Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review
Abstract
:1. Introduction
1.1. Background
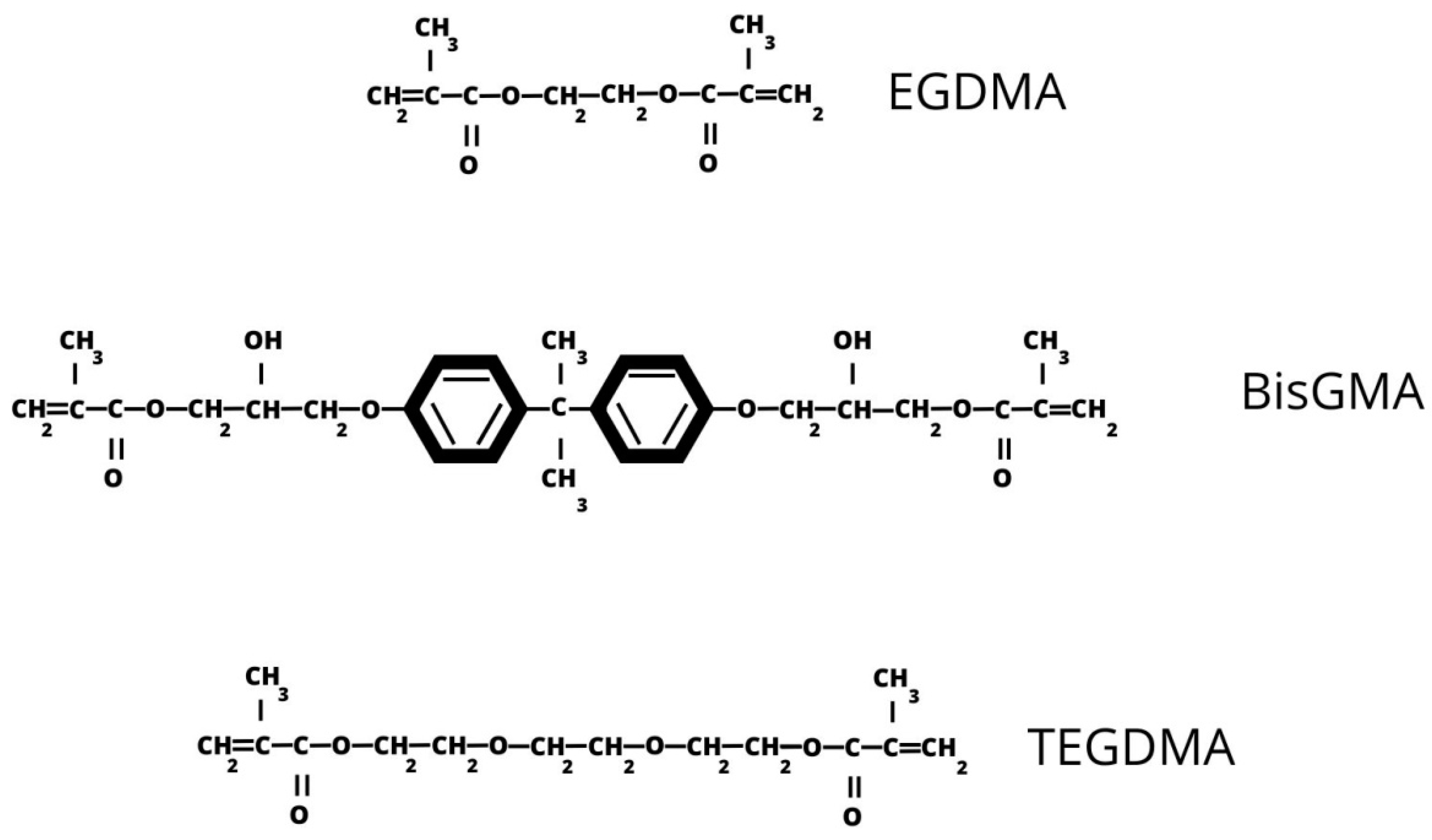
1.2. Basic Characteristics of Resin-Based Composites
1.3. Biological Properties of Resin-Based Composites
2. Methods
2.1. Research Strategy
2.2. Study Selection
2.3. Risk of Bias
3. Results
3.1. Study Selection
3.2. Risk of Bias
3.3. Cytotoxicity Results
3.4. Summary of Study Characteristics
4. Discussion
4.1. Mechanism of Toxicity
4.2. Impact on Cell Viability
4.3. Impact on Cell Cycle and Mechanism of Cell Death
4.4. Prevention Strategies
4.5. Analysis of Methodologies
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Bin-Jardan, L.I.; Almadani, D.I.; Almutairi, L.S.; Almoabid, H.A.; Alessa, M.A.; Almulhim, K.S.; AlSheikh, R.N.; Al-Dulaijan, Y.A.; Ibrahim, M.S.; Al-Zain, A.O.; et al. Inorganic Compounds as Remineralizing Fillers in Dental Restorative Materials: Narrative Review. Int. J. Mol. Sci. 2023, 24, 8295. [Google Scholar] [CrossRef] [PubMed]
- Al-hijazi, A.Y.; Hasan, N.; Nasr, B.K.; Jasim Al-Khafaji, H.H.; Al-Khafaji, B.; Abdah Alanssari, B.F.; Jalil, A.T. Recent Advances in the Use of Inorganic Nanomaterials as Anti Caries Agents. Heliyon 2023, 9, e15326. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yu, Y.; Gao, S.; Zhang, Z.; Zhao, H. Biodegradation of Dental Resin-Based Composite—A Potential Factor Affecting the Bonding Effect: A Narrative Review. Biomedicines 2022, 10, 2313. [Google Scholar] [CrossRef] [PubMed]
- Berghaus, E.; Klocke, T.; Maletz, R.; Petersen, S. Degree of Conversion and Residual Monomer Elution of 3D-Printed, Milled and Self-Cured Resin-Based Composite Materials for Temporary Dental Crowns and Bridges. J. Mater. Sci. Mater. Med. 2023, 34, 23. [Google Scholar] [CrossRef] [PubMed]
- Riva, Y.R.; Rahman, S.F. Dental Composite Resin: A Review. AIP Conf. Proc. 2019, 2193, 020011. [Google Scholar]
- Demarco, F.F.; Cenci, M.S.; Montagner, A.F.; De Lima, V.P.; Correa, M.B.; Moraes, R.R.; Opdam, N.J.M. Longevity of Composite Restorations Is Definitely Not Only about Materials. Dent. Mater. 2023, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gitalis, R.; Zhou, L.; Marashdeh, M.Q.; Sun, C.; Glogauer, M.; Finer, Y. Human Neutrophils Degrade Methacrylate Resin Composites and Tooth Dentin. Acta Biomater. 2019, 88, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Ali, M.; Agrissais, M.; Mulligan, S.; Koh, L.; Martin, N. A Comparative Life Cycle Assessment of Dental Restorative Materials. Dent. Mater. 2023, 39, 13–24. [Google Scholar] [CrossRef]
- Arbildo-Vega, H.I.; Lapinska, B.; Panda, S.; Khan, A.S.; Lukomska-Szymanska, M. Clinical Effectiveness of Bulk-Fill and Conventional Resin Composite Restorations: Systematic Review and Meta-Analysis. Polymers 2020, 12, 1786. [Google Scholar] [CrossRef]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin Based Restorative Dental Materials: Characteristics and Future Perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef]
