Enhancement of Immune Functions by Limosilactobacillus reuteri KBL346: In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Results
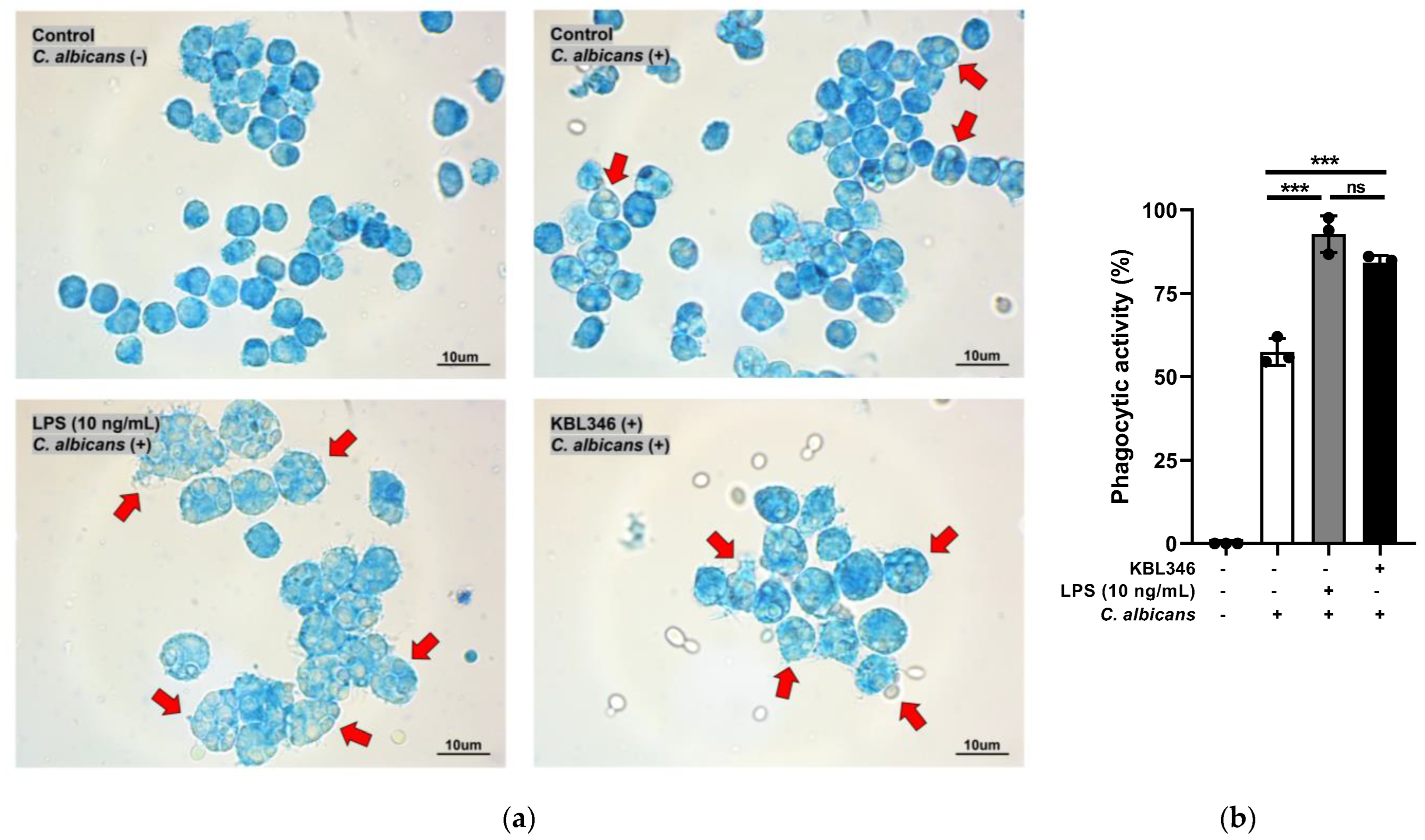
2.1. KBL346 Increased the Phagocytic Activity of Macrophages
2.2. KBL346 Increased Secretion of Nitric Oxide and Prostaglandin Secretion by Macrophages
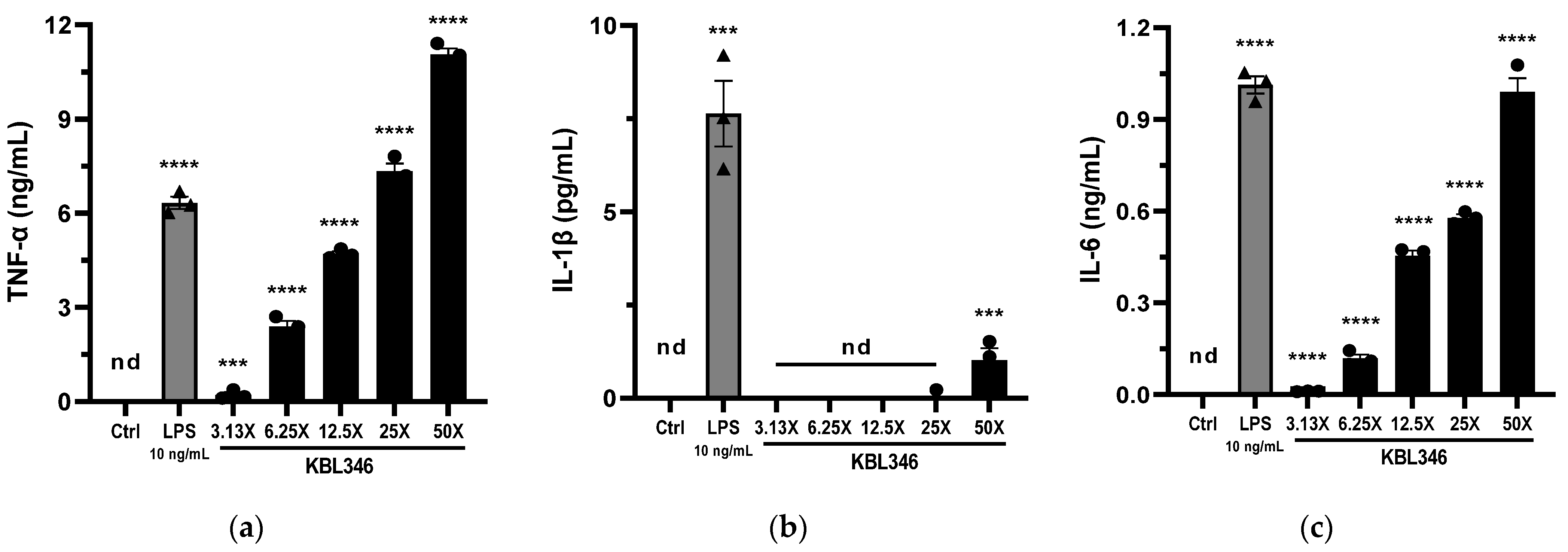
2.3. KBL346 Increased the Production of Cytokines by Macrophages
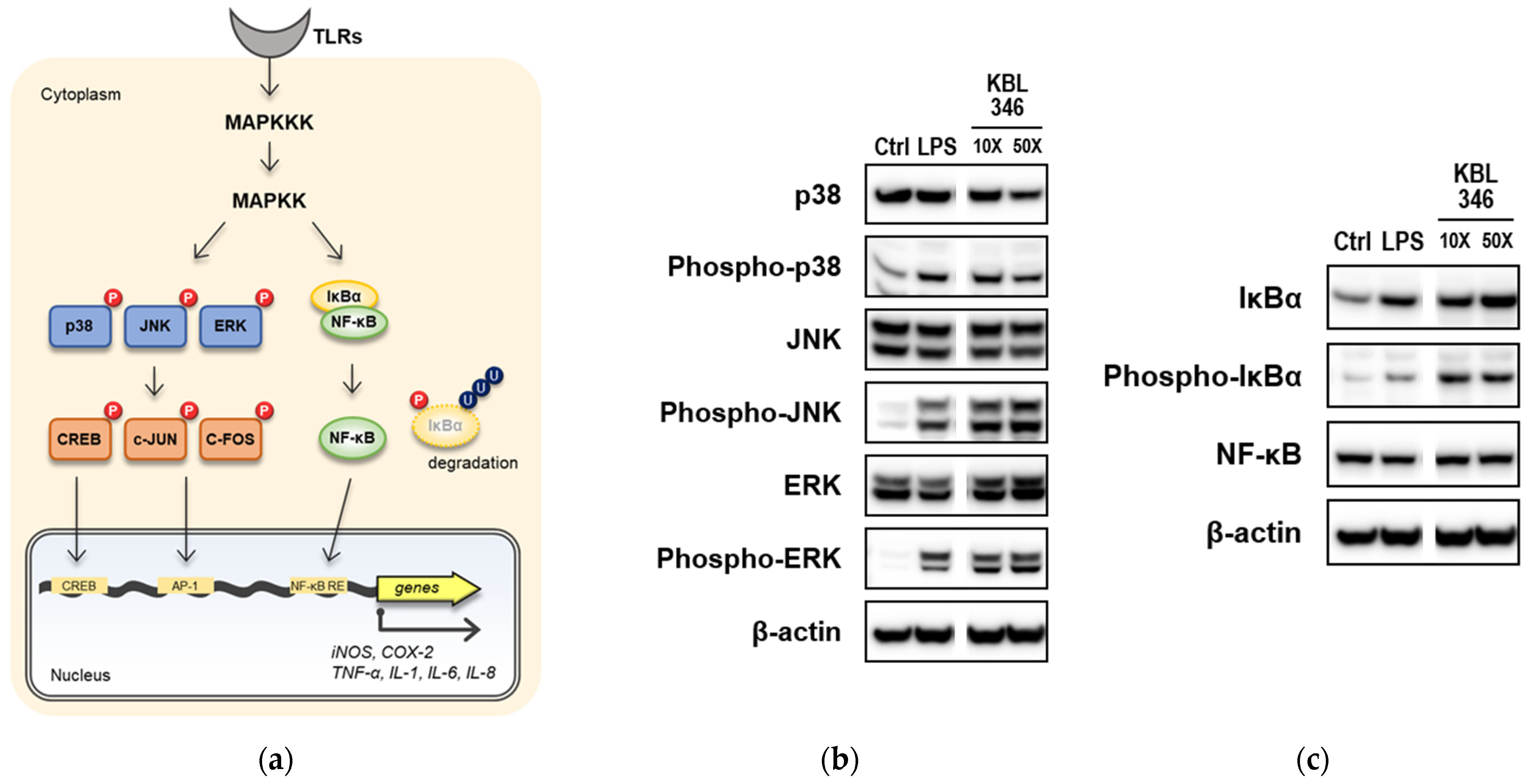
2.4. KBL346 Activated Immune Signaling Pathways and Increased Expression of Their Target Genes in Macrophages
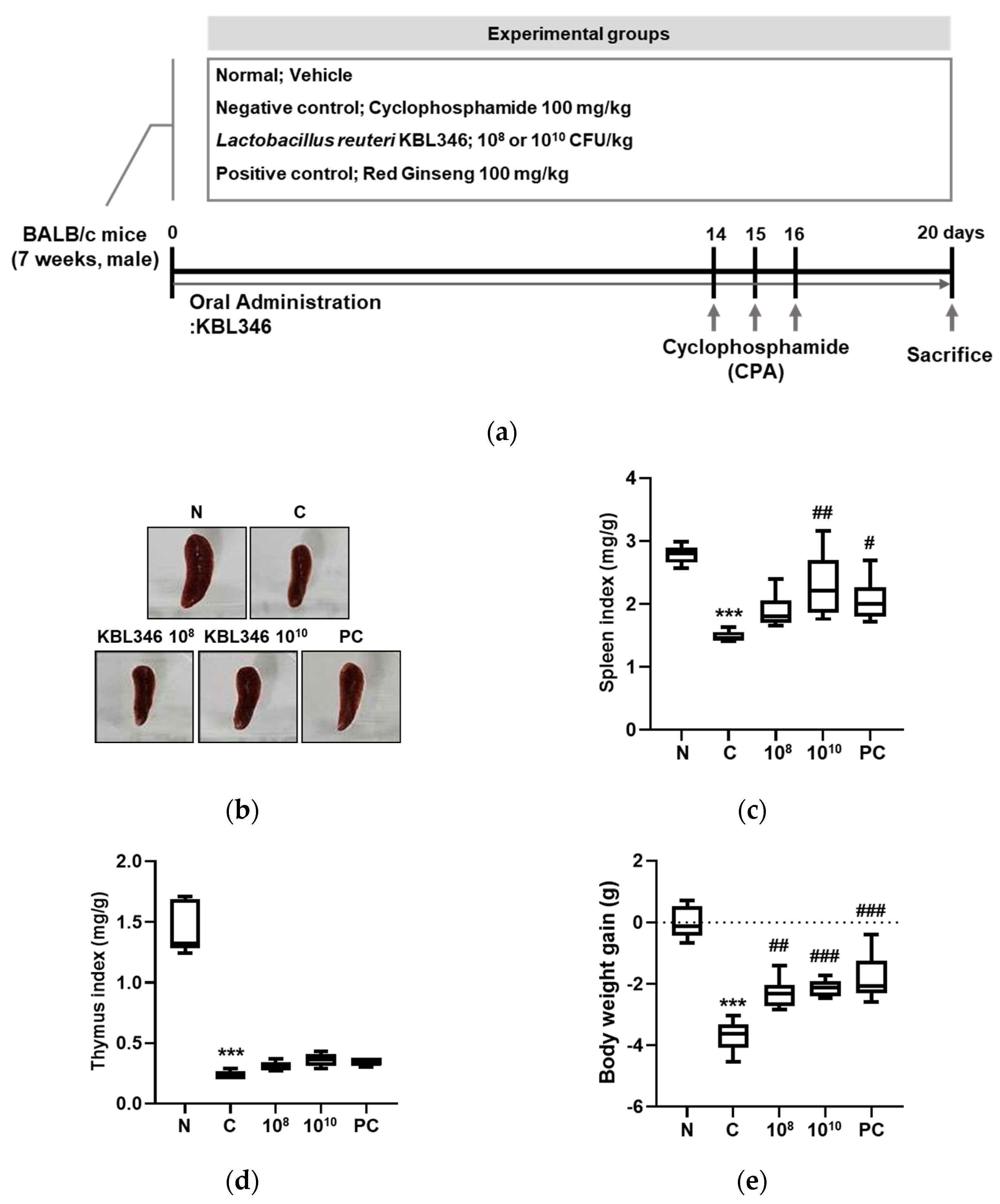
2.5. KBL346 Protected against Spleen and Body Weight Changes Decreased by Cyclophosphamide
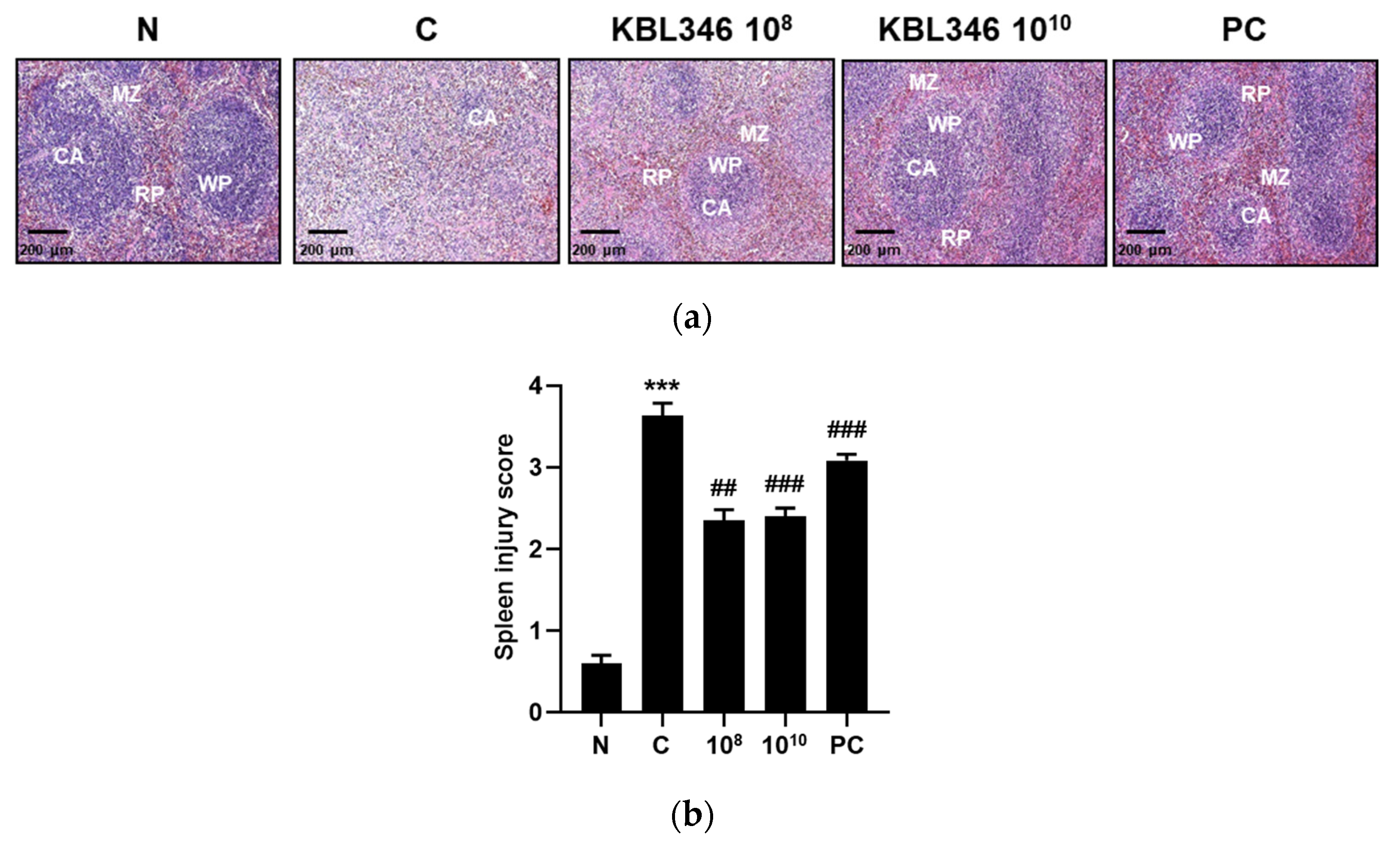
2.6. KBL346 Alleviated Spleen Tissue Damage Induced by Cyclophosphamide
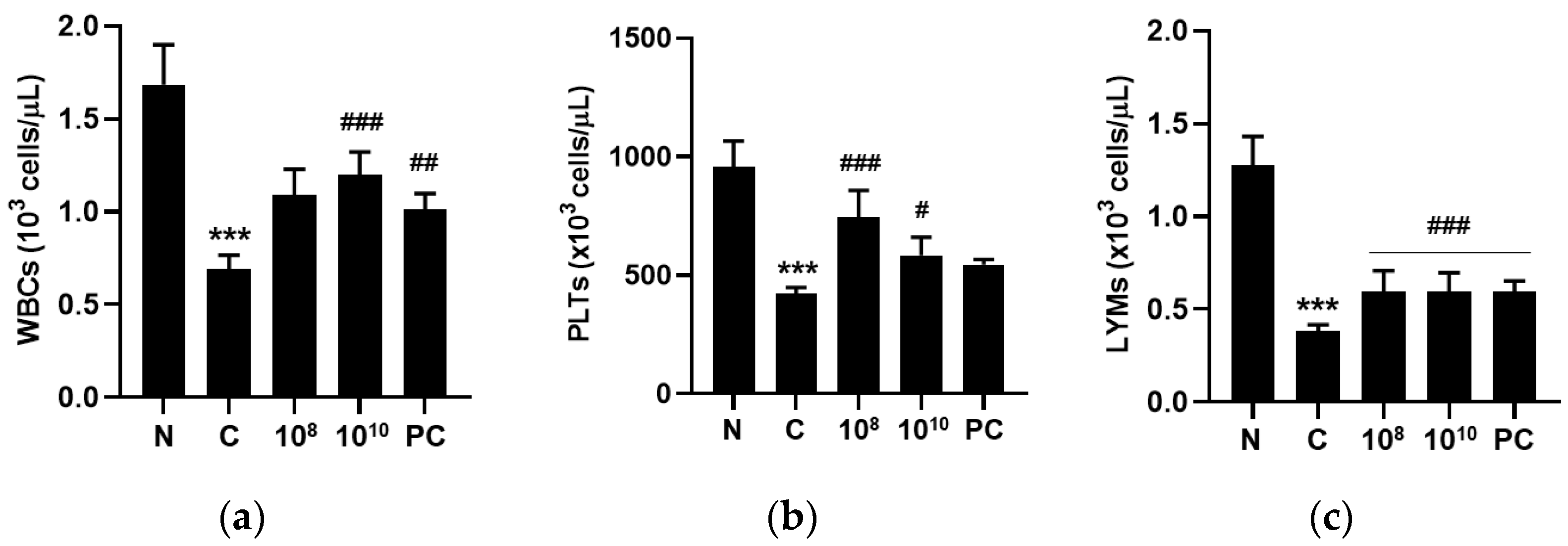
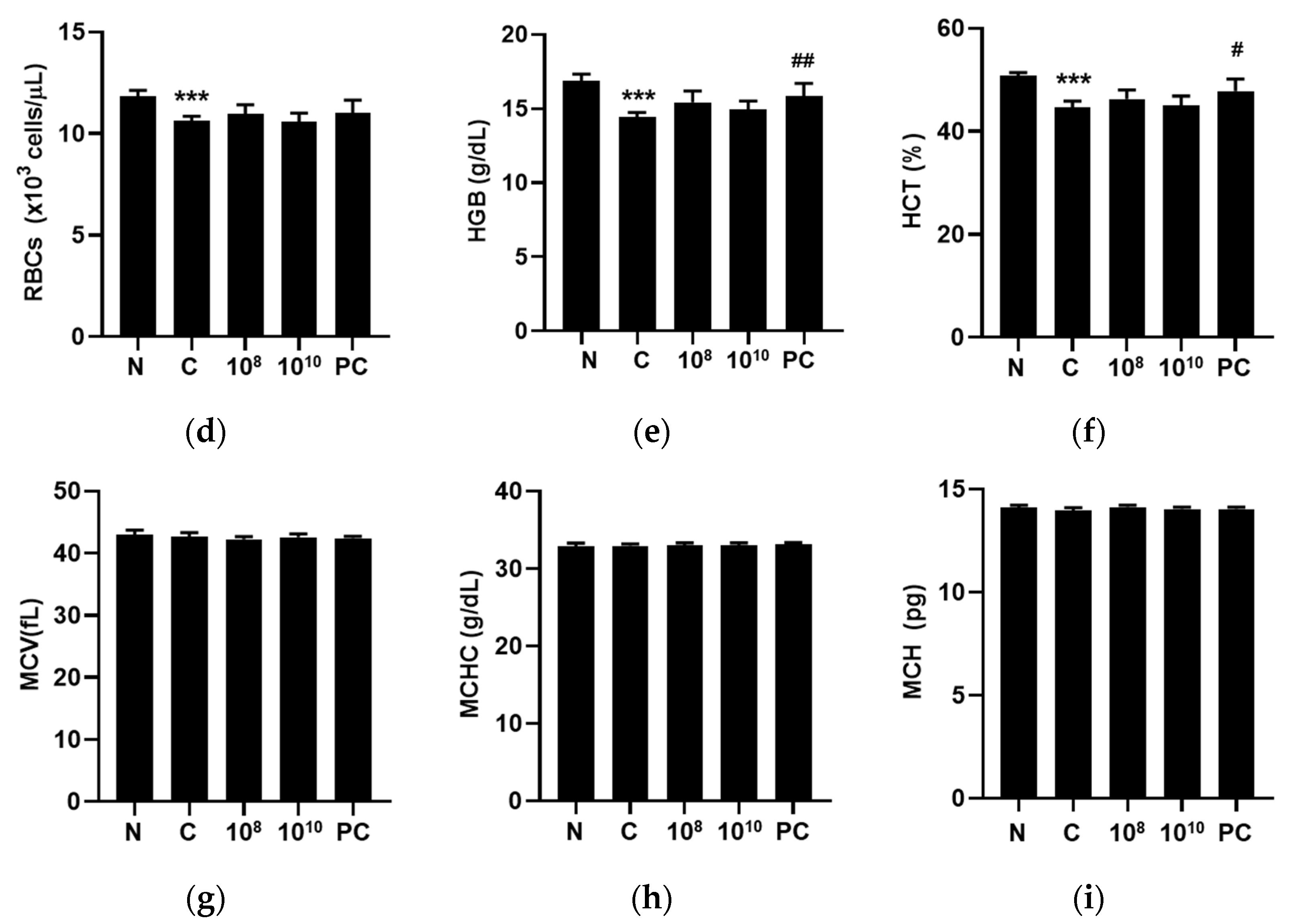
2.7. KBL346 Enhanced Blood Cell Counts Decreased by Cyclophosphamide
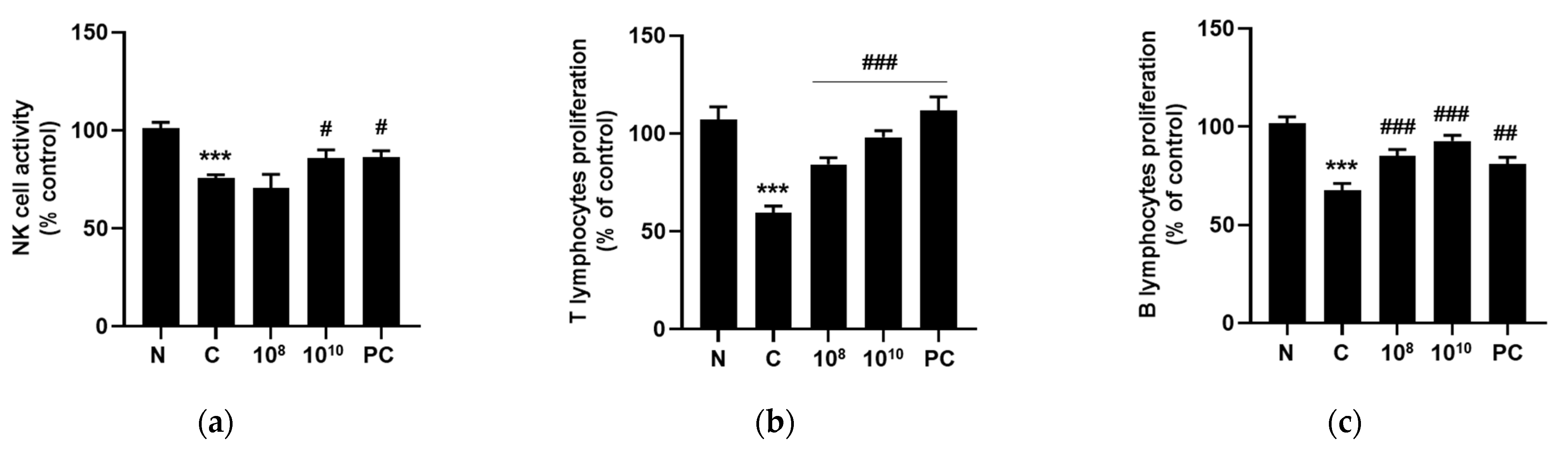
2.8. KBL346 Promoted Lymphocyte Proliferation and NK Cell Activity
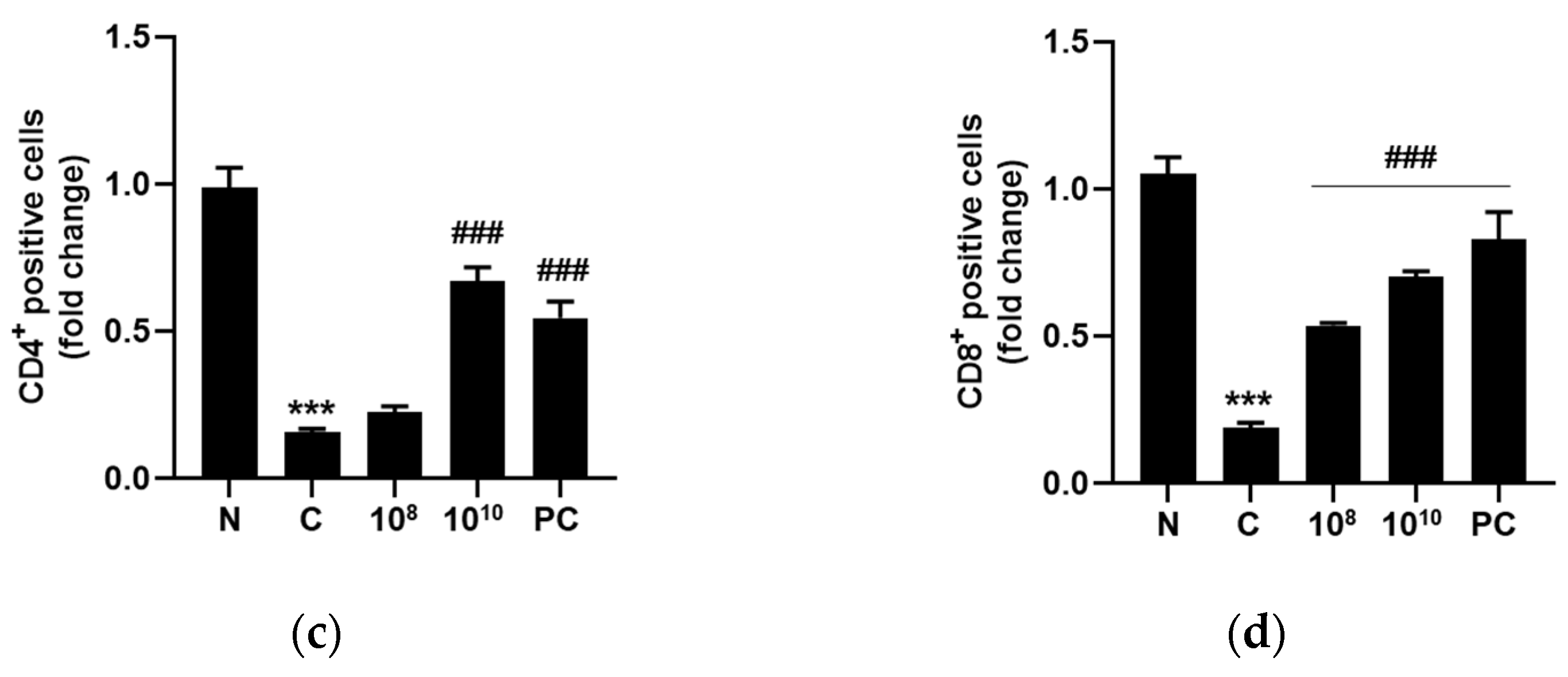
2.9. KBL346 Elevated CD4+ and CD8+ T Cell Numbers in Splenocytes Decreased by Cyclophosphamide
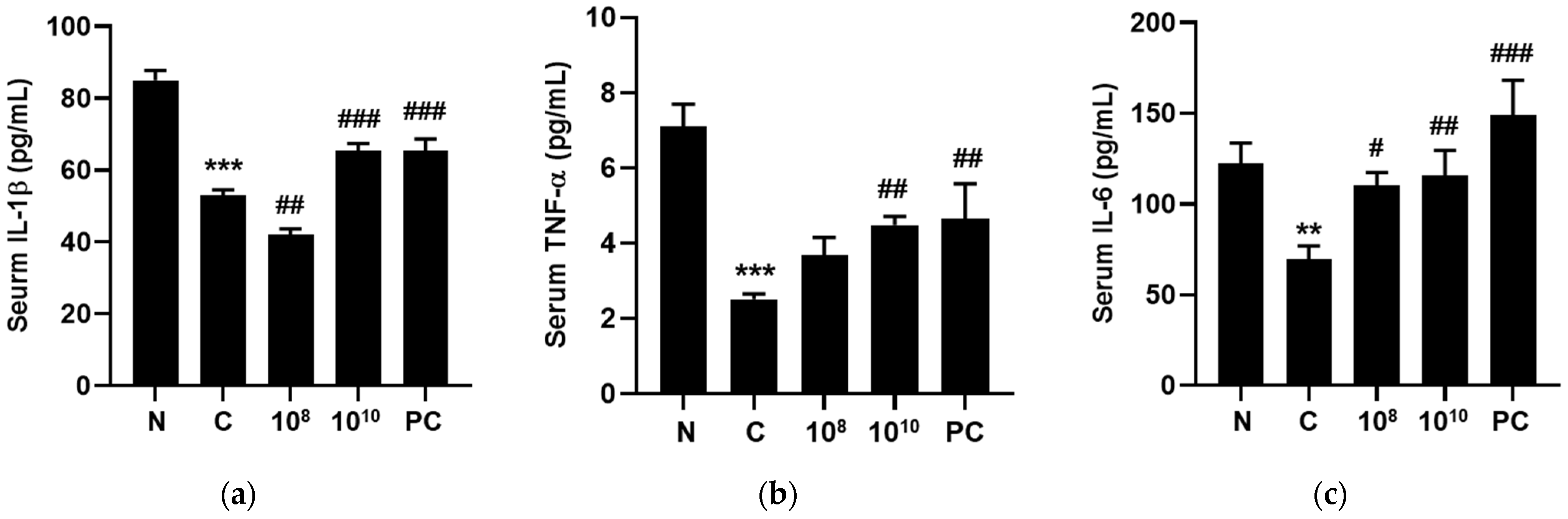
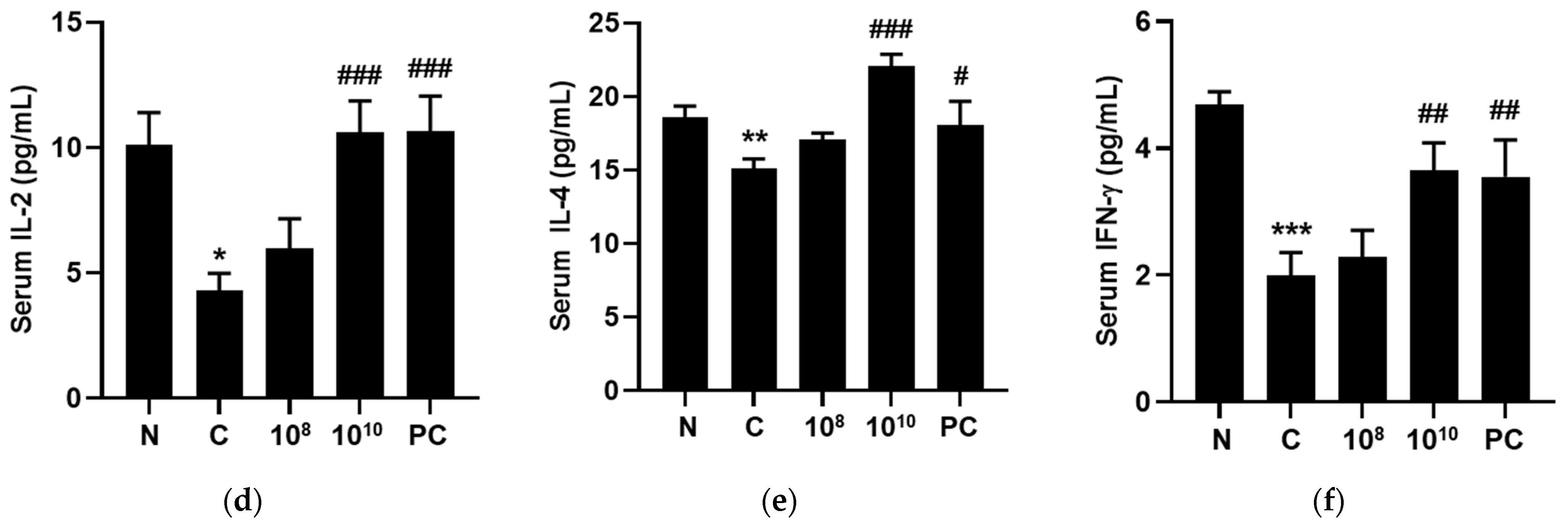
2.10. KBL346 Stimulated Secretion of Immune-Associated Cytokines
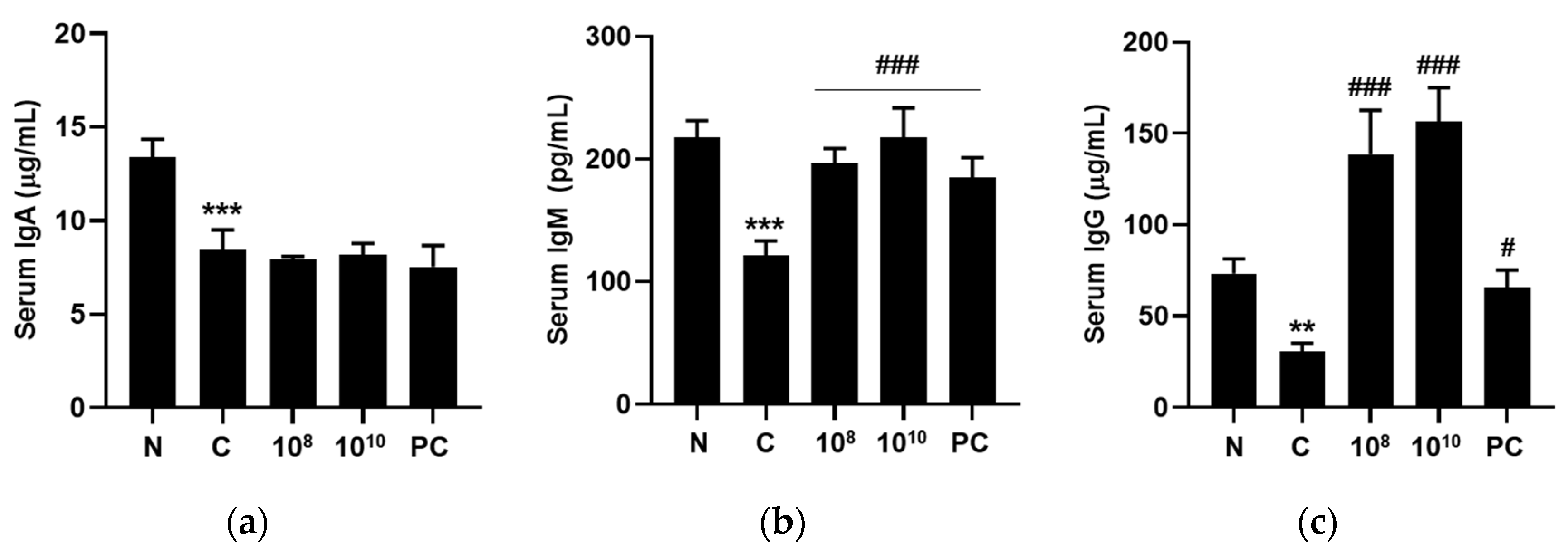
2.11. KBL346 Stimulated Secretion of Immune-Related Immunoglobulins
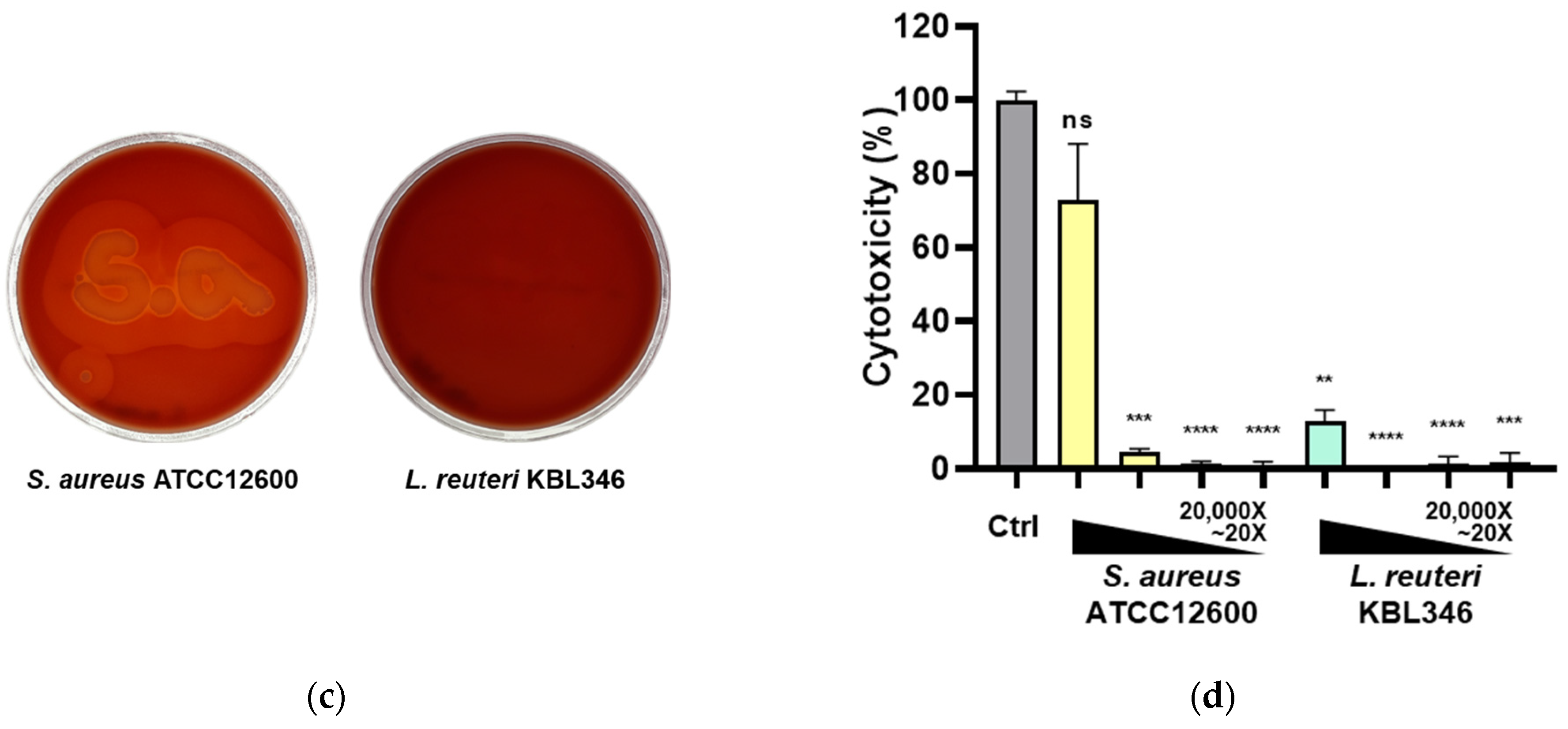
2.12. Safety Profile of KBL346
