Perfluorooctanoic Acid (PFOA) Exposure Compromises Fertility by Affecting Ovarian and Oocyte Development
Abstract
1. Introduction
2. Results
2.1. Acute Toxicity Test of PFOA on Zebrafish
2.2. PFOA Exposure Compromises the Fertility
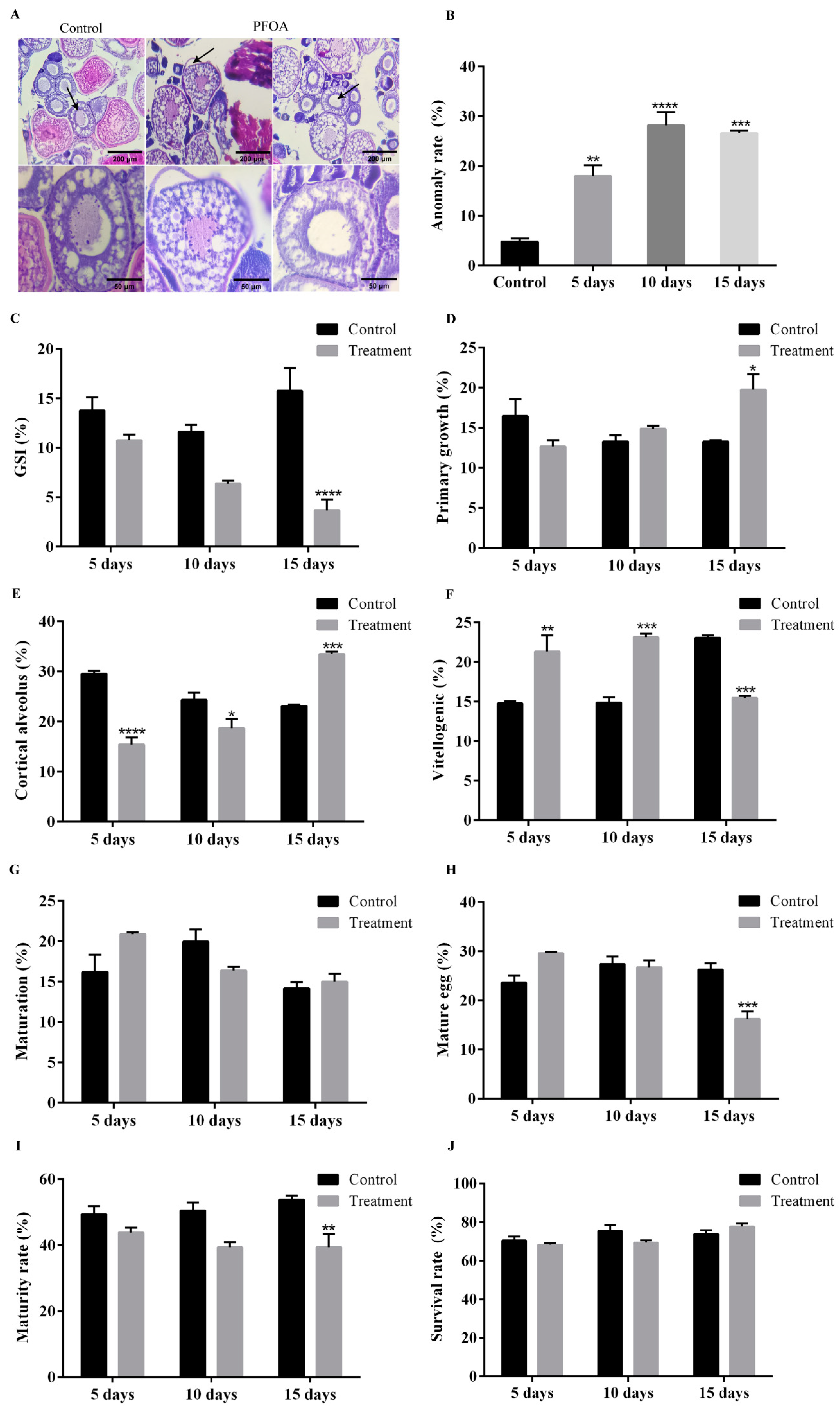
2.3. Effect of PFOA on Ovary Morphology and Oocytes Maturation
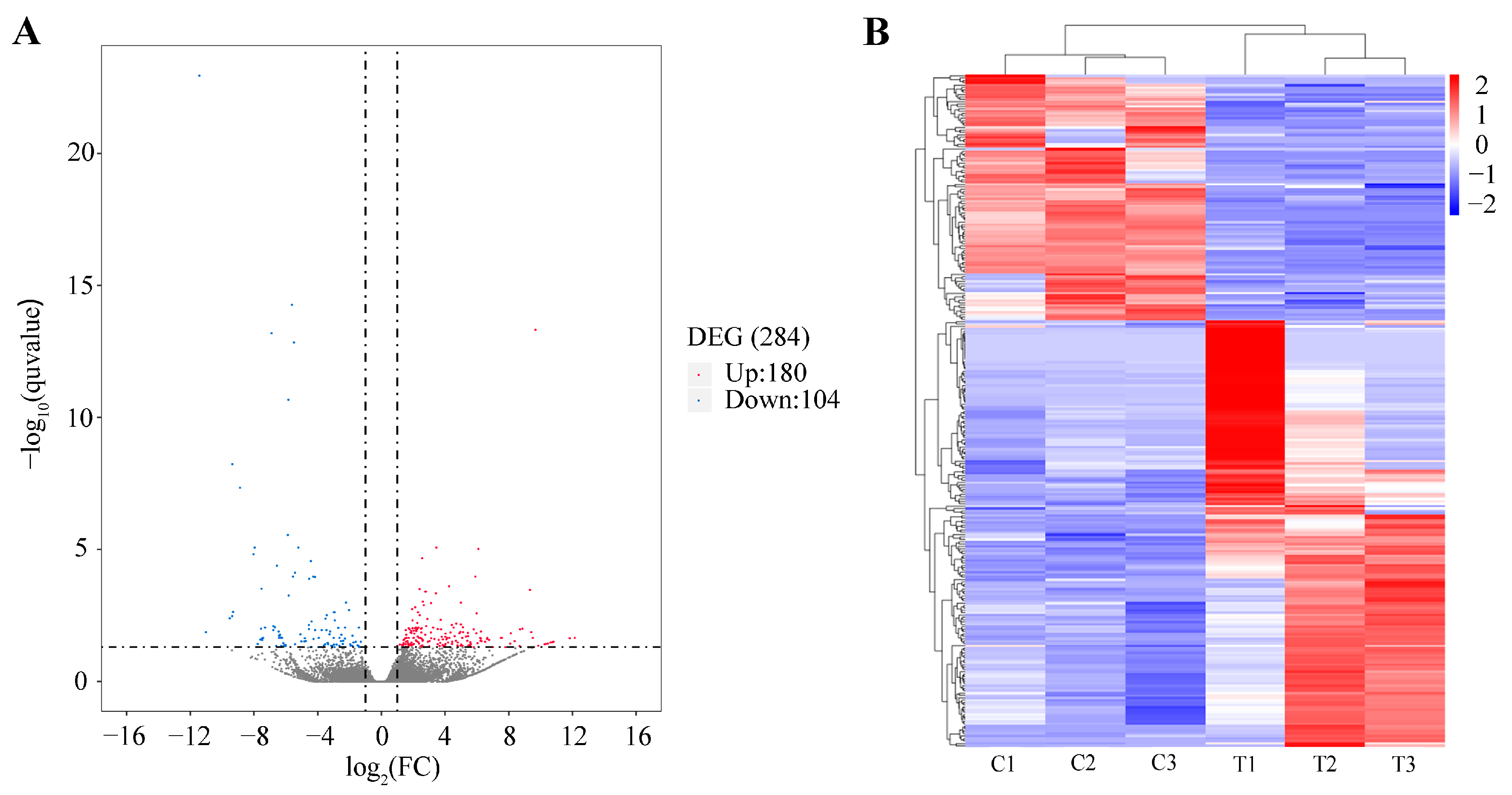
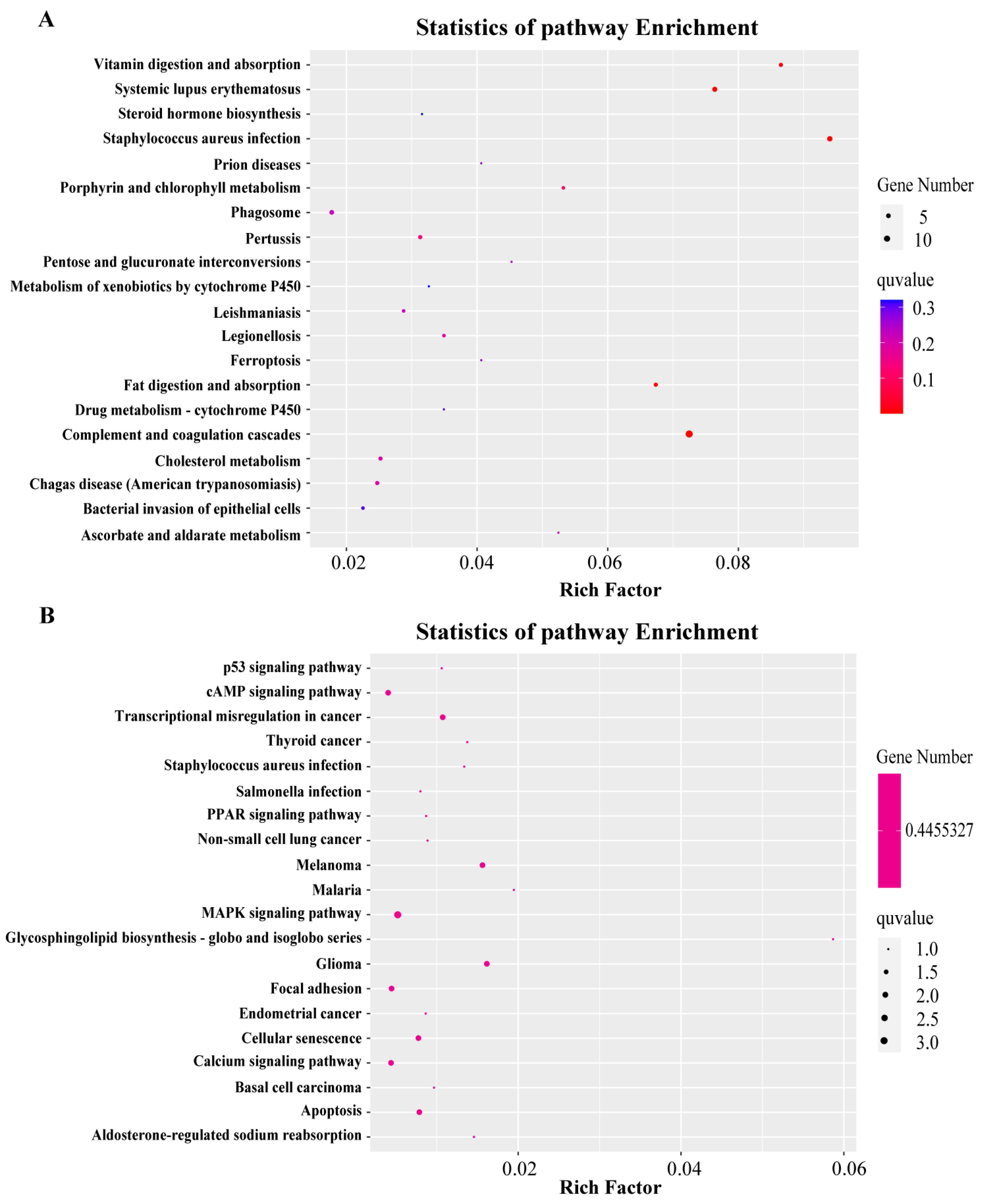
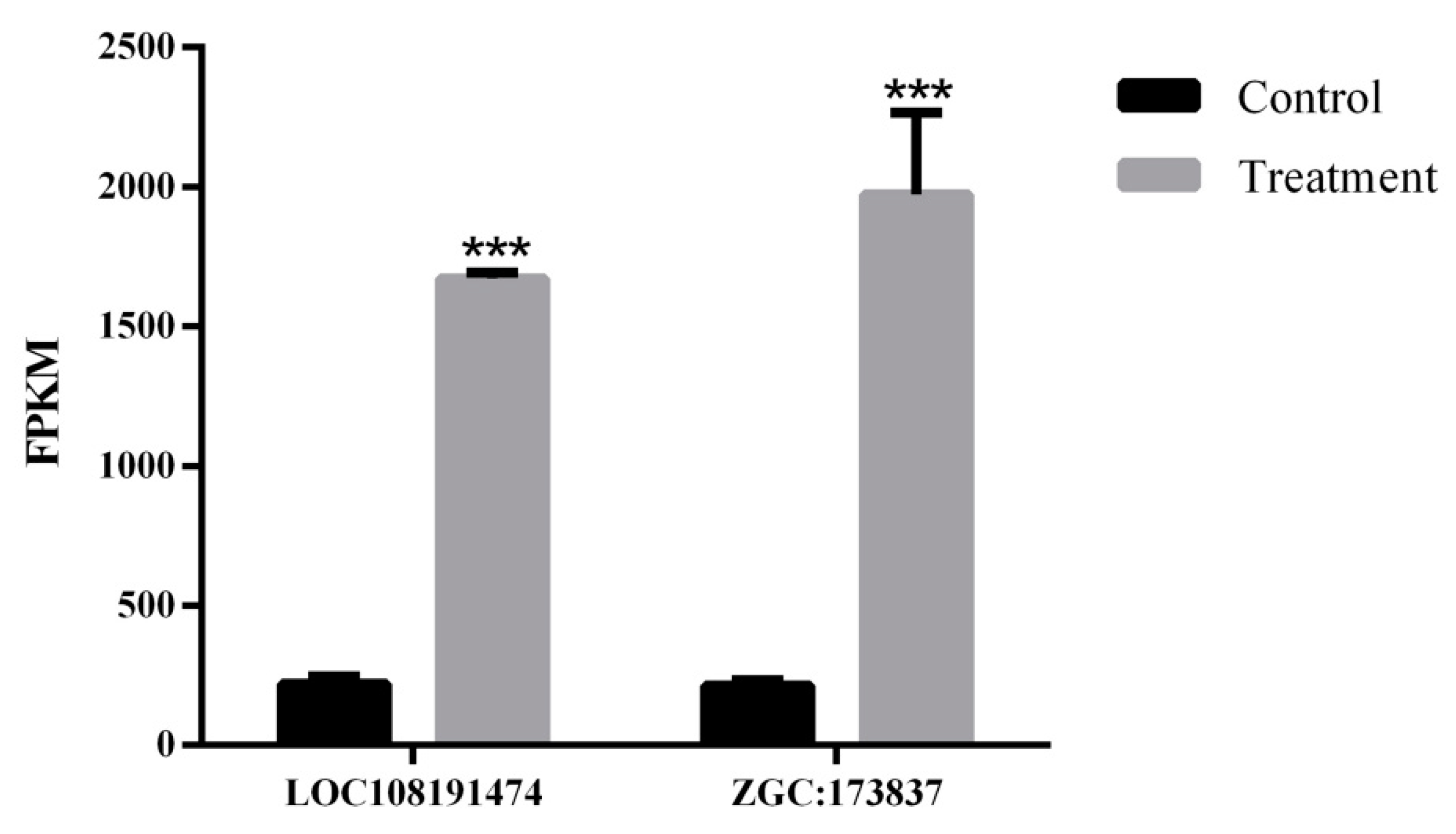
2.4. Transcriptome Analysis Identified the Potential Mechanism of PFOA-Caused Abnormal Fertility
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Exposure Regimens
4.3. Sample Collection
4.4. Reproductive Indices
4.5. Histological Analysis
4.6. In Vitro Culture of Zebrafish Oocytes
4.7. Transcriptome Sequencing
4.8. Differentially Expressed Genes and Enrichment Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, A.A.; Leffers, H. Emerging endocrine disrupters: Perfluoroalkylated substances. Int. J. Androl. 2008, 31, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.G.; Xiao, S.K.; Yu, K.F.; Wu, Q.; Wang, Y.H. Legacy and alternative per- and polyfluoroalkyl substances in a subtropical marine food web from the Beibu Gulf, South China: Fate, trophic transfer and health risk assessment. J. Hazard. Mater. 2021, 403, 123618. [Google Scholar] [CrossRef] [PubMed]
- De Felip, E.; Abballe, A.; Albano, F.L.; Battista, T.; Carraro, V.; Conversano, M.; Franchini, S.; Giambanco, L.; Iacovella, N.; Ingelido, A.M.; et al. Current exposure of Italian women of reproductive age to PFOS and PFOA: A human biomonitoring study. Chemosphere 2015, 137, 1–8. [Google Scholar] [CrossRef]
