The Molecular Basis of Male Infertility in Obesity: A Literature Review
Abstract
1. Introduction
2. Endocrine Changes
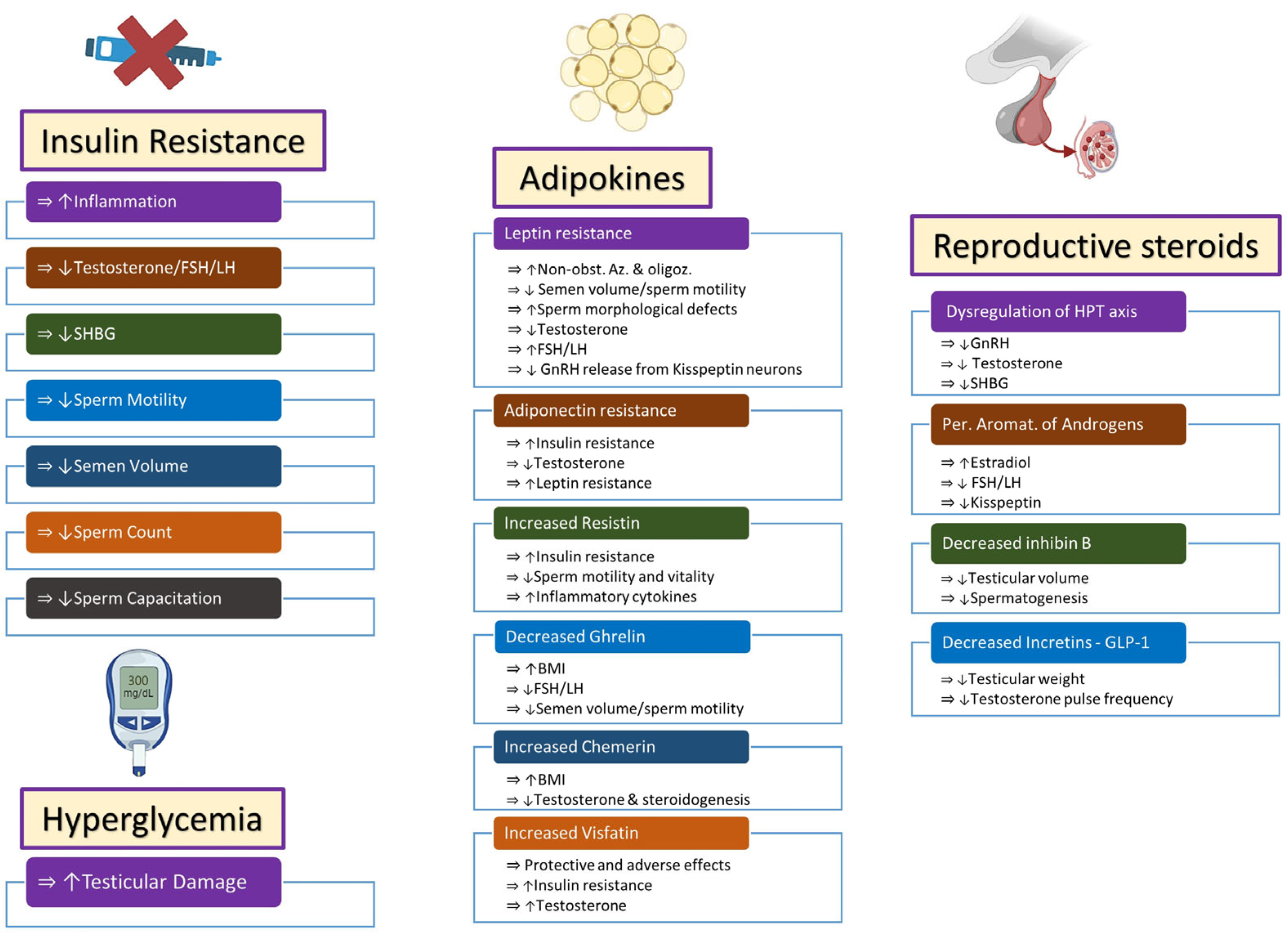
2.1. Insulin Resistance and Hyperglycemia
2.1.1. Insulin Resistance
2.1.2. Hyperglycemia
2.2. Adipokines
2.2.1. Leptin
2.2.2. Adiponectin
2.2.3. Resistin
2.2.4. Ghrelin
2.2.5. Chemerin
2.2.6. Visfatin
2.2.7. Apelin, Omentin, Hepcidin, and Vaspin
2.3. Reproductive Steroids
2.3.1. Hypothalamic–Pituitary–Testicular Axis
2.3.2. Estrogens
2.3.3. Inhibin B
2.4. Incretins