3. Discussion and Conclusions
4. Materials and Methods
4.1. Isolation and Identification of the L. reuteri KBL346
4.2. Preparation of Bacterial Samples
4.3. Cell Culture
4.4. Coculture Conditions
4.5. Evaluation of Macrophage Phagocytic Activity
4.6. Measurement of Cell Viability
4.7. Measurement of NO, PGE2, and Cytokine Levels
4.8. Western Blot
4.9. Animals
4.10. Collection of Blood Samples
4.11. Hematological Analysis
4.12. Immune-Associated Marker Analysis
4.13. Splenocytes Isolation
4.14. T and B Lymphocytes Proliferation Analysis
4.15. Natural Killer Cell Activity Measurement
4.16. Lymphocyte Subpopulation Analysis
4.17. Hematoxylin and Eosin (H&E) Staining
4.18. Safety Profile of KBL346
4.19. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo, B.C.; Chen, G.Y.; Núñez, G.; Caruso, R. Gut microbiota and systemic immunity in health and disease. Int. Immunol. 2021, 33, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Xie, C.; Xu, D.; Yang, X.; Tang, J.; Ji, X. Lactobacillus isolates from healthy volunteers exert immunomodulatory effects on activated peripheral blood mononuclear cells. J. Biomed. Res. 2013, 27, 116–126. [Google Scholar] [PubMed]
- Taweechotipatr, M.; Iyer, C.; Spinler, J.K.; Versalovic, J.; Tumwasorn, S. Lactobacillus saerimneri and Lactobacillus ruminis: Novel human-derived probiotic strains with immunomodulatory activities. FEMS Microbiol. Lett. 2009, 29, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, C.; Niu, Z.; Kang, H.; Wang, M.; Zhang, B.; Tian, H. Colonization and immunoregulation of Lactobacillus plantarum BF_15, a novel probiotic strain from the feces of breast-fed infants. Food Funct. 