- Parsons, J.R.; Saez, M.; Dolfing, J.; de Voogt, P. Biodegradation of perfluorinated compounds. Rev. Environ. Contam. Toxicol. 2008, 196, 53–71. [Google Scholar] [PubMed]
- Tian, Y.; Zhou, Y.; Miao, M.; Wang, Z.; Yuan, W.; Liu, X.; Wang, X.; Wang, Z.; Wen, S.; Liang, H. Determinants of plasma concentrations of perfluoroalkyl and polyfluoroalkyl substances in pregnant women from a birth cohort in Shanghai, China. Environ. Int. 2018, 119, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.M.; Macleod, M.; Cousins, I.T. Comparative assessment of the global fate and transport pathways of long-chain perfluorocarboxylic acids (PFCAs) and perfluorocarboxylates (PFCs) emitted from direct sources. Environ. Sci. Technol. 2009, 43, 5830–5836. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; He, M.Y.; Ling, Y.; Wang, X.J.; Zhang, F.; Feng, X.S.; Zhang, Y.; Xing, S.G.; Li, J.; Qiu, X.; et al. Tissue distribution study of perfluorooctanoic acid in exposed zebrafish using MALDI mass spectrometry imaging. Environ. Pollut. 2022, 293, 118505. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, P.; Hu, B.; Wang, C.; Li, D. Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) in Surface Water of China: National Exposure Distributions and Probabilistic Risk Assessment. Arch. Environ. Contam. Toxicol. 2021, 81, 470–481. [Google Scholar] [CrossRef]
- Barbarossa, A.; Gazzotti, T.; Farabegoli, F.; Mancini, F.R.; Zironi, E.; Busani, L.; Pagliuca, G. Assessment of Perfluorooctane Sulfonate and Perfluorooctanoic Acid Exposure Through Fish Consumption in Italy. Ital. J. Food Saf. 2016, 5, 6055. [Google Scholar] [CrossRef]
