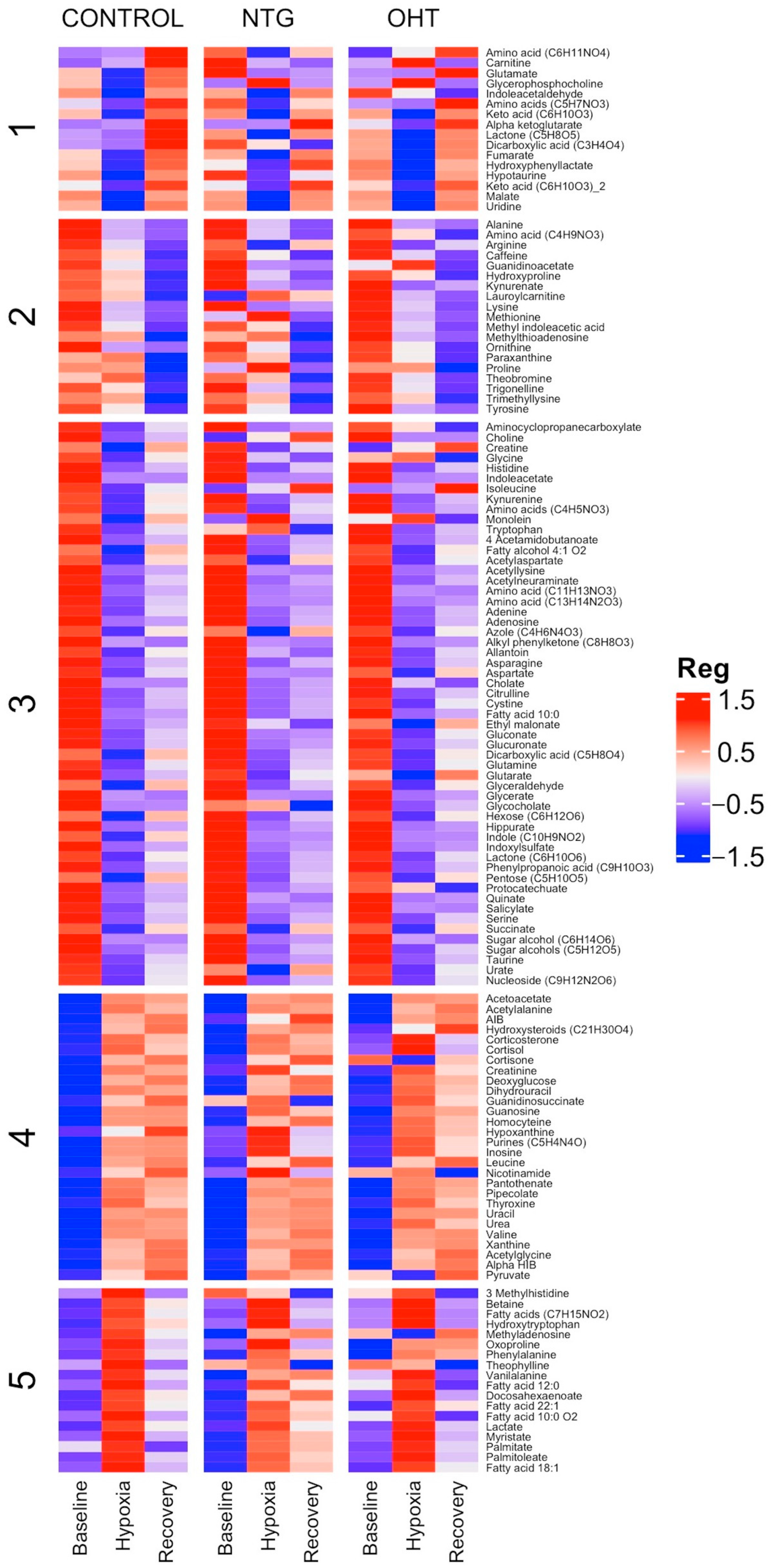
2.2. Signatures of Hypoxia
2.3. Baseline vs. Hypoxia
2.4. Hypoxia vs. Recovery
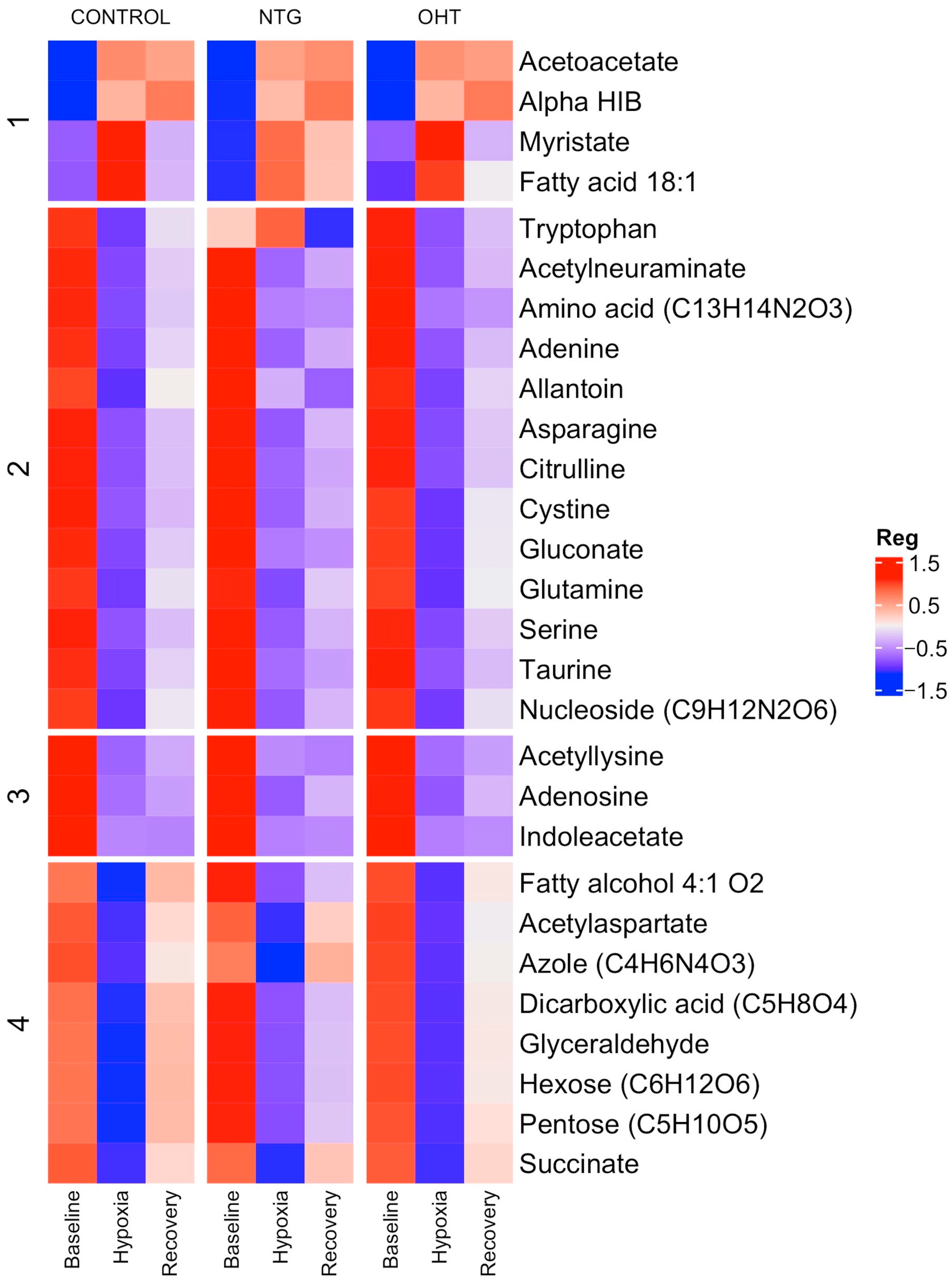
2.5. Time Point Comparisons
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Recruitment of Participants
4.3. Inclusion Criteria
4.3.1. NTG
4.3.2. OHT
4.3.3. Control Group
4.4. Exclusion Criteria
4.5. Hypoxia Model and Plasma Collection
4.6. Chemicals and Reagents
4.7. Metabolomic Analysis
4.8. Sample and Metabolite Extraction
4.9. Statistical Power
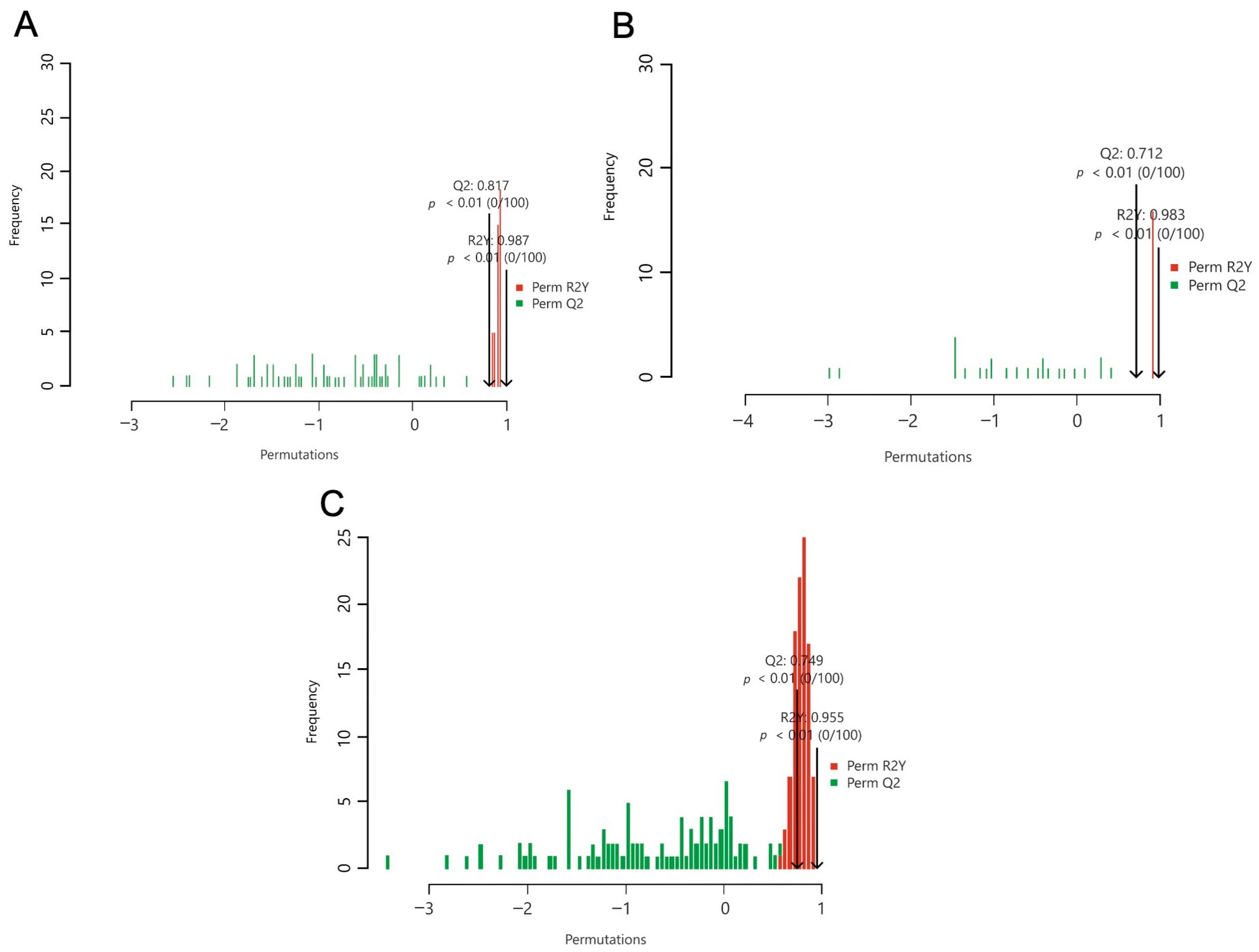
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapetanakis, V.V.; Chan, M.P.; Foster, P.J.; Cook, D.G.; Owen, C.G.; Rudnicka, A.R. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): A systematic review and meta-analysis. Br. J. Ophthalmol. 2016, 100, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.; Mullany, S.; Qassim, A.; Siggs, O.; Hassall, M.; Ridge, B.; Nguyen, T.; Awadalla, M.; Andrew, N.H.; Healey, P.R.; et al. Cardiovascular Disease Predicts Structural and Functional Progression in Early Glaucoma. Ophthalmology 2021, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Hu, H.Y.; Chu, D.; Chen, H.H.; Chang, C.K.; Chou, P. Patients with Primary Open-Angle Glaucoma May Develop Ischemic Heart Disease More Often than Those without Glaucoma: An 11-Year Population-Based Cohort Study. PLoS ONE 2016, 11, e0163210. [Google Scholar] [CrossRef]
- Cvenkel, B.; Kolko, M. Current Medical Therapy and Future Trends in the Management of Glaucoma Treatment. J. Ophthalmol. 2020, 2020, 6138132. [Google Scholar] [CrossRef]
- Dascalu, A.M.; Stana, D.; Nicolae, V.A.; Cirstoveanu, C.; Vancea, G.; Serban, D.; Socea, B. Association between vascular comorbidity and glaucoma progression: A four-year observational study. Exp. Ther. Med. 2021, 21, 283. [Google Scholar] [CrossRef]
- Han, X.; Yang, T.; Zhang, J.; Yu, S.; Guo, X.; Yan, W.; Hu, Y.; He, M. Longitudinal changes in intraocular pressure and association with systemic factors and refractive error: Lingtou Eye Cohort Study. BMJ Open 2018, 8, e019416. [Google Scholar] [CrossRef]
- He, Z.; Vingrys, A.J.; Armitage, J.A.; Bui, B.V. The role of blood pressure in glaucoma. Clin. Exp. Optom. 2011, 94, 133–149. [Google Scholar] [CrossRef]
- Leeman, M.; Kestelyn, P. Glaucoma and Blood Pressure. Hypertension 2019, 73, 944–950. [Google Scholar] [CrossRef]
- Reddy, A.; Halenda, K.; Cromer, P.; Chen, L.; Butler, J.; Raed, A.; Bhagatwala, J.; Sponseller, T.; Bollinger, K.; Zhu, H.; et al. The Association of Intraocular Pressure With Obesity and Cardiometabolic Risk in a Young Farmworker Population. J. Glaucoma 2021, 30, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Schmidl, D.; Garhofer, G.; Schmetterer, L. The complex interaction between ocular perfusion pressure and ocular blood flow—Relevance for glaucoma. Exp. Eye Res. 2011, 93, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Nemesure, B.; Hennis, A.; Leske, M.C.; Barbados Eye Studies Group. Nine-year changes in intraocular pressure: The Barbados Eye Studies. Arch. Ophthalmol. 2006, 124, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Langbøl, M.; Saruhanian, S.; Baskaran, T.; Tiedemann, D.; Mouhammad, Z.A.; Toft-Kehler, A.K.; Jun, B.; Vohra, R.; Bazan, N.G.; Kolko, M. Increased Antioxidant Capacity and Pro-Homeostatic Lipid Mediators in Ocular Hypertension-A Human Experimental Model. J. Clin. Med. 2020, 9, 2979. [Google Scholar] [CrossRef] [PubMed]