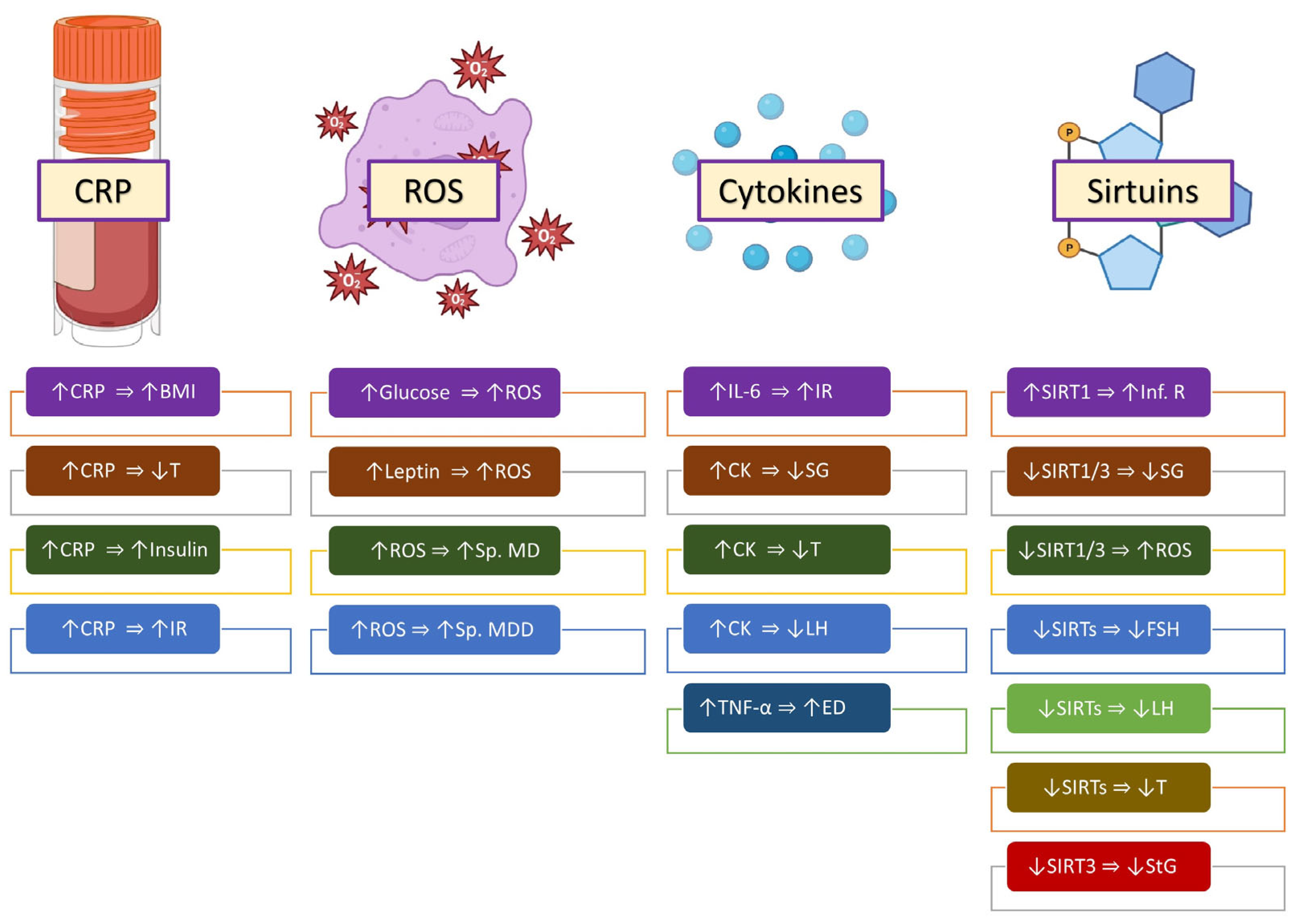
3. Inflammatory Molecules
3.1. C-Reactive Protein
3.2. Reactive Oxygen Species
3.3. Cytokines
3.4. Sirtuins
4. Intracellular Factors
4.1. Fatty Acids and Male Subfertility
4.2. Cholesterol and Sperm Capacitation
5. Obesity and Epigenetic Alterations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, R.; Haque, M. Obesity: A Doorway to a Molecular Path Leading to Infertility. Cureus 2022, 14, e30770. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 October 2023).