- Houde, M.; De Silva, A.O.; Muir, D.C.; Letcher, R.J. Monitoring of perfluorinated compounds in aquatic biota: An updated review. Environ. Sci. Technol. 2011, 45, 7962–7973. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nature reviews. Genetics 2007, 8, 353–367. [Google Scholar] [PubMed]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Yahia, D.; El-Nasser, M.A.; Abedel-Latif, M.; Tsukuba, C.; Yoshida, M.; Sato, I.; Tsuda, S. Effects of perfluorooctanoic acid (PFOA) exposure to pregnant mice on reproduction. J. Toxicol. Sci. 2010, 35, 527–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Y.T.; Li, R.; Li, S.H.; Ma, X.; Liu, L.; Niu, D.; Duan, X. Perfluorooctanoic acid (PFOA) exposure affects early embryonic development and offspring oocyte quality via inducing mitochondrial dysfunction. Environ. Int. 2022, 167, 107413. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Song, C.; Wu, H.; Chen, X.; Zhang, Y. Adverse Effects of High Concentrations of Fluoride on Characteristics of the Ovary and Mature Oocyte of Mouse. PLoS ONE 2015, 10, e0129594. [Google Scholar] [CrossRef]
- Selman, K.; Wallace, R.A.; Sarka, A.; Qi, X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J. Morphol. 1993, 218, 203–224. [Google Scholar] [CrossRef]
- Nair, S.; Lindeman, R.E.; Pelegri, F. In vitro oocyte culture-based manipulation of zebrafish maternal genes. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2013, 242, 44–52. [Google Scholar]
- Ulhaq, M.; Sundström, M.; Larsson, P.; Gabrielsson, J.; Bergman, Å.; Norrgren, L.; Örn, S. Tissue uptake, distribution and elimination of (14)C-PFOA in zebrafish (Danio rerio). Aquat. Toxicol. 2015, 163, 148–157. [Google Scholar] [CrossRef]