- Hui, F.; Tang, J.; Williams, P.A.; McGuinness, M.B.; Hadoux, X.; Casson, R.J.; Coote, M.; Trounce, I.A.; Martin, K.R.; van Wijngaarden, P.; et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin. Exp. Ophthalmol. 2020, 48, 903–914. [Google Scholar] [CrossRef]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 2021, 83, 100916. [Google Scholar] [CrossRef]
- Vohra, R.; Tsai, J.C.; Kolko, M. The role of inflammation in the pathogenesis of glaucoma. Surv. Ophthalmol. 2013, 58, 311–320. [Google Scholar] [CrossRef]
- Schuster, A.K.; Erb, C.; Hoffmann, E.M.; Dietlein, T.; Pfeiffer, N. The Diagnosis and Treatment of Glaucoma. Dtsch. Arztebl. Int. 2020, 117, 225–234. [Google Scholar] [CrossRef]
- Storgaard, L.; Tran, T.L.; Freiberg, J.C.; Hauser, A.S.; Kolko, M. Glaucoma Clinical Research: Trends in Treatment Strategies and Drug Development. Front. Med. 2021, 8, 733080. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Liu, C.J. Neuroprotection in Glaucoma: Basic Aspects and Clinical Relevance. J. Pers. Med. 2022, 12, 1884. [Google Scholar] [CrossRef]
- Allanore, Y.; Parc, C.; Monnet, D.; Brezin, A.P.; Kahan, A. Increased prevalence of ocular glaucomatous abnormalities in systemic sclerosis. Ann. Rheum. Dis. 2004, 63, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Bulotta, R.M.; Oppedisano, F.; Bosco, F.; Scarano, F.; Nucera, S.; Guarnieri, L.; Ruga, S.; Macri, R.; Caminiti, R.; et al. Potential Properties of Natural Nutraceuticals and Antioxidants in Age-Related Eye Disorders. Life 2022, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.Y.; Van Bergen, N.J.; Trounce, I.A.; Crowston, J.G. Mitochondrial dysfunction and glaucoma. J. Glaucoma 2009, 18, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jassim, A.H.; Inman, D.M.; Mitchell, C.H. Crosstalk Between Dysfunctional Mitochondria and Inflammation in Glaucomatous Neurodegeneration. Front. Pharmacol. 2021, 12, 699623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Xie, Z.; Chen, S.Y.; Zhang, X. Mitochondrial dysfunction in glaucomatous degeneration. Int. J. Ophthalmol. 2023, 16, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N. Mitochondria: Their role in ganglion cell death and survival in primary open angle glaucoma. Exp. Eye Res. 2010, 90, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, A.H.; Miao, J.H.; Sun, H.; Han, Y.; Yan, G.L.; Wu, F.F.; Wang, X.J. Metabolomics biotechnology, applications, and future trends: A systematic review. RSC Adv. 2019, 9, 37245–37257. [Google Scholar] [CrossRef]
- Burgess, L.G.; Uppal, K.; Walker, D.I.; Roberson, R.M.; Tran, V.; Parks, M.B.; Wade, E.A.; May, A.T.; Umfress, A.C.; Jarrell, K.L.; et al. Metabolome-Wide Association Study of Primary Open Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5020–5028. [Google Scholar] [CrossRef]
- Tang, Y.; Pan, Y.; Chen, Y.; Kong, X.; Chen, J.; Zhang, H.; Tang, G.; Wu, J.; Sun, X. Metabolomic Profiling of Aqueous Humor and Plasma in Primary Open Angle Glaucoma Patients Points Towards Novel Diagnostic and Therapeutic Strategy. Front. Pharmacol. 2021, 12, 621146. [Google Scholar] [CrossRef] [PubMed]