- Liu, B.; Du, Y.; Wu, Y.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Trends in obesity and adiposity measures by race or ethnicity among adults in the United States 2011-18: Population based study. BMJ 2021, 372, n365. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Cimino, L.; Magagnini, M.C.; Crafa, A.; La Vignera, S.; Calogero, A.E. Molecular Mechanisms Underlying the Relationship between Obesity and Male Infertility. Metabolites 2021, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Richard, R.A. The impact of obesity on male fertility. Hormones 2015, 14, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.E.; Brannigan, R.E. Obesity and male infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Elkin, E.P.; Fenster, L. The question of declining sperm density revisited: An analysis of 101 studies published 1934–1996. Environ. Health Perspect. 2000, 108, 961–966. [Google Scholar] [CrossRef]
- Sermondade, N.; Faure, C.; Fezeu, L.; Lévy, R.; Czernichow, S. Obesity and increased risk for oligozoospermia and azoospermia. Arch. Intern. Med. 2012, 172, 440–442. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, male infertility, and the sperm epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.G.; Jesus, T.T.; Sousa, M.; Goldberg, E.; Silva, B.M.; Oliveira, P.F. Male fertility and obesity: Are ghrelin, leptin and glucagon-like peptide-1 pharmacologically relevant? Curr. Pharm. Des. 2016, 22, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Pollet-Villard, X.; Reverchon, M.; Mellouk, N.; Levy, R. Adipokines in human reproduction. Horm. Mol. Biol. Clin. Investig. 2015, 24, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Kawwass, J.F.; Summer, R.; Kallen, C.B. Direct effects of leptin and adiponectin on peripheral reproductive tissues: A critical review. Mol. Hum. Reprod. 2015, 21, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, H.; Horie, H.; Inoue, T.; Kameyama, M.; Kanao, K.; Ishihara, S.; Tsujimura, T.; Nunotani, H.; Minami, T.; Okazaki, Y.; et al. Low testosterone levels in diabetic men and animals: A possible role in testicular impotence. Diabetes Res. Clin. Pract. 1989, 6, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Carrageta, D.F.; Oliveira, P.F.; Alves, M.G.; Monteiro, M.P. Obesity and male hypogonadism: Tales of a vicious cycle. Obes. Rev. 2019, 20, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Mongioì, L.M.; Garofalo, V.; Leanza, C.; Marino, M.; Calogero, A.E.; Condorelli, R.A. Obesity and Male Reproduction: Do Sirtuins Play a Role? Int. J. Mol. Sci. 2022, 23, 973. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.D.; Majzoub, A.; Agawal, A. Metabolic Syndrome and Male Fertility. World J. Mens. Health 2019, 37, 113–127. [Google Scholar] [CrossRef]
- Zańko, A.; Siewko, K.; Krętowski, A.J.; Milewski, R. Lifestyle, Insulin Resistance and Semen Quality as Co-Dependent Factors of Male Infertility. Int. J. Environ. Res. Public Health 2022, 20, 732. [Google Scholar] [CrossRef]
- Ma, J.; Han, R.Y.; Mei, X.A.; Qi, Y.N.; Ma, J.Y.; Liu, W.J.; Wang, S.S. Correlation of insulin resistance with male reproductive hormone levels and semen parameters. Zhonghua Nan Ke Xue 2018, 24, 695–699. [Google Scholar]
- Mansour, R.; El-Faissal, Y.; Kamel, A.; Kamal, O.; Aboulserour, G.; Aboulghar, M.; Fahmy, I. Increased insulin resistance in men with unexplained infertility. Reprod. Biomed. Online 2017, 35, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.D.; Brannigan, R.E. Metabolic syndrome and infertility in men. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 507–515. [Google Scholar] [CrossRef] [PubMed]