- Palma-Vera, S.E.; Schoen, J.; Chen, S. Periovulatory follicular fluid levels of estradiol trigger inflammatory and DNA damage responses in oviduct epithelial cells. PLoS ONE 2017, 12, e0172192. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Schock, H.; Poole, E.M.; Terry, K.L.; Tamimi, R.M.; Hankinson, S.E.; Rosner, B.A.; Tworoger, S.S. A prospective cohort study of oral contraceptive use and ovarian cancer among women in the United States born from 1947 to 1964. Cancer Causes Control. 2017, 28, 371–383. [Google Scholar] [CrossRef]
- Singh, A.P.; Senapati, S.; Ponnusamy, M.P.; Jain, M.; Lele, S.M.; Davis, J.S.; Remmenga, S.; Batra, S.K. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. The Lancet. Oncology 2008, 9, 1076–1085. [Google Scholar] [PubMed]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nature reviews. Cancer 2004, 4, 45–60. [Google Scholar] [PubMed]
- Kumar, S.; Das, S.; Rachagani, S.; Kaur, S.; Joshi, S.; Johansson, S.L.; Ponnusamy, M.P.; Jain, M.; Batra, S.K. NCOA3-mediated upregulation of mucin expression via transcriptional and post-translational changes during the development of pancreatic cancer. Oncogene 2015, 34, 4879–4889. [Google Scholar] [CrossRef] [PubMed]
- Pecquet, A.M.; Maier, A.; Kasper, S.; Sumanas, S.; Yadav, J. Exposure to perfluorooctanoic acid (PFOA) decreases neutrophil migration response to injury in zebrafish embryos. BMC Res. Notes 2020, 13, 408. [Google Scholar] [CrossRef]
- Valsecchi, S.; Conti, D.; Crebelli, R.; Polesello, S.; Rusconi, M.; Mazzoni, M.; Preziosi, E.; Carere, M.; Lucentini, L.; Ferretti, E.; et al. Deriving environmental quality standards for perfluorooctanoic acid (PFOA) and related short chain perfluorinated alkyl acids. J. Hazard. Mater. 2017, 323, 84–98. [Google Scholar] [CrossRef]
- Ye, L.; Wu, L.L.; Jiang, Y.X.; Zhang, C.J.; Chen, L. Toxicological study of PFOS/PFOA to zebrafish (Danio rerio) embryos. Huanjing Kexue 2009, 30, 1727–1732. [Google Scholar]