- Kouassi Nzoughet, J.; Chao de la Barca, J.M.; Guehlouz, K.; Leruez, S.; Coulbault, L.; Allouche, S.; Bocca, C.; Muller, J.; Amati-Bonneau, P.; Gohier, P.; et al. Nicotinamide Deficiency in Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Kouassi Nzoughet, J.; Guehlouz, K.; Leruez, S.; Gohier, P.; Bocca, C.; Muller, J.; Blanchet, O.; Bonneau, D.; Simard, G.; Milea, D.; et al. A Data Mining Metabolomics Exploration of Glaucoma. Metabolites 2020, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Prokosch, V. Energy Metabolism in the Inner Retina in Health and Glaucoma. Int. J. Mol. Sci. 2021, 22, 3689. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.N. Neuroinflammatory Mechanisms of Mitochondrial Dysfunction and Neurodegeneration in Glaucoma. J. Ophthalmol. 2021, 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Morales, J.; Bosley, T.M. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2533–2541. [Google Scholar] [CrossRef]
- Izzotti, A.; Sacca, S.C.; Cartiglia, C.; De Flora, S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am. J. Med. 2003, 114, 638–646. [Google Scholar] [CrossRef]
- Li, G.; Luna, C.; Liton, P.B.; Navarro, I.; Epstein, D.L.; Gonzalez, P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol. Vision. 2007, 13, 2282–2288. [Google Scholar]
- Nakazawa, T.; Nakazawa, C.; Matsubara, A.; Noda, K.; Hisatomi, T.; She, H.; Michaud, N.; Hafezi-Moghadam, A.; Miller, J.W.; Benowitz, L.I. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J. Neurosci. 2006, 26, 12633–12641. [Google Scholar] [CrossRef]
- Schild, L.; Blair, P.V.; Davis, I.W.; Baugh, S. Effect of adenine nucleotide pool size in mitochondria on intramitochondrial ATP levels. Biochim. Et Biophys. Acta 1999, 1413, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Li, L.Y.; Patil, R.V.; Wax, M.B. TNF-a and TNF-a Receptor-1 in the Retina of Normal and Glaucomatous Eyes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1787–1794. [Google Scholar]
- Tezel, G.; Yang, X.; Yang, J.; Wax, M.B. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004, 996, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Lascaratos, G.; Chau, K.Y.; Zhu, H.; Gkotsi, D.; King, R.; Gout, I.; Kamal, D.; Luthert, P.J.; Schapira, A.H.V.; Garway-Heath, D.F. Resistance to the most common optic neuropathy is associated with systemic mitochondrial efficiency. Neurobiol. Dis. 2015, 82, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M. Fatty acids. In Nutrient Metabolism, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and Cyclooxygenase Activities of Fatty Acids Found in Food. J. Agric. Food Chem. 2002, 50, 2231–2234. [Google Scholar] [CrossRef]
- Midha, A.D.; Zhou, Y.; Queliconi, B.B.; Barrios, A.M.; Fong, C.O.Y.; Blecha, J.E.; VanBrocklin, H.; Seo, Y.; Jain, I.H. Organ-Specific Fuel Rewiring in Acute and Chronic Hypoxia Redistributes Glucose and Fatty Acid Metabolism. Cell Metab. 2022, 35, 504–516. [Google Scholar] [CrossRef]
- Jankowska-Kulawy, A.; Klimaszewska-Lata, J.; Gul-Hinc, S.; Ronowska, A.; Szutowicz, A. Metabolic and Cellular Compartments of Acetyl-CoA in the Healthy and Diseased Brain. Int. J. Mol. Sci. 2022, 23, 10073. [Google Scholar] [CrossRef]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Freitas, H.R.; Ferreira, G.D.C.; Trevenzoli, I.H.; Oliveira, K.J.; de Melo Reis, R.A. Fatty Acids, Antioxidants and Physical Activity in Brain Aging. Nutrients 2017, 9, 1263. [Google Scholar] [CrossRef]