- Caldas, I.P.; Alves, G.G.; Barbosa, I.B.; Scelza, P.; de Noronha, F.; Scelza, M.Z. In Vitro Cytotoxicity of Dental Adhesives: A Systematic Review. Dent. Mater. 2019, 35, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Parolia, A.; Chaubal, T.; Dharamadhikari, S.; Abdulla, A.M.; Sakkir, N.; Arora, S.; Bapat, P.; Sindi, A.M.; Kesharwani, P. Recent Update on Potential Cytotoxicity, Biocompatibility and Preventive Measures of Biomaterials Used in Dentistry. Biomater. Sci. 2021, 9, 3244–3283. [Google Scholar] [CrossRef] [PubMed]
- Wuersching, S.N.; Högg, C.; Kohl, L.; Reichl, F.-X.; Hickel, R.; Kollmuss, M. Leaching Components and Initial Biocompatibility of Novel Bioactive Restorative Materials. Dent. Mater. 2023, 39, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Lankes, V.; Reymus, M.; Mayinger, F.; Coldea, A.; Liebermann, A.; Hoffmann, M.; Stawarczyk, B. Three-Dimensional Printed Resin: Impact of Different Cleaning Protocols on Degree of Conversion and Tensile Bond Strength to a Composite Resin Using Various Adhesive Systems. Materials 2023, 16, 3580. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Ro, S.T.; Kang, S.W.; Lee, H.; Lee, J.S.; Chae, Y.K.; Lee, K.E.; Lee, H.-S.; Kwack, K.H.; Kim, S.K.; et al. Bisphenol A Release from Commercially Available 3-Dimensionally Printed Resins and Human Cell Apoptosis to Bisphenol A: An in-Vitro Study. J. Clin. Pediatr. Dent. 2023, 47, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Vulović, S.; Nikolić-Jakoba, N.; Radunović, M.; Petrović, S.; Popovac, A.; Todorović, M.; Milić-Lemić, A. Biofilm Formation on the Surfaces of CAD/CAM Dental Polymers. Polymers 2023, 15, 2140. [Google Scholar] [CrossRef] [PubMed]
- Tiu, J.; Belli, R.; Lohbauer, U. A Step toward Bio-Inspired Dental Composites. Biomater. Investig. Dent. 2023, 10, 1–7. [Google Scholar] [CrossRef]
- Khan, A.A.; Zafar, M.S.; Fareed, M.A.; AlMufareh, N.A.; Alshehri, F.; AlSunbul, H.; Lassila, L.; Garoushi, S.; Vallittu, P.K. Fiber-Reinforced Composites in Dentistry—An Insight into Adhesion Aspects of the Material and the Restored Tooth Construct. Dent. Mater. 2023, 39, 141–151. [Google Scholar] [CrossRef]
- He, J.; Lassila, L.; Garoushi, S.; Vallittu, P. Tailoring the Monomers to Overcome the Shortcomings of Current Dental Resin Composites—Review. Biomater. Investig. Dent. 2023, 10, 2191621. [Google Scholar] [CrossRef]
- Hardan, L.; Devoto, W.; Bourgi, R.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Fernández-Barrera, M.Á.; Cornejo-Ríos, E.; Monteiro, P.; Zarow, M.; Jakubowicz, N.; et al. Immediate Dentin Sealing for Adhesive Cementation of Indirect Restorations: A Systematic Review and Meta-Analysis. Gels 2022, 8, 175. [Google Scholar] [CrossRef]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of Degradation of the Hybrid Layer in Adhesive Dentistry and Therapeutic Agents to Improve Bond Durability—A Literature Review. Dent. Mater. 