- Colombo, I.; de Wolf, W.; Thompson, R.S.; Farrar, D.G.; Hoke, R.A.; L’Haridon, J. Acute and chronic aquatic toxicity of ammonium perfluorooctanoate (APFO) to freshwater organisms. Ecotoxicol. Environ. Saf. 2008, 71, 749–756. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Kundu, G.K.; Al-Mamun, M.H.; Sarkar, S.K.; Akter, M.S.; Khan, M.S. Chromium (VI) induced acute toxicity and genotoxicity in freshwater stinging catfish, Heteropneustes fossilis. Ecotoxicol. Environ. Saf. 2013, 92, 64–70. [Google Scholar] [CrossRef]
- Jantzen, C.E.; Toor, F.; Annunziato, K.A.; Cooper, K.R. Effects of chronic perfluorooctanoic acid (PFOA) at low concentration on morphometrics, gene expression, and fecundity in zebrafish (Danio rerio). Reprod. Toxicol. 2017, 69, 34–42. [Google Scholar] [CrossRef]
- Estefania Gonzalez-Alvarez, M.; Severin, A.; Sayadi, M.; Keating, A.F. PFOA-Induced Ovotoxicity Differs Between Lean and Obese Mice with Impacts on Ovarian Reproductive and DNA Damage Sensing and Repair Proteins. Toxicol. Sci. Off. J. Soc. Toxicol. 2022, 190, 173–188. [Google Scholar] [CrossRef]
- Heffernan, A.L.; Cunningham, T.K.; Drage, D.S.; Aylward, L.L.; Thompson, K.; Vijayasarathy, S.; Mueller, J.F.; Atkin, S.L.; Sathyapalan, T. Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. Int. J. Hyg. Environ. Health 2018, 221, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Gao, F.; Zhang, X.; Wang, L.; Liu, J.; Fu, M.; Zhang, S.; Wan, Y.; Shen, H.; Hu, J. Nontargeted identification of per- and polyfluoroalkyl substances in human follicular fluid and their blood-follicle transfer. Environ. Int. 2020, 139, 105686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qi, C.; Ma, Z.; Wang, Y.; Zhang, L.; Hou, X. Perfluorooctanoic acid exposure in vivo perturbs mitochondrial metabolic during oocyte maturation. Environ. Toxicol. 