- Mylonis, I.; Simos, G.; Paraskeva, E. Hypoxia-Inducible Factors and the Regulation of Lipid Metabolism. Cells 2019, 8, 214. [Google Scholar] [CrossRef]
- Krishnan, J.; Suter, M.; Windak, R.; Krebs, T.; Felley, A.; Montessuit, C.; Tokarska-Schlattner, M.; Aasum, E.; Bogdanova, A.; Perriard, E.; et al. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 2009, 9, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.R.; Wheaton, W.W.; Jin, E.S.; Chen, P.H.; Sullivan, L.B.; Cheng, T.; Yang, Y.; Linehan, W.M.; Chandel, N.S.; DeBerardinis, R.J. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 2011, 481, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, P.A.; Yang, J.; Metelo, A.M.; Perez-Carro, R.; Baker, R.; Wang, Z.; Arreola, A.; Rathmell, W.K.; Olumi, A.; Lopez-Larrubia, P.; et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab. 2013, 17, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Tribble, J.R.; Hui, F.; Joe, M.; Bell, K.; Chrysostomou, V.; Crowston, J.G.; Williams, P.A. Targeting Diet and Exercise for Neuroprotection and Neurorecovery in Glaucoma. Cells 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Metghalchi, S.; Ponnuswamy, P.; Simon, T.; Haddad, Y.; Laurans, L.; Clement, M.; Dalloz, M.; Romain, M.; Esposito, B.; Koropoulis, V.; et al. Indoleamine 2,3-Dioxygenase Fine-Tunes Immune Homeostasis in Atherosclerosis and Colitis through Repression of Interleukin-10 Production. Cell Metab. 2015, 22, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Ikeda, Y.; Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Morikawa, S.; Nakashima, M.; Asai, T.; Matsuda, S. The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases. Int. J. Mol. Sci. 2023, 24, 5742. [Google Scholar] [CrossRef]
- Hilmas, C.; Pereira, E.F.R.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The Brain Metabolite Kynurenic Acid Inhibits a7 Nicotinic Receptor Activity and Increases Non-a7 Nicotinic Receptor Expression: Physiopathological Implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef]
- Moroni, F.; Fossati, S.; Chiarugi, A.; Cozzi, A. Kynurenic acid actions in brain and periphery. Int. Congr. Ser. 2007, 1304, 305–313. [Google Scholar] [CrossRef]
- Kaszaki, J.; Palasthy, Z.; Erczes, D.; Racz, A.; Torday, C.; Varga, G.; Vecsei, L.; Boros, M. Kynurenic acid inhibits intestinal hypermotility and xanthine oxidase activity during experimental colon obstruction in dogs. Neurogastroenterol. Motil. 2008, 20, 53–62. [Google Scholar] [CrossRef]
- Fang, Y.; Tan, J.; Zhang, Q. Signaling pathways and mechanisms of hypoxia-induced autophagy in the animal cells. Cell Biol. Int. 2015, 39, 891–898. [Google Scholar] [CrossRef]