2020, 11, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Shin, Y.J.; Lee, D.Y.; Kim, K.M.; Yang, S.J.; Kim, D.S.; Choi, J.W.; Lee, S.; Kim, D.H. Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation. BMC Microbiol. 2021, 21, 146. [Google Scholar] [CrossRef] [PubMed]
- Ladda, B.; Jantararussamee, C.; Pradidarcheep, W.; Kasorn, A.; Matsathit, U.; Taweechotipatr, M. Anti-inflammatory and gutmicrobiota modulating effects of probiotic Lactobacillus paracasei MSMC39-1 on dextransulfate sodium-induced colitis in rats. Nutrients. 2023, 15, 1388. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, H.; Tsao, R.; Mine, Y. Lactobacillus pentosus S-PT84 prevents low-grade chronic inflammation-associated metabolic disorders in a lipopolysaccharide and high-fat diet C57/BL6J mouse model. J. Agric. Food Chem. 2020, 68, 4374–4386. [Google Scholar] [CrossRef]
- Tripathy, A.; Swain, N.; Padhan, P.; Raghav, S.K.; Gupta, B. Lactobacillus rhamnosus reduces CD8+T cell mediated inflammation in patients with rheumatoid arthritis. Immunobiology. 2023, 228, 152415. [Google Scholar] [CrossRef]
- Min, F.; Hu, J.; Huang, T.; Huang, Y.; Nie, S.; Xiong, T.; Xie, M. Effects of Lactobacillus casei NCU011054 on immune response and gut microbiota of cyclophosphamide induced immunosuppression mice. Food Chem Toxicol. 2023, 174, 113662. [Google Scholar] [CrossRef]