2022, 37, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhao, Z.; Zhao, K.; Huang, J.; Ding, L.; Huang, X.; Meng, L.; Li, L.; Wei, H.; Zhang, S. Perfluorooctanoic acid inhibits the maturation rate of mouse oocytes cultured in vitro by triggering mitochondrial and DNA damage. Birth Defects Res. 2021, 113, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chang, M.X.; Ma, J.; LaPatra, S.E.; Hu, Y.W.; Huang, L.; Nie, P.; Zeng, L. Transcriptomic analysis of the host response to an iridovirus infection in Chinese giant salamander, Andrias davidianus. Vet. Res. 2015, 46, 136. [Google Scholar] [CrossRef] [PubMed]
- Oikonomopoulou, K.; Ricklin, D.; Ward, P.A.; Lambris, J.D. Interactions between coagulation and complement--their role in inflammation. Semin. Immunopathol. 2012, 34, 151–165. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Xie, H.; Liu, W.; Fu, Q.; Yao, D.; Xu, J.; Gu, J. High mucin 5AC expression predicts adverse postoperative recurrence and survival of patients with clear-cell renal cell carcinoma. Oncotarget 2017, 8, 59777–59790. [Google Scholar] [CrossRef][Green Version]
- Yang, B.; Wu, A.; Hu, Y.; Tao, C.; Wang, J.M.; Lu, Y.; Xing, R. Mucin 17 inhibits the progression of human gastric cancer by limiting inflammatory responses through a MYH9-p53-RhoA regulatory feedback loop. J. Exp. Clin. Cancer Res. CR 2019, 38, 283. [Google Scholar] [CrossRef]
- Pan, W.Y. Biotoxicological Effects and Risk Assessment of Zebrafish by PFOS and PFOA; Hebei University of Science & Technology: Hebei, China, 2020; pp. 17–20. [Google Scholar]