- Schauer, R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hashimoto, A.; Izawa, M.; Miyazaki, K.; Chen, G.Y.; Takematsu, H.; Kozutsumi, Y.; Suzuki, A.; Furuhata, K.; Cheng, F.L.; et al. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. 2006, 66, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.J.; Wodschow, H.Z.; Nilsson, M.; Rungby, J. Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8767. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shan, W.; Zhu, F.; Wu, J.; Wang, Q. Ketone Bodies in Neurological Diseases: Focus on Neuroprotection and Underlying Mechanisms. Front. Neurol. 2019, 10, 585. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, K.; Przeliorz, A. The Effect of the Ketogenic Diet on the Therapy of Neurodegenerative Diseases and Its Impact on Improving Cognitive Functions. Dement. Geriatr. Cogn. Dis. Extra 2022, 12, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zarnowski, T.; Tulidowicz-Bielak, M.; Kosior-Jarecka, E.; Zarnowska, I.; Turski, W.A.; Gasior, M. A ketogenic diet may offer neuroprotection in glaucoma and mitochondrial diseases of the optic nerve. Med. Hypothesis Discov. Innov. Ophthalmol. 2012, 1, 45–49. [Google Scholar]
- Gough, S.M.; Casella, A.; Ortega, K.J.; Hackam, A.S. Neuroprotection by the Ketogenic Diet: Evidence and Controversies. Front. Nutr. 2021, 8, 782657. [Google Scholar] [CrossRef]
- Vesga-Jimenez, D.J.; Martin, C.; Barreto, G.E.; Aristizabal-Pachon, A.F.; Pinzon, A.; Gonzalez, J. Fatty Acids: An Insight into the Pathogenesis of Neurodegenerative Diseases and Therapeutic Potential. Int. J. Mol. Sci. 2022, 23, 2577. [Google Scholar] [CrossRef]
- Estes, R.E.; Lin, B.; Khera, A.; Davis, M.Y. Lipid Metabolism Influence on Neurodegenerative Disease Progression: Is the Vehicle as Important as the Cargo? Front. Mol. Neurosci. 2021, 14, 788695. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.; Zhang, L.; Fang, Y.; Zheng, Q.; Liu, X.; Yu, W.; Chen, S.; Ying, J.; Hua, F. Lipid metabolism and storage in neuroglia: Role in brain development and neurodegenerative diseases. Cell Biosci. 2022, 12, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, J.; Venneti, S.; Cross, J.R.; Takagi, T.; Bhinder, B.; Djaballah, H.; Kanai, M.; Cheng, E.H.; Judkins, A.R.; et al. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol. Cell 2014, 56, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Buurman, E.T.; Voorde, G.J.T.; Mattos, M.J.T.D. The physiological function of periplasmic glucose oxidation in phosphate-limited chemostat cultures of Klebsiella pneumoniae NCTC 418. Microbiology 1994, 140, 2451–2458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schaffer, S.W.; Shimada-Takaura, K.; Jong, C.J.; Ito, T.; Takahashi, K. Impaired energy metabolism of the taurine-deficient heart. Amino Acids 2016, 48, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.H.; Andersen, M.L.; Cornett, C.; Gradinaru, R.; Grunnet, N. A role for taurine in mitochondrial function. J. Biomed. Sci. 2010, 17, 23–31. [Google Scholar] [CrossRef]
- Qi, Q.; Li, J.; Yu, B.; Moon, J.Y.; Chai, J.C.; Merino, J.; Hu, J.; Ruiz-Canela, M.; Rebholz, C.; Wang, Z.; et al. Host and gut microbial tryptophan metabolism and type 2 diabetes: An integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut 2022, 71, 1095–1105. [Google Scholar] [CrossRef]
- Guieu, R.; Deharo, J.C.; Maille, B.; Crotti, L.; Torresani, E.; Brignole, M.; Parati, G. Adenosine and the Cardiovascular System: The Good and the Bad. J. Clin. Med. 2020, 9, 1366. [Google Scholar] [CrossRef]
- Gorlach, A. Control of adenosine transport by hypoxia. Circ. Res. 2005, 97, 1–3. [Google Scholar] [CrossRef]
- Coney, A.M.; Marshall, J.M. Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. J. Physiol. 1998, 509, 507–518. [Google Scholar] [CrossRef]