- Zhang, N.; Tian, Y.; Wang, Y.; Fan, Y.; Zhang, Y.; Xing, X.; Nan, B.; Ai, Z.; Li, X.; Wang, Y. Ameliorative effect of Lactobacillus plantarum Lp2 against cyclophosphamide-induced liver injury in mice. Food Chem Toxicol. 2022, 169, 113433. [Google Scholar] [CrossRef]
- Kim, K.J.; Paik, H.D.; Kim, J.Y. Immune-enhancing effects of Lactobacillus plantarum 200655 isolated from Korean Kimchi in a cyclophosphamide-induced immunocompromised mouse model. J. Microbiol. Biotechnol. 2021, 31, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fan, S.; Huang, D.; Xiong, T.; Nie, S.; Xie, M. Polysaccharides from fermented Asparagus officinalis with Lactobacillus plantarum NCU116 alleviated liver injury via modulation of glutathione homeostasis, bile acid metabolism, and SCFA production. Food Funct. 2020, 11, 7681–7695. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Li, Z.; Xue, J.; Wang, H.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. Lactobacillus acidophilus LA85 ameliorates cyclophosphamide-induced immunosuppression by modulating Notch and TLR4/NF-κB signal pathways and remodeling the gut microbiota. Food Funct. 2022, 13, 8107–8118. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Britton, R.A.; Roos, S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4645–4652. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Widyarman, A.S.; Theodorea, C.F. Novel indigenous probiotic Lactobacillus reuteri strain produces anti-biofilm reuterin against pathogenic periodontal bacteria. Eur. J. Dent. 2022, 16, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rivera, Y.; Sánchez-Vega, R.; Gutiérrez-Méndez, N.; León-Félix, J.; Acosta-Muñiz, C.; Sepulveda, D.R. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268. [Google Scholar] [CrossRef] [PubMed]