| Exposure Duration (h) | LC50 (mg/L) | 95% Confidence Interval |
|---|---|---|
| 24 | 423.654 | 394.104–450.290 |
| 48 | 410.980 | 385.357–441.295 |
| 72 | 393.741 | 359.000–418.490 |
| 96 | 375.578 | 347.802–404.769 |
| Concentration (mg/L) | Fertilization Rate (%) | Hatching Rate (%) | Deformation Rate (%) |
|---|---|---|---|
| 0 | 78.00 ± 2.65 | 88.67 ± 2.19 | 0.33 ± 0.33 |
| 25 | 79.33 ± 1.20 | 90.33 ± 0.67 | 0.00 ± 0.00 |
| 50 | 76.33 ± 0.67 | 87.00 ± 4.04 | 2.33 ± 0.67 |
| 75 | 63.00 ± 6.00 | 81.00 ± 3.00 | 8.33 ± 0.88 |
| 100 | 51.67 ± 1.20 | 56.74 ± 1.43 | 13.61 ± 1.41 |
| Day 5 | Day 10 | Day 15 | ||||
|---|---|---|---|---|---|---|
| Control | Treatment | Control | Treatment | Control | Treatment | |
| GSI (%) | 13.80 ± 1.33 | 10.77 ± 0.60 | 11.65 ± 0.68 | 6.40 ± 0.27 | 15.78 ± 2.32 | 3.68 ± 1.07 |
| Primary growth (%) | 16.47 ± 2.14 | 12.67 ± 0.80 | 13.33 ± 0.75 | 14.90 ± 0.36 | 13.30 ± 0.17 | 19.75 ± 1.97 |
| Cortical alveolus (%) | 29.59 ± 0.51 | 15.45 ± 1.40 | 24.35 ± 1.39 | 18.70 ± 1.86 | 23.12 ± 0.29 | 33.50 ± 0.50 |
| Vitellogenic (%) | 14.80 ± 0.26 | 21.35 ± 2.04 | 14.88 ± 0.67 | 23.21 ± 0.42 | 23.12 ± 0.29 | 15.48 ± 0.24 |
| Maturation (%) | 16.21 ± 2.16 | 20.88 ± 0.22 | 19.97 ± 1.51 | 16.40 ± 0.45 | 14.18 ± 0.82 | 15.02 ± 0.95 |
| Mature egg (%) | 23.61 ± 1.49 | 29.66 ± 0.26 | 27.46 ± 1.53 | 26.79 ± 1.35 | 26.27 ± 1.31 | 16.24 ± 1.55 |
| Maturity rate (%) | 49.44 ± 2.42 | 43.89 ± 1.47 | 50.56 ± 2.42 | 39.45 ± 1.47 | 53.89 ± 1.11 | 39.45 ± 4.00 |
| Survival rate (%) | 70.56 ± 2.00 | 68.33 ± 0.96 | 75.56 ± 2.94 | 69.44 ± 1.11 | 73.89 ± 2.00 | 77.78 ± 1.47 |
| Sample | Clean Reads | Clean Bases (bp) | GC (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|