- Feoktistov, I.; Ryzhov, S.; Zhong, H.; Goldstein, A.E.; Matafonov, A.; Zeng, D.; Biaggioni, I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension 2004, 44, 649–654. [Google Scholar] [CrossRef]
- Kozlik, P.; Hasikova, L.; Stiburkova, B.; Zavada, J.; Kalikova, K. Rapid and reliable HILIC-MS/MS method for monitoring allantoin as a biomarker of oxidative stress. Anal. Biochem. 2020, 589, 113509–113514. [Google Scholar] [CrossRef] [PubMed]
- Kand’ar, R.; Zakova, P. Allantoin as a marker of oxidative stress in human erythrocytes. Clin. Chem. Lab. Med. 2008, 46, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; El Masri, Y.; Turkieh, A.; Amouyel, P.; Pinet, F.; Annicotte, J.S. Cardiac Acetylation in Metabolic Diseases. Biomedicines 2022, 10, 1834. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Pathek, R.R.; Mujtaba, S. The biology of lysine acetylation integrates transcriptional programming and metabolism. Nutr. Metab. 2011, 8, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Baeza, J.; Smallegan, M.J.; Denu, J.M. Site-specific reactivity of nonenzymatic lysine acetylation. ACS Chem. Biol. 2015, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Vohra, R.; Dalgaard, L.M.; Vibæk, J.; Langbol, M.A.; Bergersen, L.H.; Olsen, N.V.; Hassel, B.; Chaudhry, F.A.; Kolko, M. Potential metabolic markers in glaucoma and their regulation in response to hypoxia. Acta Ophthalmol. 2019, 97, 567–576. [Google Scholar] [CrossRef]
- Akbar, M.; Essa, M.M.; Daradkeh, G.; Abdelmegeed, M.A.; Choi, Y.; Mahmood, L.; Song, B.J. Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res. 2016, 1637, 34–55. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Shen, W.C.; Huang, B.Q.; Yang, J. Regulatory mechanisms of retinal ganglion cell death in normal tension glaucoma and potential therapies. Neural Regen. Res. 2023, 18, 87–93. [Google Scholar] [CrossRef]
- Piotrowska-Nowak, A.; Kosior-Jarecka, E.; Schab, A.; Wrobel-Dudzinska, D.; Bartnik, E.; Zarnowski, T.; Tonska, K. Investigation of whole mitochondrial genome variation in normal tension glaucoma. Exp. Eye Res. 2019, 178, 186–197. [Google Scholar] [CrossRef]
- Hara, Y.; Kume, S.; Kataoka, Y.; Watanabe, N. Changes in TCA cycle and TCA cycle-related metabolites in plasma upon citric acid administration in rats. Heliyon 2021, 7, 8501–8507. [Google Scholar] [CrossRef] [PubMed]
- Beetsch, J.W.; Olson, J.E. Taurine synthesis and cysteine metabolism in cultured rat astrocytes: Effects of hyperosmotic exposure. Am. J. Physiol. 1998, 274, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Hagopian, K.; Ramsey, J.J.; Weindruch, R. Enzymes of glycerol and glyceraldehyde metabolism in mouse liver: Effects of caloric restriction and age on activities. Biosci. Rep. 2008, 28, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.R.; Arun, P.; Ariyannur, P.S.; Namboodiri, A.M. N-Acetylaspartate reductions in brain injury: Impact on post-injury neuroenergetics, lipid synthesis, and protein acetylation. Front. Neuroenergetics 2013, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Hofer, D.C.; Zirkovits, G.; Pelzmann, H.J.; Huber, K.; Pessentheiner, A.R.; Xia, W.; Uno, K.; Miyazaki, T.; Kon, K.; Tsuneki, H.; et al. N-acetylaspartate availability is essential for juvenile survival on fat-free diet and determines metabolic health. FASEB J. 2019, 33, 13808–13824. [Google Scholar] [CrossRef] [PubMed]