- Valeur, N.; Engel, P.; Carbajal, N.; Connolly, E.; Ladefoged, K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl. Environ. Microbiol. 2004, 70, 1176–1181. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front Immunol 2022, 13, 812774. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat Rev Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Pallmer, K.; Oxenius, A. Recognition and regulation of T Cells by NK Cells. Front Immunol. 2016, 7, 251. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, S.G.; Whitmire, J.K.; Jones, N.R.; Galic, Z.; Kitchen, C.M.; Ahmed, R.; Zack, J.A. The CD4 molecule on CD8+ T lymphocytes directly enhances the immune response to viral and cellular antigens. Proc. Natl. Acad. Sci. USA 2005, 102, 3794–3799. [Google Scholar] [CrossRef] [PubMed]
- Un-Nisa, A.; Khan, A.; Zakria, M.; Siraj, S.; Ullah, S.; Tipu, M.K.; Ikram, M.; Kim, M.O. Updates on the role ofprobiotics against different health issues: Focus on Lactobacillus. Int. J. Mol. Sci. 2022, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Capurso, L. Thirty Years of Lactobacillus rhamnosus GG: A Review. J. Clin. Gastroenterol. 2019, 53 (Suppl. S1), S1–S41. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Yang, Y.; Zhou, G.; Tu, Z.K. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World, J. Gastroenterol. 2019, 25, 3527–3537. [Google Scholar] [CrossRef]