2016, 32, e41–e53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Meng, X.; Ye, Y.; Feng, D.; Xue, J.; Wang, H.; Huang, H.; Wang, M.; Wang, J. Rheological and Mechanical Properties of Resin-Based Materials Applied in Dental Restorations. Polymers 2021, 13, 2975. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Cicciù, M.; Herford, A.S.; Germanà, A.; Fiorillo, L. Biological and Chemo-Physical Features of Denture Resins. Materials 2020, 13, 3350. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, S.; Hatton, P.V.; Martin, N. Resin-Based Composite Materials: Elution and Pollution. Br. Dent. J. 2022, 232, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Özcan, M.; Maleki Dizaj, S.; Sharifi, S.; Al-Haj Husain, N.; Eftekhari, A.; Ahmadian, E. A Review on Potential Toxicity of Dental Material and Screening Their Biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Pałka, K.; Miazga-Karska, M.; Pawłat, J.; Kleczewska, J.; Przekora, A. The Effect of Liquid Rubber Addition on the Physicochemical Properties, Cytotoxicity, and Ability to Inhibit Biofilm Formation of Dental Composites. Materials 2021, 14, 1704. [Google Scholar] [CrossRef] [PubMed]
- Szczesio-Wlodarczyk, A.; Domarecka, M.; Kopacz, K.; Sokolowski, J.; Bociong, K. An Evaluation of the Properties of Urethane Dimethacrylate-Based Dental Resins. Materials 2021, 14, 2727. [Google Scholar] [CrossRef]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The Photoinitiators Used in Resin Based Dental Composite—A Review and Future Perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef]
- Mulla, S.A.; Kondkari, S.A.; Patil, A.; Jain, A.; Mali, S.; Jaiswal, H.C.; Jakhar, A.; Ansari, Z.M.; Agarwal, S.; Yadav, P. A Look Into the Cytotoxicity of Composite Fillings: Friend or Foe? Cureus 2023, 15, e46327. [Google Scholar] [CrossRef]
- Schmalz, G.; Galler, K.M. Biocompatibility of Biomaterials—Lessons Learned and Considerations for the Design of Novel Materials. Dent. Mater. 2017, 33, 382–393. [Google Scholar] [CrossRef]
- Arab-Nozari, M.; Zamani, E.; Evazalipour, M.; Soleimani, B.; Jorbonian, A.; Nahvi, A. Histocompatibility of Light-Curing Composites Used in Pediatric Dentistry in Human Oral Fibroblast Cells. J. Dent. 2023, 24, 112–117. [Google Scholar] [CrossRef]
- Folwaczny, M.; Ahantab, R.; Kessler, A.; Ern, C.; Frasheri, I. Cytotoxicity of 3D Printed Resin Materials for Temporary Restorations on Human Periodontal Ligament (PDL-hTERT) Cells. Dent. Mater. 2023, 39, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, N.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H.K. Bioactive Dental Composites and Bonding Agents Having Remineralizing and Antibacterial Characteristics. Dent. Clin. N. Am. 2017, 61, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and Validation of a Risk-of-Bias Tool for Assessing in Vitro Studies Conducted in Dentistry: The QUIN. J. Prosthet. Dent. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.O.; Magalhães, L.M.D.; Corrêa, J.D.; Dutra, W.O.; Gollob, K.J.; Silva, T.A.; Horta, M.C.R.; Souza, P.E.A. Composite-Derived Monomers Affect Cell Viability and Cytokine Expression in Human Leukocytes Stimulated with Porphyromonas Gingivalis. J. Appl. Oral. Sci. 2019, 27, e20180529. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, S.; Velioğlu, N.; Cengiz, M.İ.; Çakmak Özlü, F.; Akbal, A.U.; Çoban, A.Y.; Özcan, M. Cytotoxicity of Acrylic Resins, Particulate Filler Composite Resin and Thermoplastic Material in Artificial Saliva with and without Melatonin. Materials 2022, 15, 1457. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Cotto, R.; Etges, A.; Jardim, P.S.; Torre, E.; Kaizer, M.R.; Ferrúa, C.P.; Nedel, F.; Cuevas-Suárez, C.E.; Moraes, R.R. Cytotoxicity of Contemporary Resin-based Dental Materials in Contact with Dentin. Eur. J. Oral Sci. 2020, 128, 436–443. [Google Scholar] [CrossRef]
- Beltrami, R.; Colombo, M.; Rizzo, K.; Di Cristofaro, A.; Poggio, C.; Pietrocola, G. Cytotoxicity of Different Composite Resins on Human Gingival Fibroblast Cell Lines. Biomimetics 2021, 6, 26. [Google Scholar] [CrossRef]
- Kavuncu, G.; Yilmaz, A.M.; Karademir Yilmaz, B.; Yilmaz Atali, P.; Altunok, E.C.; Kuru, L.; Agrali, O.B. Cytotoxicity of Different Nano Composite Resins on Human Gingival and Periodontal Ligament Fibroblast Cell Lines: An In Vitro Study. Biomedicines 2020, 8, 48. [Google Scholar] [CrossRef]
- Sulek, J.; Luczaj-Cepowicz, E.; Marczuk-Kolada, G.; Rosłan, M.; Holownia, A. Cytotoxicity of Methacrylate Dental Resins to Human Gingival Fibroblasts. J. Funct. Biomater. 2022, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.R.; Hakami-Tafreshi, R.; Tomasino-Perez, A.; Tayebi, L.; Lobner, D. Effects of Dental Composite Resin Monomers on Dental Pulp Cells. Dent. Mater. J. 2019, 38, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Lovász, B.V.; Lempel, E.; Szalma, J.; Sétáló, G.; Vecsernyés, M.; Berta, G. Influence of TEGDMA Monomer on MMP-2, MMP-8, and MMP-9 Production and Collagenase Activity in Pulp Cells. Clin. Oral Investig. 2021, 25, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Agnes, A.; Long, A.; Best, S.; Lobner, D. Pulp Capping Materials Alter the Toxicity and Oxidative Stress Induced by Composite Resins in Dental Pulp Culture. Eur. Endod. J. 2017, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Lovász, B.V.; Berta, G.; Lempel, E.; Sétáló, G.; Vecsernyés, M.; Szalma, J. TEGDMA (Triethylene Glycol Dimethacrylate) Induces Both Caspase-Dependent and Caspase-Independent Apoptotic Pathways in Pulp Cells. Polymers 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, G.-L.; Huang, Y.; Diwu, H.-L.; Luo, Y.-C.; Su, J.; Xiao, Y.-H. The Effects of 2-Hydroxyethyl Methacrylate on Matrix Metalloproteinases 2 and 9 in Human Pulp Cells and Odontoblast-like Cells in Vitro. Int. Endod. J. 2018, 51, e157–e166. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-C.; Chang, Y.-C.; Yeh, K.-L.; Huang, F.-M.; Su, N.-Y.; Kuan, Y.-H. Protective Effect of Rutin on Triethylene Glycol Dimethacrylate-Induced Toxicity through the Inhibition of Caspase Activation and Reactive Oxygen Species Generation in Macrophages. Int. J. Mol. Sci. 2022, 23, 11773. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Ho, Y.-C.; Lee, S.-S.; Li, Y.-C.; Lai, M.-Y.; Kuan, Y.-H. Cytotoxicity and Apoptotic Mechanism of 2-Hydroxyethyl Methacrylate via Genotoxicity and the Mitochondrial-Dependent Intrinsic Caspase Pathway and Intracellular Reactive Oxygen Species Accumulation in Macrophages. Polymers 2022, 14, 3378. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Chiang, C.-Y.; Chiang, Y.-W.; Lee, M.-W.; Lee, C.-Y.; Chen, H.-Y.; Lin, H.-W.; Kuan, Y.-H. Toxic Effects of Urethane Dimethacrylate on Macrophages Through Caspase Activation, Mitochondrial Dysfunction, and Reactive Oxygen Species Generation. Polymers 2020, 12, 1398. [Google Scholar] [CrossRef]
- Wawrzynkiewicz, A.; Rozpedek-Kaminska, W.; Galita, G.; Lukomska-Szymanska, M.; Lapinska, B.; Sokolowski, J.; Majsterek, I. The Toxicity of Universal Dental Adhesives: An In Vitro Study. Polymers 2021, 13, 2653. [Google Scholar] [CrossRef]
- Wawrzynkiewicz, A.; Rozpedek-Kaminska, W.; Galita, G.; Lukomska-Szymanska, M.; Lapinska, B.; Sokolowski, J.; Majsterek, I. The Cytotoxicity and Genotoxicity of Three Dental Universal Adhesives—An In Vitro Study. Int. J. Mol. Sci. 2020, 21, 3950. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; Balkis, M.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Uses and Limitations of the XTT Assay in Studies of Candida Growth and Metabolism. J. Clin. Microbiol. 2003, 41, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Scarcello, E.; Lambremont, A.; Vanbever, R.; Jacques, P.J.; Lison, D. Mind Your Assays: Misleading Cytotoxicity with the WST-1 Assay in the Presence of Manganese. PLoS ONE 2020, 15, e0231634. [Google Scholar] [CrossRef] [PubMed]
- Kaja, S.; Payne, A.J.; Naumchuk, Y.; Koulen, P. Quantification of Lactate Dehydrogenase for Cell Viability Testing Using Cell Lines and Primary Cultured Astrocytes. Curr. Protoc. Toxicol. 2017, 72, 2.26.1–2.26.10. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Reiter, R.J.; Topal, T.; Manchester, L.C.; Oter, S.; Tan, D.-X. Melatonin: An Established Antioxidant Worthy of Use in Clinical Trials. Mol. Med. 2009, 15, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, J.T.; Dahl, J.E. Biological Aspects of Modern Dental Composites. Biomater. Investig. Dent. 2023, 10, 2223223. [Google Scholar] [CrossRef]

| Phase | Function | Composition |
|---|---|---|
| Resin matrix | Polymerization | BisGMA, TEGDMA, UDMA, HEMA, BisEMA, EGDMA |
| Filler particles | Improvement in mechanical and wear properties, aesthetic qualities, and reduction in polymerization shrinkage | Soft and hard glass: borosilicate, quartz, aluminum silicate, lithium aluminum silicate, ytterbium fluoride, barium, strontium, zirconium and zinc glass |
| Coupling agent | Combining resin matrix and filler particles, reduced water sorption | Silanes, zirconates, titanates |