- AbbasiHormozi, S.; Kouhkan, A.; Shahverdi, A.; Parikar, A.; Shirin, A.; Vesali, S. How much obesity and diabetes do impair male fertility? Reprod. Biol. Endocrinol. 2023, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, A.O.; Gibson, M.; Peterson, C.M.; Meikle, A.W.; Carrell, D.T. Impact of male obesity on infertility: A critical review of the current literature. Fertil. Steril. 2008, 90, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.K.; Yang, W.X. Factors and pathways involved in capacitation: How are they regulated? Oncotarget 2017, 8, 3600–3627. [Google Scholar] [CrossRef]
- Okabe, M. Sperm-egg interaction and fertilization: Past, present, and future. Biol. Reprod. 2018, 99, 134–146. [Google Scholar] [CrossRef]
- Aquila, S.; Gentile, M.; Middea, E.; Catalano, S.; Andò, S. Autocrine regulation of insulin secretion in human ejaculated spermatozoa. Endocrinology 2005, 146, 552–557. [Google Scholar] [CrossRef]
- Aitken, R.J.; Curry, B.J.; Shokri, S.; Pujianto, D.A.; Gavriliouk, D.; Gibb, Z.; Whiting, S.; Connaughton, H.S.; Nixon, B.; Salamonsen, L.A.; et al. Evidence that extrapancreatic insulin production is involved in the mediation of sperm survival. Mol. Cell. Endocrinol. 2021, 526, 111193. [Google Scholar] [CrossRef]
- Pasquali, R.; Patton, L.; Gambineri, A. Obesity and infertility. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 482–487. [Google Scholar] [CrossRef]
- Lampiao, F.; Agarwal, A.; du Plessis, S.S. Invited review The role of insulin and leptin in male reproduction. Arch. Med. Sci. Spec. Issues 2009, 5, S48–S54. [Google Scholar]
- Maresch, C.C.; Stute, D.C.; Alves, M.G.; Oliveira, P.F.; de Kretser, D.M.; Linn, T. Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Hum. Reprod. Update 2018, 24, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Facondo, P.; Di Lodovico, E.; Delbarba, A.; Anelli, V.; Pezzaioli, L.C.; Filippini, E.; Cappelli, C.; Corona, G.; Ferlin, A. The impact of diabetes mellitus type 1 on male fertility: Systematic review and meta-analysis. Andrology 2022, 10, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.L.; Liu, Y.; Liu, M.E.; Pan, J.X.; Guo, M.X.; Sheng, J.Z.; Huang, H.F. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J. Androl. 2015, 17, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Maresch, C.C.; Stute, D.C.; Fleming, T.; Lin, J.; Hammes, H.P.; Linn, T. Hyperglycemia induces spermatogenic disruption via major pathways of diabetes pathogenesis. Sci. Rep. 2019, 9, 13074. [Google Scholar] [CrossRef] [PubMed]
- Omolaoye, T.S.; du Plessis, S.S. Male infertility: A proximate look at the advanced glycation end products. Reprod. Toxicol. 2020, 93, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Roumaud, P.; Martin, L.J. Roles of leptin, adiponectin and resistin in the transcriptional regulation of steroidogenic genes contributing to decreased Leydig cells function in obesity. Horm. Mol. Biol. Clin. Investig. 2015, 24, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Tsatsanis, C.; Dermitzaki, E.; Avgoustinaki, P.; Malliaraki, N.; Mytaras, V.; Margioris, A.N. The impact of adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG) axis. Hormones 2015, 14, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The Importance of Leptin to Reproduction. Endocrinology 2021, 162, bqaa204. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Parmar, M.; Khosravizadeh, Z.; Kuchakulla, M.; Manoharan, M.; Arora, H. The role of leptin and obesity on male infertility. Curr. Opin. Urol. 2020, 30, 334–339. [Google Scholar] [CrossRef]
- Ghaderpour, S.; Ghiasi, R.; Heydari, H.; Keyhanmanesh, R. The relation between obesity, kisspeptin, leptin, and male fertility. Horm. Mol. Biol. Clin. Investig. 2021, 43, 235–247. [Google Scholar] [CrossRef]