| Control.1 | 50,483,288 | 7,572,493,200 | 47.99; 48.06 | 96.79; 96.64 | 91.98; 91.46 |
| Control.2 | 52,995,288 | 7,949,293,200 | 47.89; 47.89 | 96.95; 97.23 | 92.47; 92.88 |
| Control.3 | 41,232,102 | 6,184,815,300 | 48.24; 48.33 | 96.92; 95.81 | 92.35; 89.66 |
| Treatment.1 | 48,007,202 | 7,201,080,300 | 48.65; 48.77 | 96.77; 96.06 | 91.93; 90.26 |
| Treatment.2 | 39,874,770 | 5,981,215,500 | 48.07; 48.13 | 96.84; 96.50 | 92.18; 91.23 |
| Treatment.3 | 42,874,982 | 6,431,247,300 | 48.14; 48.16 | 96.95; 95.85 | 92.54; 89.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Han, L.; Qiu, L.; Zhao, B.; Gao, Y.; Chu, Z.; Dai, X. Perfluorooctanoic Acid (PFOA) Exposure Compromises Fertility by Affecting Ovarian and Oocyte Development. Int. J. Mol. Sci. 2024, 25, 136. https://doi.org/10.3390/ijms25010136
Zhang H, Han L, Qiu L, Zhao B, Gao Y, Chu Z, Dai X. Perfluorooctanoic Acid (PFOA) Exposure Compromises Fertility by Affecting Ovarian and Oocyte Development. International Journal of Molecular Sciences. 2024; 25(1):136. https://doi.org/10.3390/ijms25010136
Chicago/Turabian StyleZhang, Han, Lulu Han, Lijun Qiu, Bo Zhao, Yang Gao, Zhangjie Chu, and Xiaoxin Dai. 2024. "Perfluorooctanoic Acid (PFOA) Exposure Compromises Fertility by Affecting Ovarian and Oocyte Development" International Journal of Molecular Sciences 25, no. 1: 136. https://doi.org/10.3390/ijms25010136
APA StyleZhang, H., Han, L., Qiu, L., Zhao, B., Gao, Y., Chu, Z., & Dai, X. (2024). Perfluorooctanoic Acid (PFOA) Exposure Compromises Fertility by Affecting Ovarian and Oocyte Development. International Journal of Molecular Sciences, 25(1), 136. https://doi.org/10.3390/ijms25010136