- Fendt, S.M.; Verstreken, P. Neurons eat glutamate to stay alive. J. Cell Biol. 2017, 216, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Gotoh, T. Arginine metabolic enzymes, nitric oxide and infection. J. Nutr. 2004, 134, 2820–2825. [Google Scholar] [CrossRef]
- Cobb, J.; Eckhart, A.; Motsinger-Reif, A.; Carr, B.; Groop, L.; Ferrannini, E. alpha-Hydroxybutyric Acid Is a Selective Metabolite Biomarker of Impaired Glucose Tolerance. Diabetes Care 2016, 39, 988–995. [Google Scholar] [CrossRef]
- Trico, D.; Prinsen, H.; Giannini, C.; de Graaf, R.; Juchem, C.; Li, F.; Caprio, S.; Santoro, N.; Herzog, R.I. Elevated alpha-Hydroxybutyrate and Branched-Chain Amino Acid Levels Predict Deterioration of Glycemic Control in Adolescents. J. Clin. Endocrinol. Metab. 2017, 102, 2473–2481. [Google Scholar] [CrossRef]
- Harizman, N.; Oliveira, C.; Chiang, A.; Tello, C.; Marmor, M.; Ritch, R.; Liebmann, J.M. The ISNT rule and differentiation of normal from glaucomatous eyes. Arch. Ophthalmol. 2006, 124, 1579–1583. [Google Scholar] [CrossRef]
- Kouassi Nzoughet, J.; Bocca, C.; Simard, G.; Prunier-Mirebeau, D.; Chao de la Barca, J.M.; Bonneau, D.; Procaccio, V.; Prunier, F.; Lenaers, G.; Reynier, P. A Nontargeted UHPLC-HRMS Metabolomics Pipeline for Metabolite Identification: Application to Cardiac Remote Ischemic Preconditioning. Anal. Chem. 2017, 89, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Bocca, C.; Kouassi Nzoughet, J.; Leruez, S.; Amati-Bonneau, P.; Ferre, M.; Kane, M.S.; Veyrat-Durebex, C.; Chao de la Barca, J.M.; Chevrollier, A.; Homedan, C.; et al. A Plasma Metabolomic Signature Involving Purine Metabolism in Human Optic Atrophy 1 (OPA1)-Related Disorders. Investig. Ophthalmol. Vis. Sci. 2018, 59, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Klein, R.; Klein, B.E.; Meuer, S.M.; Hubbard, L.D. Retinal vessel diameters and their associations with age and blood pressure. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4644–4650. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
| Control | NTG | OHT | |
|---|---|---|---|
| N | 10 | 10 | 10 |
| Age (years) | 67 (8.1) | 73 (6.8) | 72 (4.3) |
| BMI | 27.3 (7.1) | 24.0 (2.6) | 25.3 (2.4) |
| Sex (men/women) | 7/3 | 6/4 | 5/5 |
| IOP OD (mmHg) | 13.6 (1.8) | 12.1 (2.0) | 29.3 (5.7) |
| IOP OS (mmHg) | 13.6 (2.0) | 12.5 (2.4) | 31.2 (5.5) |
| MD OD (dB) | 0.9 (1.6) | 7.2 (7.0) | 1.4 (1.7) |
| MD OS (dB) | 1.0 (2.6) | 11.1 (6.4) | 0.9 (1.4) |
| Control | NTG | OHT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite | FC | FDR | VIP | O-VIP | FC | FDR | VIP | O-VIP | FC | FDR | VIP | O-VIP |
| Acetylaspartate | 0.7105 | 0.0278 | 1.7095 | 0.6008 | 0.7641 | 0.0258 | 0.8629 | 0.6428 | 0.7239 | 0.0119 | 1.7776 | 0.9227 |
| Adenine | 0.7149 | 0.0029 | 1.9385 | 0.6912 | 0.7845 | 0.0110 | 1.9661 | 0.7799 | 0.7948 | 0.0119 | 1.8624 | 0.9491 |
| Alpha HIB | 1.7137 | 0.0110 | 1.8708 | 0.6450 | 1.5854 | 0.0083 | 2.1538 | 0.4206 | 1.6238 | 0.0119 | 1.9119 | 0.6514 |
| Citrulline | 0.6038 | 0.0074 | 1.8991 | 0.8186 | 0.6123 | 0.0326 | 1.8805 | 0.8689 | 0.6678 | 0.0119 | 1.9123 | 0.8883 |
| Glutamine | 0.7188 | 0.0070 | 1.9642 | 0.9315 | 0.7405 | 0.0044 | 2.3512 | 0.7687 | 0.7627 | 0.0119 | 1.7434 | 0.9683 |