- Isidori, A.M.; Caprio, M.; Strollo, F.; Moretti, C.; Frajese, G.; Isidori, A.; Fabbri, A. Leptin and androgens in male obesity: Evidence for leptin contribution to reduced androgen levels. J. Clin. Endocrinol. Metab. 1999, 84, 3673–3680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, S.; Kratzsch, D.; Schaab, M.; Scholz, M.; Grunewald, S.; Thiery, J.; Paasch, U.; Kratzsch, J. Seminal plasma adipokine levels are correlated with functional characteristics of spermatozoa. Fertil. Steril. 2013, 99, 1256–1263.e3. [Google Scholar] [CrossRef] [PubMed]
- Page, S.T.; Herbst, K.L.; Amory, J.K.; Coviello, A.D.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J. Testosterone administration suppresses adiponectin levels in men. J. Androl. 2005, 26, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.; Brown, R.; Imran, S.A.; Ur, E. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology 2007, 86, 191–209. [Google Scholar] [CrossRef]
- Heydari, H.; Ghiasi, R.; Ghaderpour, S.; Keyhanmanesh, R. The Mechanisms Involved in Obesity-Induced Male Infertility. Curr. Diabetes Rev. 2021, 17, 259–267. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Liu, X. Ghrelin alleviates endoplasmic reticulum stress and inflammation-mediated reproductive dysfunction induced by stress. J. Assist. Reprod. Genet. 2019, 36, 2357–2366. [Google Scholar] [CrossRef]
- Kheradmand, A.; Dezfoulian, O.; Alirezaei, M.; Rasoulian, B. Ghrelin modulates testicular germ cells apoptosis and proliferation in adult normal rats. Biochem. Biophys. Res. Commun. 2012, 419, 299–304. [Google Scholar] [CrossRef]
- Kluge, M.; Schussler, P.; Uhr, M.; Yassouridis, A.; Steiger, A. Ghrelin suppresses secretion of luteinizing hormone in humans. J. Clin. Endocrinol. Metab. 2007, 92, 3202–3205. [Google Scholar] [CrossRef]
- Kluge, M.; Uhr, M.; Bleninger, P.; Yassouridis, A.; Steiger, A. Ghrelin suppresses secretion of FSH in males. Clin. Endocrinol. 2009, 70, 920–923. [Google Scholar] [CrossRef]
- Wang, C.; Jackson, G.; Jones, T.H.; Matsumoto, A.M.; Nehra, A.; Perelman, M.A.; Swerdloff, R.S.; Traish, A.; Zitzmann, M.; Cunningham, G. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care 2011, 34, 1669–1675. [Google Scholar] [CrossRef]
- Kısa, Ü.; Başar, M.M.; Şipal, T.; Ceylan, Ö.D. Ghrelin and orexin levels in infertile male: Evaluation of effects on varicocele pathophysiology, relationship seminal and hormonal parameter. Turk. J. Biochem. 2020, 45, 877–882. [Google Scholar] [CrossRef]
- Li, L.; Huang, C.; Zhang, X.; Wang, J.; Ma, P.; Liu, Y.; Xiao, T.; Zabel, B.A.; Zhang, J.V. Chemerin-derived peptide C-20 suppressed gonadal steroidogenesis. Am. J. Reprod. Immunol. 2014, 71, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, P.; Huang, C.; Liu, Y.; Zhang, Y.; Gao, C.; Xiao, T.; Ren, P.G.; Zabel, B.A.; Zhang, J.V. Expression of chemerin and its receptors in rat testes and its action on testosterone secretion. J. Endocrinol. 2014, 220, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yang, Y.; Huang, C.; Ge, L.; Xue, L.; Xiao, Z.; Xiao, T.; Zhao, H.; Ren, P.; Zhang, J.V. Chemerin: A Functional Adipokine in Reproductive Health and Diseases. Biomedicines 2022, 10, 1910. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Choubey, M.; Bora, P.; Krishna, A. Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reprod. Sci. 2018, 25, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Jachimowicz-Duda, O.; Slezak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Wang, W.D.; Xing, L.; Teng, J.R.; Li, S.; Mi, N.A. Effects of basal insulin application on serum visfatin and adiponectin levels in type 2 diabetes. Exp. Ther. Med. 2015, 9, 2219–2224. [Google Scholar] [CrossRef][Green Version]