- Whiteside, T.L. Immune suppression in cancer: Effects on immune cells, mechanisms and future therapeutic intervention. Semin. Cancer Biol. 2006, 16, 3–15. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Ponticelli, C.; Glassock, R.J. Prevention of complications from use of conventional immunosuppressants: A critical review. J. Nephrol. 2019, 32, 851–870. [Google Scholar] [CrossRef]
- Iles, K.E.; Forman, H.J. Macrophage signaling and respiratory burst. Immunol Res. 2002, 26, 95–105. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Sahni, S.; Lok, H.; Davies, M.J.; Wink, D.A.; Richardson, D.R. Regulation and control of nitric oxide (NO) in macrophages: Protecting the “professional killer cell” from its own cytotoxic arsenal via MRP1 and GSTP1. Biochim Biophys Acta Gen Subj. 2017, 1861, 995–999. [Google Scholar] [CrossRef]
- Salva, S.; Marranzino, G.; Villena, J.; Agüero, G.; Alvarez, S. Probiotic Lactobacillus strains protect against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int. Immunopharmacol. 2014, 22, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Lee, E.B.; Quah, Y.; Birhanu, B.T.; Suk, K.; Lim, S.K.; Park, S.C. Heat-killed Limosilactobacillus reuteri PSC102 ameliorates impaired immunity in cyclophosphamide-induced immunosuppressed mice. Front Microbiol. 2022, 13, 820838. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Galdeano, C.M.; Perdigón, G. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J. Appl. Microbiol. 2004, 97, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.I.; Park, H.O.; Kang, J.H. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009, 107, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Lazerenko, L.M.; Babenko, L.P.; Gichka, S.G.; Sakhno, L.O.; Demchenko, O.M.; Bubnov, R.C.; Sichel, L.M.; Spivak, M. Assessment of the safety of Lactobacillus casei IMV B-7280 probiotic strain on a mouse model. Probiotics Antimicrob. Proteins. 2021, 13, 1644–1657. [Google Scholar]
- Park, S.; Kim, H.; Kim, J.; Lee, J.; Kim, S.; Shin, H.; Yi, T. Immunostimulatory effect of fermented red ginseng in the mouse model. Prev. Nutr. Food Sci. 2014, 19, 10–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoon, S.H.; Lee, S.M.; Han, C.; In, G.; Park, C.; Hyun, S.H. Immune activity of polysaccharide fractions isolated from Korean red ginseng. Molecules 2020, 25, 3569. [Google Scholar] [CrossRef]
- Criswell, K.A.; Bock, J.H.; Wildeboer, S.E.; Johnson, K.; Giovanelli, R.P. Comparison of the Sysmex XT-2000iV and microscopic bone marrow differential counts in Wistar rats treated with cyclophosphamide, erythropoietin, or serial phlebotomy. Vet. Clin. Pathol. 2014, 43, 137–153. [Google Scholar] [CrossRef]
- Jeong, M.K.; Kim, B.H. Grading criteria of histopathological evaluation in BCOP assay by various staining methods. Toxicol Res. 2021, 38, 9–17. [Google Scholar] [CrossRef]



| Antibiotic (mg/L) | AM | GM | KM | SM | EM | CM | TC | CL | |
|---|---|---|---|---|---|---|---|---|---|
| Susceptibility | EFSA BP | 2 | 8 | 64 | 64 | 1 | 1 | 16 | 4 |
| KBL346 | 2 | 0.38 | 12 | 4 | 0.023 | <0.016 | 12 | 0.75 | |