| Allantoin | 0.6548 | 0.0186 | 1.7486 | 0.6592 | ||||||||
| Azole (C4H6N4O3) | 0.8270 | 0.0261 | 1.6694 | 0.8197 | ||||||||
| Gluconate | 0.8071 | 0.0405 | 1.6574 | 0.7318 | ||||||||
| Indoleacetate | 0.5937 | 0.0278 | 1.5122 | 1.0439 | ||||||||
| Acetoacetate | 3.2305 | 0.0119 | 1.8952 | 0.4330 | ||||||||
| Acetylneuraminate | 0.8243 | 0.0119 | 1.8315 | 0.9941 | ||||||||
| Fatty acid 18:1 | 1.3764 | 0.0189 | 1.7271 | 1.0603 | ||||||||
| Myristate | 1.3488 | 0.0119 | 1.9050 | 0.5487 | ||||||||
| Tryptophan | 0.8775 | 0.0103 | 2.0147 | 0.4868 | ||||||||
| Cystine | 0.6916 | 0.0070 | 1.8868 | 0.0800 | 0.6626 | 0.0044 | 2.2478 | 0.6675 | ||||
| Dicarboxylic acid (C5H8O4) | 0.8970 | 0.0405 | 1.5093 | 1.0341 | 0.8358 | 0.0083 | 2.0548 | 0.6598 | ||||
| Fatty alcohol 4.1 O2 | 0.8933 | 0.0278 | 1.5150 | 1.0399 | 0.8319 | 0.0083 | 2.0977 | 0.6549 | ||||
| Glyceraldehyde | 0.8881 | 0.0405 | 1.5000 | 1.0871 | 0.8189 | 0.0044 | 2.1532 | 0.6640 | ||||
| Hexose (C6H12O6) | 0.8961 | 0.0261 | 1.4972 | 0.9774 | 0.8320 | 0.0083 | 2.1303 | 0.6301 | ||||
| Pentose (C5H10O5) | 0.8840 | 0.0278 | 1.6628 | 1.0000 | 0.8280 | 0.0044 | 2.1664 | 0.6613 | ||||
| Serine | 0.7051 | 0.0219 | 1.6542 | 1.0954 | 0.7576 | 0.0205 | 1.8690 | 1.0349 | ||||
| Acetyllysine | 0.6862 | 0.0186 | 1.7629 | 1.1218 | 0.7376 | 0.0119 | 1.8707 | 0.8031 | ||||
| Adenosine | 0.8066 | 0.0405 | 1.5172 | 1.0711 | 0.7683 | 0.0119 | 1.7795 | 0.8201 | ||||
| Amino acid (C13H14N2O3) | 0.5119 | 0.0219 | 1.6143 | 0.5305 | 0.5210 | 0.0157 | 1.5710 | 0.9532 | ||||
| Asparagine | 0.6709 | 0.0095 | 1.7561 | 1.1434 | 0.7338 | 0.0311 | 1.7116 | 0.9124 | ||||
| Nucleoside (C9H12N2O6) | 0.8357 | 0.0110 | 1.8266 | 0.4729 | 0.8461 | 0.0189 | 1.7018 | 0.9529 | ||||
| Succinate | 0.6415 | 0.0074 | 1.8306 | 0.6296 | 0.6895 | 0.0119 | 1.9347 | 0.9058 | ||||
| Taurine | 0.7413 | 0.0029 | 2.0438 | 0.3861 | 0.7927 | 0.0245 | 1.5723 | 0.9030 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langbøl, M.; Rovelt, J.; Saruhanian, A.; Saruhanian, S.; Tiedemann, D.; Baskaran, T.; Bocca, C.; Vohra, R.; Cvenkel, B.; Lenaers, G.; et al. Distinct Metabolic Profiles of Ocular Hypertensives in Response to Hypoxia. Int. J. Mol. Sci. 2024, 25, 195. https://doi.org/10.3390/ijms25010195
Langbøl M, Rovelt J, Saruhanian A, Saruhanian S, Tiedemann D, Baskaran T, Bocca C, Vohra R, Cvenkel B, Lenaers G, et al. Distinct Metabolic Profiles of Ocular Hypertensives in Response to Hypoxia. International Journal of Molecular Sciences. 2024; 25(1):195. https://doi.org/10.3390/ijms25010195
Chicago/Turabian StyleLangbøl, Mia, Jens Rovelt, Arevak Saruhanian, Sarkis Saruhanian, Daniel Tiedemann, Thisayini Baskaran, Cinzia Bocca, Rupali Vohra, Barbara Cvenkel, Guy Lenaers, and et al. 2024. "Distinct Metabolic Profiles of Ocular Hypertensives in Response to Hypoxia" International Journal of Molecular Sciences 25, no. 1: 195. https://doi.org/10.3390/ijms25010195
APA StyleLangbøl, M., Rovelt, J., Saruhanian, A., Saruhanian, S., Tiedemann, D., Baskaran, T., Bocca, C., Vohra, R., Cvenkel, B., Lenaers, G., & Kolko, M. (2024). Distinct Metabolic Profiles of Ocular Hypertensives in Response to Hypoxia. International Journal of Molecular Sciences, 25(1), 195. https://doi.org/10.3390/ijms25010195