- Chang, Y.C.; Chang, T.J.; Lee, W.J.; Chuang, L.M. The relationship of visfatin/pre-B-cell colony-enhancing factor/nicotinamide phosphoribosyltransferase in adipose tissue with inflammation, insulin resistance, and plasma lipids. Metabolism 2010, 59, 93–99. [Google Scholar] [CrossRef]
- Hameed, W.; Yousaf, I.; Latif, R.; Aslam, M. Effect of visfatin on testicular steroidogenesis in purified Leydig cells. J. Ayub. Med. Coll. Abbottabad 2012, 24, 62–64. [Google Scholar]
- Elfassy, Y.; Bastard, J.P.; McAvoy, C.; Fellahi, S.; Dupont, J.; Levy, R. Adipokines in Semen: Physiopathology and Effects on Spermatozoas. Int. J. Endocrinol. 2018, 2018, 3906490. [Google Scholar] [CrossRef]
- Wozniak, S.E.; Gee, L.L.; Wachtel, M.S.; Frezza, E.E. Adipose tissue: The new endocrine organ? A review article. Dig. Dis. Sci. 2009, 54, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Masaki, T.; Gotoh, K.; Chiba, S.; Katsuragi, I.; Tanaka, K.; Kakuma, T.; Yoshimatsu, H. Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology 2007, 148, 2690–2697. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Signorini, C.; Noto, D.; Tripodi, S.A.; Menchiari, A.; Sorrentino, E.; Collodel, G. Seminal Levels of Omentin-1/ITLN1 in Inflammatory Conditions Related to Male Infertility and Localization in Spermatozoa and Tissues of Male Reproductive System. J. Inflamm. Res. 2022, 15, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- de Souza Batista, C.M.; Yang, R.Z.; Lee, M.J.; Glynn, N.M.; Yu, D.Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Bekri, S.; Gual, P.; Anty, R.; Luciani, N.; Dahman, M.; Ramesh, B.; Iannelli, A.; Staccini-Myx, A.; Casanova, D.; Ben Amor, I.; et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 2006, 131, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Sokol, R.Z. Endocrinology of male infertility: Evaluation and treatment. Semin. Reprod. Med. 2009, 27, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Anawalt, B.D. Approach to Male Infertility and Induction of Spermatogenesis. J. Clin. Endocrinol. Metab. 2013, 98, 3532–3542. [Google Scholar] [CrossRef]
- Genchi, V.A.; Rossi, E.; Lauriola, C.; D’Oria, R.; Palma, G.; Borrelli, A.; Caccioppoli, C.; Giorgino, F.; Cignarelli, A. Adipose Tissue Dysfunction and Obesity-Related Male Hypogonadism. Int. J. Mol. Sci. 2022, 23, 8194. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Maghsoumi-Norouzabad, L.; James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Becerra-Tomás, N.; Javid, A.Z.; Abed, R.; Torres, P.J.; et al. Male adiposity, sperm parameters and reproductive hormones: An updated systematic review and collaborative meta-analysis. Obes. Rev. 2021, 22, e13082. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Kaufman, J.M.; Vermeulen, A. Pathogenesis of the decreased androgen levels in obese men. J. Clin. Endocrinol. Metab. 1994, 79, 997–1000. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.P.; Bélanger, A.; Dupont, A.; Prud’homme, D.; Moorjani, S.; Lupien, P.J.; Labrie, F. Reduced testosterone and adrenal C19 steroid levels in obese men. Metabolism 1995, 44, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M. Metabolic regulation of kisspeptin—The link between energy balance and reproduction. Nat. Rev. Endocrinol. 2020, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Harter, C.J.L.; Kavanagh, G.S.; Smith, J.T. The role of kisspeptin neurons in reproduction and metabolism. J. Endocrinol. 2018, 238, R173–R183. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.A.; Stanczyk, F.Z.; Sokol, R.Z. Serum inhibin B levels in males with gonadal dysfunction. Fertil. Steril. 