| Photo-initiator | Initiation of resin matrix polymerization by providing free radicals upon exposure to external energy | CQ, PQ, TPO |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Publication in English Published since 2017 In vitro study Containing the following keyword combinations: “cytotoxicity of dental materials” OR “cytotoxicity of resin composite” OR “biocompatibility of dental materials” OR “biocompatibility of resin composite” OR “oxidative stress of dental materials” OR “oxidative stress of resin composite” OR “genotoxicity of dental materials” OR “genotoxicity of resin composite” OR “mutagenicity of dental materials” OR “mutagenicity of resin composite” | Publication not in English Published before 2017 Review articles, abstracts, book chapters, live animal studies |
| Study | Final Score (%) | Risk of Bias |
|---|---|---|
| Neves et al., 2019 [36] | 65 | Medium |
| Cengiz et al., 2022 [37] | 80 | Low |
| Carrillo-Cotto et al., 2020 [38] | 80 | Low |
| Beltrami et al., 2021 [39] | 70 | Medium |
| Kavuncu et al., 2020 [40] | 70 | Medium |
| Sulek et al., 2022 [41] | 80 | Low |
| Schneider et al., 2019 [42] | 55 | Medium |
| Lovász et al., 2021 [43] | 55 | Medium |
| Agnes et al., 2017 [44] | 80 | Low |
| Lovász et al., 2021 [45] | 80 | Low |
| Sun et al., 2018 [46] | 75 | Low |
| Yang et al., 2022 [47] | 85 | Low |
| Lee et al., 2022 [48] | 75 | Low |
| Chang et al., 2020 [49] | 75 | Low |
| Wawrzynkiewicz et al., 2021 [50] | 85 | Low |
| Wawrzynkiewicz et al., 2020 [51] | 85 | Low |
| Study | Cell Viability Assay | Other Methods | Cell Line | Resin-Based Materials |
|---|---|---|---|---|
| Neves et al., 2019 [36] | MTT assay | APC Annexin V apoptosis detection kit, ELISA assay | Human peripheral blood mononuclear cells (hPBMC) | Monomers: BisGMA, TEGDMA, UDMA |
| Schneider et al., 2019 [42] | LDH assay | MCB assay, DCF assay | Human dental pulp cells (hDPC) | Monomers: BisGMA, TEGDMA, UDMA |
| Lovász et al., 2021 [43] | WST-1 assay | EncCheckGelatinolyticCollagenolitic activity assay, Western blotting | Human dental pulp cells (hDPC) | Monomers: TEGDMA |
| Lovász et al., 2021 [45] | WST-1 assay | Hemocytometer, fluorescence microscopy, Western blotting | Human dental pulp cells (hDPC) | Monomers: TEGDMA |
| Sun et al., 2018 [46] | MTT assay | RT-PCR, gelatinezymography, transwell migration assay, Western blotting | Human dental pulp cells (hDPC) | Monomers: HEMA |
| Yang et al., 2022 [47] | MTT assay | FITC Annexin V, comet assay | Murine macrophages | Monomers: TEGDMA |
| Lee et al., 2022 [48] | MTT assay | FITC Annexin V, micronucleus assay, comet assay | Murine macrophages | Monomers: HEMA |
| Chang et al., 2020 [49] | LDH assay | FITC Annexin V, MN assay, comet assay | Murine macrophage | Monomers: UDMA |
| Cengiz et al., 2022 [37] | MTT assay | None | Murine fibroblasts (mF) | Eluates of polymerized specimens: Signum (S), Adoro (A) |