| Resistance gene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Contig Name | Size (bp) | GC (%) | CDS | tRNA | rRNA |
|---|---|---|---|---|---|
| Contig 1 | 2,227,886 | 39.1 | 2151 | 69 | 18 |
| Contig 2 | 19,058 | 36.9 | 17 | 0 | 0 |
| Total | 2,246,944 | 39.0 | 2168 | 69 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Ji, S.Y.; Hwangbo, H.; Shin, S.-y.; Kim, M.Y.; Lee, K.; Kim, D.H.; Cho, B.-R.; Lee, H.; Choi, Y.H.; et al. Enhancement of Immune Functions by Limosilactobacillus reuteri KBL346: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2024, 25, 141. https://doi.org/10.3390/ijms25010141
Park C, Ji SY, Hwangbo H, Shin S-y, Kim MY, Lee K, Kim DH, Cho B-R, Lee H, Choi YH, et al. Enhancement of Immune Functions by Limosilactobacillus reuteri KBL346: In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 2024; 25(1):141. https://doi.org/10.3390/ijms25010141
Chicago/Turabian StylePark, Chanseop, Seon Yeong Ji, Hyun Hwangbo, Seung-yeon Shin, Min Yeong Kim, Kiuk Lee, Da Hye Kim, Bo-Ram Cho, Hyesook Lee, Yung Hyun Choi, and et al. 2024. "Enhancement of Immune Functions by Limosilactobacillus reuteri KBL346: In Vitro and In Vivo Studies" International Journal of Molecular Sciences 25, no. 1: 141. https://doi.org/10.3390/ijms25010141
APA StylePark, C., Ji, S. Y., Hwangbo, H., Shin, S.-y., Kim, M. Y., Lee, K., Kim, D. H., Cho, B.-R., Lee, H., Choi, Y. H., & You, H. J. (2024). Enhancement of Immune Functions by Limosilactobacillus reuteri KBL346: In Vitro and In Vivo Studies. International Journal of Molecular Sciences, 25(1), 141. https://doi.org/10.3390/ijms25010141