2000, 74, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Meachem, S.J.; Nieschlag, E.; Simoni, M. Inhibin B in male reproduction: Pathophysiology and clinical relevance. Eur. J. Endocrinol. 2001, 145, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.M.; Skakkebaek, N.E. Serum inhibin B levels during male childhood and puberty. Mol. Cell. Endocrinol. 2001, 180, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Farilla, L.; Merkel, P.; Perfetti, R. The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects. Eur. J. Endocrinol. 2002, 146, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Calogero, A.E.; Condorelli, R.A.; Greco, E.A.; Aversa, A.; La Vignera, S. Is there a role for glucagon-like peptide-1 receptor agonists in the treatment of male infertility? Andrology 2021, 9, 1499–1503. [Google Scholar] [CrossRef]
- Leisegang, K.; Henkel, R.; Agarwal, A. Obesity and metabolic syndrome associated with systemic inflammation and the impact on the male reproductive system. Am. J. Reprod. Immunol. 2019, 82, e13178. [Google Scholar] [CrossRef]
- Yeap, B.B.; Knuiman, M.W.; Divitini, M.L.; Handelsman, D.J.; Beilby, J.P.; Beilin, J.; McQuillan, B.; Hung, J. Differential associations of testosterone, dihydrotestosterone and oestradiol with physical, metabolic and health-related factors in community-dwelling men aged 17-97 years from the Busselton Health Survey. Clin. Endocrinol. 2014, 81, 100–108. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Rohrmann, S.; McGlynn, K.A.; Nyante, S.J.; Lopez, D.S.; Bradwin, G.; Feinleib, M.; Joshu, C.E.; Kanarek, N.; Nelson, W.G.; et al. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology 2013, 1, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Christeff, N.; Benassayag, C.; Carli-Vielle, C.; Carli, A.; Nunez, E.A. Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. J. Steroid. Biochem. 1988, 29, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Baxter, C.R.; Parker, C.R.J. Effect of burn trauma on adrenal and testicular steroid hormone production. J. Clin. Endocrinol. Metab. 1987, 64, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J.; Wojcicka, G.; Jamroz, A. Leptin decreases plasma paraoxonase 1 (PON1) activity and induces oxidative stress: The possible novel mechanism for proatherogenic effect of chronic hyperleptinemia. Atherosclerosis 2003, 170, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction 2017, 154, R123–R131. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhao, C.; Guo, H.; Liu, T.; Li, Y.; Qi, Y.; Deussing, J.M.; Zhang, Y.; Tan, J.; Han, H.; et al. Obesity induces male mice infertility via oxidative stress, apoptosis, and glycolysis. Reproduction 2023, 166, 27–36. [Google Scholar] [CrossRef]
- Nijhawan, P.; Behl, T. Role of sirtuins in obesity. Obes. Med. 2020, 17, 100156. [Google Scholar] [CrossRef]
- Costa, C.d.S.; Hammes, T.O.; Rohden, F.; Margis, R.; Bortolotto, J.W.; Padoin, A.V.; Mottin, C.C.; Guaragna, R.M. SIRT1 Transcription Is Decreased in Visceral Adipose Tissue of Morbidly Obese Patients with Severe Hepatic Steatosis. Obes. Surg. 2010, 20, 633–639. [Google Scholar] [CrossRef]
- Petrangeli, E.; Coroniti, G.; Brini, A.T.; de Girolamo, L.; Stanco, D.; Niada, S.; Silecchia, G.; Morgante, E.; Lubrano, C.; Russo, M.A.; et al. Hypoxia Promotes the Inflammatory Response and Stemness Features in Visceral Fat Stem Cells From Obese Subjects. J. Cell. Physiol. 2016, 231, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Silva, B.M.; Sousa, M.; Oliveira, P.F. Sirtuins: Novel Players in Male Reproductive Health. Curr. Med. Chem. 2016, 23, 1084–1099. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Du, C.; Shi, Y.; Wei, J.; Wu, H.; Cui, H. The Sirt1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int. J. Mol. Med. 2017, 39, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Picardo, M.; Gandini, L.; Dondero, F. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 1996, 2, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Zubi, Z.B.H.; Alfarisi, H.A.H. Hyperlipidemia and male infertility. Egypt. J. Basic Appl. Sci. 2021, 8, 385–396. [Google Scholar] [CrossRef]