| Carillo-Cotto et al., 2020 [38] | MTT assay | Fourier-transform infrared spectroscopy | Human keratinocytes (hK) | Eluates of polymerized specimens: OptiBond FL (OB), Clearfil SE Bond (CB), Adper Single Bond Universal (AS), Filtek Z350 XT (FZ3), Filtek Flow Z350 XT (FFZ3), Dyad Flow (DF), Variolink II (VII), RelyXU200 (RX) |
| Beltrami et al., 2021 [39] | MTT assay | None | Human gingival fibroblasts (hGF) | Eluates of polymerized specimens: Omnichroma (OC), Omnichroma Blocker (OCB), Admira Fusion x-tra (AFX), Enamel Plus Hri Bio Function Enamel (EPE), Enamel Plus Hri (EP), G-aenial (GA), G-aenial Flo X (GAF), Enamel Plus Hri Bio Function Bio Dentine (EPD) |
| Kavuncu et al., 2020 [40] | MTT assay | None | Human gingival fibroblasts (hGF), human periodontal ligament fibroblasts (hPLF) | Polymerized samples placed directly in cell culture medium: Admira Fusion (AF), Charisma Topaz (CT), Estelite Sigma Quick (ESQ) |
| Sulek et al., 2022 [41] | MTT assay, LDH assay | Flow cytometry, FITC Annexin V, Western blotting | Human gingival fibroblasts (hGF) | Eluates of polymerized specimens: Charisma (CH), Estelite Sigma Quick (ESQ), Filtek Z550 (FZ5) |
| Agnes et al., 2017 [44] | LDH assay | DCF assay | Human dental pulp cells (hDPC) | Polymerized samples placed directly in cell culture medium: Flow Line (FL), Durafill VS (DF) |
| Wawrzynkiewicz et al., 2021 [50] | XTT assay | Comet assay, flow cytometry, FITC Annexin V | Human monocytes/macrophage peripheral blood cells | Eluates of polymerized specimens: All-Bond Universal, CLEARFIL Universal Bond Quick, G-Premio BOND, Single Bond Universal |
| Wawrzynkiewicz et al., [51] | XTT assay | Comet assay, flow cytometry, FITC Annexin V | Human monocytes/macrophage peripheral blood cells | Eluates of polymerized specimens: OptiBond Universal, Prime&Bond Universal, AdheseUniversal |
| Method | Characteristics |
|---|---|
| Direct contact test | Direct contact between the material and cell culture, typically in mono-layer. |
| Indirect contact test | Separation of the material and cell culture with an intermediate layer, e.g., agar gel, Millipore filter, dentin layer. |
| Extract test | Application of eluates from the material to cell culture. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiertelak-Makała, K.; Szymczak-Pajor, I.; Bociong, K.; Śliwińska, A. Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 152. https://doi.org/10.3390/ijms25010152
Wiertelak-Makała K, Szymczak-Pajor I, Bociong K, Śliwińska A. Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(1):152. https://doi.org/10.3390/ijms25010152
Chicago/Turabian StyleWiertelak-Makała, Kacper, Izabela Szymczak-Pajor, Kinga Bociong, and Agnieszka Śliwińska. 2024. "Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review" International Journal of Molecular Sciences 25, no. 1: 152. https://doi.org/10.3390/ijms25010152
APA StyleWiertelak-Makała, K., Szymczak-Pajor, I., Bociong, K., & Śliwińska, A. (2024). Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review. International Journal of Molecular Sciences, 25(1), 152. https://doi.org/10.3390/ijms25010152