- Lewis, S.E. Is sperm evaluation useful in predicting human fertility? Reproduction 2007, 134, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hambly, B.D.; McLachlan, C.S. FTO associations with obesity and telomere length. J. Biomed. Sci. 2017, 24, 65. [Google Scholar] [CrossRef]
- Raee, P.; Shams Mofarahe, Z.; Nazarian, H.; Abdollahifar, M.A.; Ghaffari Novin, M.; Aghamiri, S.; Ghaffari Novin, M. Male obesity is associated with sperm telomere shortening and aberrant mRNA expression of autophagy-related genes. Basic Clin. Androl. 2023, 33, 13. [Google Scholar] [CrossRef]
- Li, L.; Law, C.; Lo Conte, R.; Power, C. Intergenerational influences on childhood body mass index: The effect of parental body mass index trajectories. Am. J. Clin. Nutr. 2009, 89, 551–557. [Google Scholar] [CrossRef]
- Fullston, T.; Ohlsson Teague, E.M.; Palmer, N.O.; DeBlasio, M.J.; Mitchell, M.; Corbett, M.; Print, C.G.; Owens, J.A.; Lane, M. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013, 27, 4226–4243. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L.; Strong, T.D.; Trock, B.J.; Jin, L.; Bivalacqua, T.J.; Musicki, B. Serum biomarker measurements of endothelial function and oxidative stress after daily dosing of sildenafil in type 2 diabetic men with erectile dysfunction. J. Urol. 2009, 181, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Arana Rosainz Mde, J.; Ojeda, M.O.; Acosta, J.R.; Elias-Calles, L.C.; Gonzalez, N.O.; Herrera, O.T.; Garcia Alvarez, C.T.; Rodriguez, E.M.; Baez, M.E.; Seijas, E.A.; et al. Imbalanced low-grade inflammation and endothelial activation in patients with type 2 diabetes mellitus and erectile dysfunction. J. Sex. Med. 2011, 8, 2017–2030. [Google Scholar] [CrossRef] [PubMed]
- McPherson, N.O.; Fullston, T.; Bakos, H.W.; Setchell, B.P.; Lane, M. Obese father’s metabolic state, adiposity, and reproductive capacity indicate son’s reproductive health. Fertil. Steril. 2014, 101, 865–873. [Google Scholar] [CrossRef]
- Hunter, E.; Avenell, A.; Maheshwari, A.; Stadler, G.; Best, D. The effectiveness of weight-loss lifestyle interventions for improving fertility in women and men with overweight or obesity and infertility: A systematic review update of evidence from randomized controlled trials. Obes. Rev. 2021, 22, e13325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, B.T.; Jhancy, M.; Dube, R.; Kar, S.S.; Annamma, L.M. The Molecular Basis of Male Infertility in Obesity: A Literature Review. Int. J. Mol. Sci. 2024, 25, 179. https://doi.org/10.3390/ijms25010179
George BT, Jhancy M, Dube R, Kar SS, Annamma LM. The Molecular Basis of Male Infertility in Obesity: A Literature Review. International Journal of Molecular Sciences. 2024; 25(1):179. https://doi.org/10.3390/ijms25010179
Chicago/Turabian StyleGeorge, Biji Thomas, Malay Jhancy, Rajani Dube, Subhranshu Sekhar Kar, and Lovely Muthiah Annamma. 2024. "The Molecular Basis of Male Infertility in Obesity: A Literature Review" International Journal of Molecular Sciences 25, no. 1: 179. https://doi.org/10.3390/ijms25010179
APA StyleGeorge, B. T., Jhancy, M., Dube, R., Kar, S. S., & Annamma, L. M. (2024). The Molecular Basis of Male Infertility in Obesity: A Literature Review. International Journal of Molecular Sciences, 25(1), 179. https://doi.org/10.3390/ijms25010179









